Abstract
Interleukin-12 (IL-12) is a key cytokine, which promotes T helper type 1 (Th1) cell-mediated immunity and inhibits Th2-type responses. It has been previously shown that IL-12 administration during active immunization following a single allergen exposure can prevent antigen-induced increases in immunoglobulin E (IgE) formation, Th2 cytokine production and bronchoalveolar lavage (BAL) eosinophils in a murine model of allergic airway inflammation. Thus, these studies have now been extended and two IL-12 treatment protocols on this murine model were evaluated. Administration of IL-12 during the active immunization strikingly increased Der p I-specific serum IgG2a and transiently decreased the levels of IgG1 and IgE antibodies following multiple allergen challenges. Such early treatment of IL-12 down-regulated IL-5 production and modestly up-regulated interferon-γ production but did not effect BAL eosinophilia. These results suggest that repeated exposure to antigen and IL-12 is necessary to maintain a persistent Th1-recall response. Furthermore, administration of IL-12 to actively immunized mice, in which Th2-associated responses were established, had a significant effect on IgG2a synthesis and a modest effect on IgE levels, also down-regulation of IL-5 production, and markedly increased interferon-γ production and abolished recruitment of eosinophils. Therefore, these data indicate that IL-12 can inhibit antigen-induced eosinophil infiltration into airways, despite the existence of a Th2-associated response. Taken together, these studies suggest that IL-12 may be useful as an immunotherapeutic agent in the treatment of such pulmonary allergic disorders as bronchial asthma.
INTRODUCTION
The house dust mite is one of the most important inhalant allergens in respiratory disorders, such as bronchial asthma and allergic rhinitis.1 Among many species of mite in house dust, Dermatophagoides pteronyssinus (Dp) is dominant, as many basic and clinical studies have indicated. Der p I, a 25 000 MW glycoprotein found in mite faeces, has been purified and shown to be the predominant antigen.2 However, no study has described an animal model of airway inflammation induced by Der p I allergen. Allergic asthma is characterized mainly by elevated specific immunoglobulin E (IgE) antibody production and eosinophilic inflammation.3,4 It has been proposed that eosinophils mediate tissue injury and airway hyperresponsiveness.5 Since IgE production and eosinophil differentiation and recruitment are positively controlled by the type 2 cytokines interleukin-4 (IL-4) and IL-5, respectively.6–9 It has been recognized that T helper type 2 (Th2) cells and their cytokines are responsible for the initiation and maintenance of allergic disorders.10 Thus, agents which decrease IgE levels or Th2 cytokine production or increase Th1 cytokine production may inhibit allergen-induced disorders.
Interleukin-12 (IL-12) is a key cytokine produced by macrophages to promote Th1-type cell-mediated immune functions.11,12 Previous studies have shown that treatment with IL-12 inhibits Th2 cytokines and related antibody production in vitro and in vivo.13–16 These biological activities form the basis for many studies examining the therapeutic potential of IL-12. Recent studies in several murine models have shown that IL-12 has tremendous potential as a vaccine-adjuvant in promoting a Th1 response.17–20 However, such enthusiasm for IL-12 as a biological adjuvant, founded primarily on striking data obtained in these short-term experimental systems, makes it impossible to determine whether IL-12 has lasting impact on Th1-recall responses following repeated antigen exposure in the absence of IL-12. In addition, several studies have demonstrated that IL-12 needs to be administered early in the sensitization process to induce a Th1-mediated immune response to inhaled antigen.14,21
Collectively, a key to the development of IL-12 as a vaccine adjuvant or a clinical therapeutic agent for allergic asthma will be better understanding of modes for using IL-12 to establish long-lived immune memory of Th1 responses, to reverse or inhibit existing Th2 responses. Therefore, to address these issues, a well-defined mouse model of airway inflammation induced by Der p I allergen was used and two protocols of IL-12 treatment were tested.
MATERIALS AND METHODS
Animals
Female, 7-week-old C57BL/6 mice were obtained from and maintained in the Animal Center of the College of Medicine, National Taiwan University.
Preparation of antigens
The allergen Der p I was isolated by affinity column from spent mite media, which was kindly provided by Dr K-Y. Chua (The National University of Singapore). Firstly, 5 g of spent mite media were mixed with 100 ml of 0·1 m Tris–HCl (pH 7·6) and then stirred overnight at 4°. The mite extract was collected after centrifugation at 19 000 g for 30 min at 4° and passed through the anti-Der p I affinity column. The column was washed with phosphate-buffered saline (PBS) and then Der p I protein was eluted with NH4OH (pH 11) at 4°. Immediately, 0·1 m Tris–HCl (pH 6·8) was added to neutralize the eluted fractions. The pooled fractions were dialysed against PBS and further concentrated. Finally, the concentrated product was monitored at optical density (OD) 562 nm and stored at −20° before use.
The lyophilized house dust mite, Dp, was purchased from Allergon (Angelholm, Sweden). The allergen was prepared as described.22 Briefly, 1 g of lyophilized mite body was defatted with 100 ml ether, then homogenized and stirred continuously in 25 ml PBS for 48 hr at 4°. After centrifugation (12 000 g, 30 min), the crude extract was dialysed with PBS, then the mite extract was dissolved in PBS and stored at −20°.
Immunization and inhalation exposure of mice
For systemic immunization, 10 μg Der p I was mixed with 2 mg alum plus 400 ng pertussis toxin (List Biological Lab. Inc., Campbell, CA) as the adjuvant and injected intraperitoneally in a volume of 100 μl.
To examine the effects of recombinant mouse IL-12 (rIL-12; R & D, Minneapolis, MN), two groups of mice were in addition treated with IL-12 for 5 days (day −1 to +3) simultaneously with immunization indicated. IL-12 was administered intraperitoneally at 1 μg/mouse/day. Control mice received PBS instead of IL-12.
Aerosol immunization was performed with crude mite extract (Dermatophagoides pteronyssinus) solution. The aerosols were generated into the chamber using an ultrasonic nebulizer (DeVilbiss, Somerset, PA). The output of the nebulizer was 0·3 ml/min, and the produced particles had a size range of 0·5–5 μm. The concentration of crude mite extract in the nebulizer was 0·1% (w/v). The mice were exposed to 8 ml suspension over a 20-min period, by placing them in a chamber, which could contain six to eight mice concurrently.
Experimental design
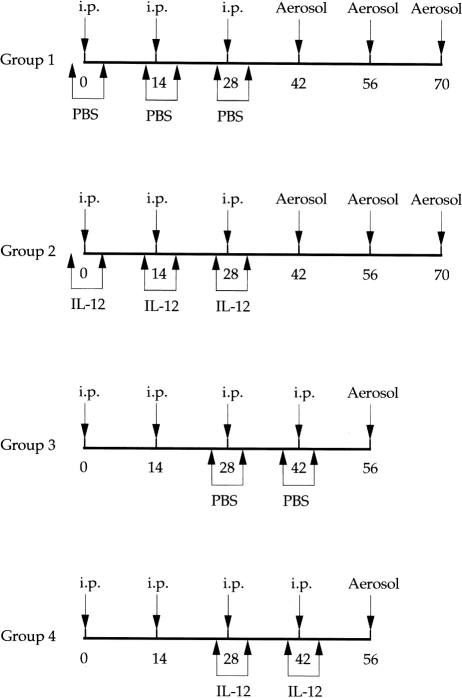
The experimental design is summarized in Fig. 1. Group 1 (n =8) was immunized three times intraperitoneally with Der p I in Al(OH)3 plus pertussis toxin at days 0, 14 and 28. On day 42, day 56 and day 70, the mice were aerosolized with crude mite extract. As a control, one group was given PBS on days −1 to 3, days 13 to 17 and days 27 to 31.
Figure 1.
Immunization protocol of the four experimental groups of C57BL/6 mice (n =8). Intraperitoneal (i.p.) Der p I injections consisted of 10 μg of Der p I, 2 mg Al(OH)3 and 400 ng pertussis toxin dissolved in 100 μl of PBS/dose. Dp inhalation was performed with 8 mg of Dp dissolved in 8 ml of PBS/time. Intraperitoneal IL-12 consisted of 1 μg of IL-12 dissolved in 50 μl of PBS/dose and treated mice for 5 days (day −1 to +3) simultaneously with immunization indicated; i.p. PBS consisted of 50 μl of PBS/dose.
Group 2 (n =8) was immunized intraperitoneally with Der p I in Al(OH)3 plus pertussis toxin at days 0, 14 and 28 and subsequently aerosolized with the allergen as described above. In addition, rIL-12 was administered for 5 days each time (days −1 to 3, days 13 to 17, days 27 to 31).
Group 3 (n =8) was immunized intraperitoneally with Der p I in Al(OH)3 plus pertussis toxin at days 0, 14, 28 and 42 and thereafter aerosolized with crude mite extract on day 56. As control group, PBS was given for 5 days (days 27–31) and after 2 weeks for another 5 days (days 41–45).
Group 4 (n =8) was immunized intraperitoneally with Der p I in Al(OH)3 plus pertussis toxin at days 0, 14, 28 and 42 and subsequently aerosolized with the allergen on day 56. These mice were treated with rIL-12, administered from days 27 to 31 and from days 41 to 45. Our preliminary study has found that administration of IL-12 caused more severe adverse effects in Group 4 mice than in mice of Group 2. Furthermore, a short course of IL-12 delivery was designed for the purpose of treatment. Therefore, there were only two occasions of IL-12 treatment in Group 4 mice to explore the therapeutic effects of IL-12 on airway inflammation.
Mite allergen-specific antibody and total antibody assays
Der p I-specific IgE, IgG1 and IgG2a sera antibody titres were determined by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well microtitre plates were coated with 4 μg/ml Der p I diluted in NaHCO3 buffer, pH 8·2. After overnight incubation at 4°, plates were washed twice and blocked with 3% bovine serum albumin (BSA) in PBS for 2 hr at 37°. Serial dilution of sera were added to each well for an overnight period at 4°. Plates were washed and incubated with biotin-conjugated anti-mouse IgE (0·4 μg/ml, Serotec, Raleigh, NC) or IgG1 (1:500, PharMingen, San Diego, CA) or IgG2a (1:500, PharMingen) diluted in 3%BSA–PBS buffer for 1 hr at 37°. After further washes, streptavidin-conjugated alkaline phosphatase (1:2000, Sigma, St Louis, MO) was added for an additional 2 hr at room temperature. After extensive washing, wells were developed by phosphatase substrate p-nitrophenyl phosphate (pNPP) and absorbance at 405 nm was determined using a microplate reader. The levels of antibody were compared with IgG1, IgE and IgG2a standards with predetermined concentrations. (immunoglobulin concentrations: IgG1=28·2 μg/ml, IgE=1·1 μg/ml, IgG2a=16·7 μg/ml). For determination of serum total IgE level, microtitre plates were coated with 2 μg/ml of anti-mouse IgE (PharMingen) and blocked as described above. Serial dilutions of the sera and the IgE standard were added for 1 hr at 37° and then incubated with a biotin-conjugated anti-mouse IgE (1:500, PharMingen), followed by 1:2000 dilution of alkaline phosphatase-conjugated avidin and the substrate pNPP in reaction buffer. The levels of total IgG2a were measured by radial immunodiffusion (RID, The Binding Site, Birmingham, UK) method. The concentration was determined by measuring the ring diameter of the tested samples and reading off a RID reference table.
Antigen-specific proliferative assay
To measure the Der p I-specific T-cell proliferative response, the spleens were removed aseptically from rIL-12-treated or control (PBS-treated) mice 24 hr after the last allergen inhalation. The cells were plated into 96-well round-bottomed plates at a concentration of 3×105/well and were stimulated with 10 μg/ml of Der p I. In addition, phytohaemagglutinin (PHA; 10 μg/ml) was used as a positive mitogenic control and ovalbumin (4 μg/ml) was used as a negative control antigen. Control wells contained cells only. After 2 days in culture, the cells were pulsed with 1 μCi/well of [3H]TdR for 15–17 hr. Specific incorporation of TdR was determined by β-counter (Packard Instrument Co., Meriden, CT) and results were expressed as c.p.m.
Cytokines assay
To measure cytokine secretion, splenocytes (1×107/well) of immunized mice treated with or without IL-12 after the last allergen inhalation were cultured in 0·5 ml AIM-V medium (serum-free lymphocyte medium, Gibco BRL, Grand Island, NY) supplemented with 2% TCM (mouse serum replacement, Celox Lab., Hopkins, MN) in the presence of 10 μg/ml Der p I or PHA (10 μg/ml) in a 48-well microtitre plate at 37° for 48 hr. After the culture, the culture supernatants were collected and centrifuged at 400 g at 4°. The cell-free supernatants were stored at −20° until they were used for the cytokine assay. The quantities of IL-5 and interferon-γ (IFN-γ) in the culture supernatants of spleen cells were evaluated by sandwich-ELISA (PharMingen). The levels of sensitivity for the IL-5 and IFN-γ assay were 60 pg/ml and 150 pg/ml, respectively.
Bronchoalveolar lavage (BAL) and histopathological study
At 24 hr after the last aerosol exposure, all groups of mice were bled from the retro-orbital venous plexus and killed. In addition, the naive mice exposed to aerosolized allergen were used as negative controls. The lung was immediately lavaged via the trachea cannula with 3×1 ml of Hanks’ balanced salt solution (HBSS), free of ionized calcium and magnesium. The lavage fluid was centrifuged at 400 g for 10 min at 4°. After washing, the cells were resuspended in 1 ml HBSS and total cells were determined by counting in a haemocytometer. Cytocentrifuged preparations were stained with Liu’s stain for differential cell counts. A minimum of 200 cells was counted and classified as macrophages, lymphocytes, neutrophils and eosinophils, based on standard morphological criteria.
To evaluate the effects of IL-12 treatment on allergen-induced lung inflammation, each group of animals was killed for histopathological examination. After the lavage, the lungs were immediately removed and fixed in 10% neutral-buffered formalin. The tissues were subsequently embedded in paraffin and cut into 5 μm thick sections. These frozen sections were stained with haematoxylin and eosin and examined using light microscopy for histological changes.
The levels of serotonin in BAL fluids
The levels of serotonin in lavage fluids were measured by serotonin ELISA kit (IBL, Hamburg, Germany) according to the manufacturer’s recommendations. Absorbance values, read at 405 nm were converted to concentration in BAL fluids by comparison with a standard curve. The level of sensitivity for serotonin assay was 0·03 ng/ml. Because the sample preparation leads to a 207·25-fold dilution, the values read from the standard curve have to be corrected by multiplying by 207·25.
Statistical analysis
Individual experimental values were compared by the paired two-tailed Student’s t-test. Differences between two groups were considered significant at P < 0·05.
RESULTS
Effects of IL-12 treatment on long-term antibody responses to Der p I allergen
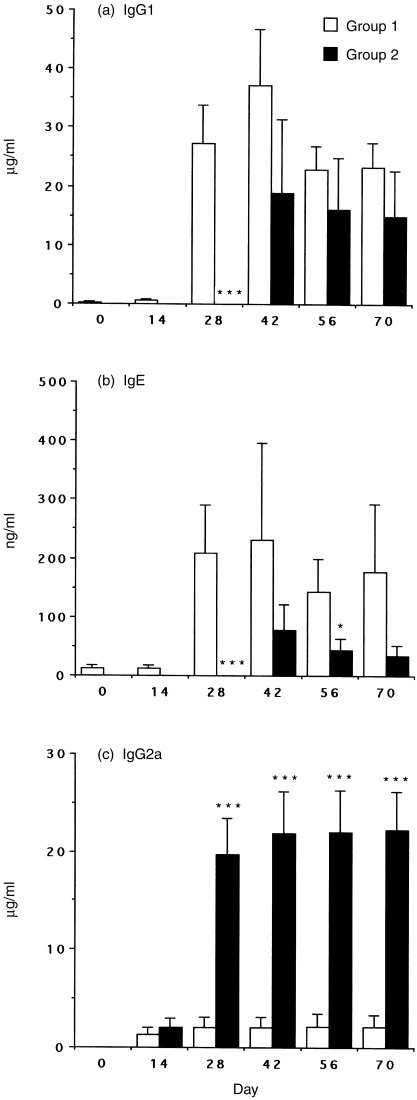
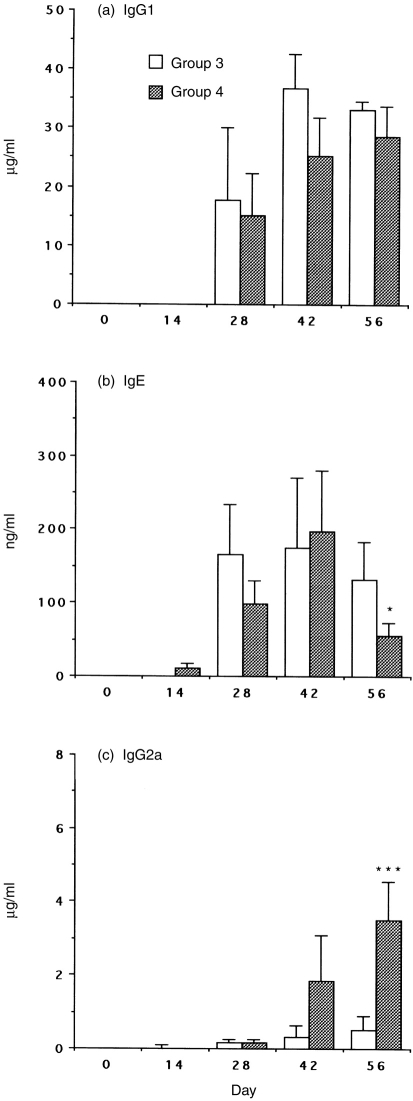
To evaluate the in vivo impact of rIL-12 as vaccine adjuvant on the maintenance of Th1 activity and the capacity of rIL-12 to enhance Th1-associated responses under Th2-dominated conditions, administration of IL-12 was timed to interfere with either initial allergen sensitization (Group 2) or boosting allergen exposure (Group 4). In Groups 1 and 3, antibodies of the IgG1 were produced in large amounts (Fig. 2a, Fig. 3a). Furthermore, substantial amounts of IgE were produced, whereas the synthesis of IgG2a was low (Fig. 2b, c; Fig. 3b, c). The data indicated allergic sensitization of these mice that normally develops in a Th2 manner. In Group 2, IL-12 treatment transiently suppressed Der p I-specific IgG1 (Fig. 2a, P < 0·001) and IgE (Fig. 2b, P < 0·005) responses but failed to show a difference from Group 3 since day 42. However, the IgG2a levels were strongly augmented and consistently maintained in Group 2 even without IL-12 treatment for a long period (Fig. 2c, P < 0·001). In Group 4, IL-12 treatment did not significantly down-regulate IgG1 production (Fig. 3a) and the serum IgE levels were not decreased until day 56 (Fig. 3b, P < 0·05). Whereas the synthesis of IgG2a was significantly up-regulated after two cycles of IL-12 treatment in Group 4 (Fig. 3c, P < 0·001).
Figure 2.
Serum IgG1, IgE and IgG2a antibody responses to Der p I. Groups 1 and 2 are described in the Materials and Methods. C57BL/6 mice were immunized i.p. three times with 10 μg/mouse Der p I adsorbed to alum plus pertussis toxin at biweekly intervals. In Group 2, IL-12 was administered i.p. at 1 μg/day for 5 days (day −1 to +3) to mice simultaneously with each systemic immunization. After 2 weeks, sensitized mice were aerosolized three times with Dp extract on days 42, 56 and 70. Blood was collected on the days indicated and sera levels of anti-Der p I antibodies IgG1 (a), IgE (b) and IgG2a (c) were assayed by using ELISA. Data are shown as mean±SEM for eight mice per group. Significant differences (*P < 0·05; ***P < 0·001) from the immunized IL-12-untreated Group 1 are indicated.
Figure 3.
Serum IgG1, IgE and IgG2a antibody responses to Der p I. Groups 3 and 4 are described in the Materials and Methods. C57BL/6 mice were immunized i.p. four times with 10 μg/mouse Der p I adsorbed to alum plus pertussis toxin at biweekly intervals. In Group 4, IL-12 was administered i.p. at 1 μg/day for 5 days (day −1 to +3) to mice simultaneously with the third and fourth systemic immunizations. After 2 weeks, each group of sensitized mice was challenged with aerosolized Dp allergen. Blood was collected on the days indicated and sera levels of anti-Der p I antibodies IgG1 (a), IgE (b) and IgG2a (c) were assayed by using ELISA. Data are shown as mean±SEM for eight mice per group. Significant differences (*P < 0·05; ***P < 0·001) from the immunized IL-12-untreated Group 3 are indicated.
The total IgE and IgG2a serum levels were summarized in Table 1. The results suggested that there was no significant difference in the level of total IgE among the four groups. It further indicated that the reduced levels of Der p I-specific IgE in mice of Group 2 and Group 4 were regulated by an antigen-specific process. In contrast, the level of total IgG2a was significantly enhanced as early as at day 28 in mice of Group 2 and such results were consistent with the level of Der p I-specific IgG2a antibodies. Nevertheless, the levels of total IgG2a showed no significant difference between the mice of Group 3 and Group 4.
Table 1.
Total serum IgE and IgG2a levels in Der p I-immunized mice
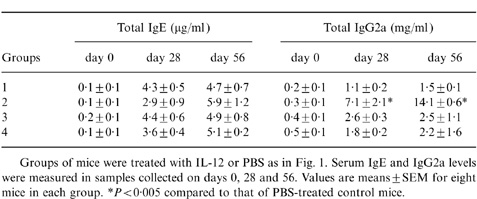
Groups of mice were treated with IL-12 or PBS as in Fig. 1 Serum IgE and IgG2a levels were measured in samples collected on days 0, 28 and 56. Values are means±SEM for eight mice in each group.
*P < 0·005 compared to that of PBS-treated control mice.
Effects of IL-12 treatment on T-cell response to Der p I allergen
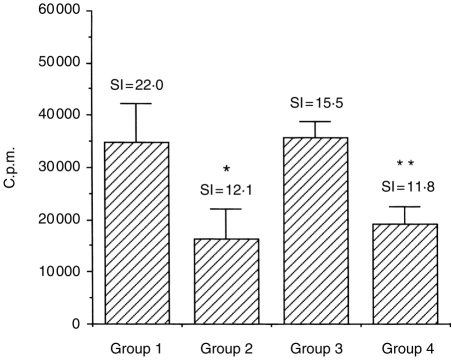
The results of Der p I-specific T-cell proliferative responses are summarized in Fig. 4. In Groups 2 and 4, proliferative responses were reduced 45% (P ≤ 0·05) and 24% (P ≤ 0·001), respectively. In some experiments, McKinght et al. have observed a 20–30% reduction of T-cell proliferation after IL-12 administration.17 However, the reason for this phenomenon is yet to be defined.
Figure 4.
The effect of IL-12 on Der p I antigen-specific T-cell proliferative response. C57BL/6 mice were treated as described in Fig. 1. Following the last inhalation, spleen cells were taken from these sensitized mice after 24 hr and restimulated with 10 μg/ml Der p I in vitro. Proliferation was measured by [3H]thymidine incorporation on day 3. The results are expressed as c.p.m. and shown as mean±SEM for seven or eight mice per group. The background values of controls were between 1450 and 2534 c.p.m. Ovalbumin (4 μg/ml) was used as a negative control antigen and the responses were between 1045 and 3138 c.p.m. in this assay. The stimulation index (SI) was calculated as the mean c.p.m. of the stimulated wells divided by the mean c.p.m. of the control wells. Significant differences (*P ≤ 0·05; **P ≤ 0·001) from the immunized untreated controls are indicated.
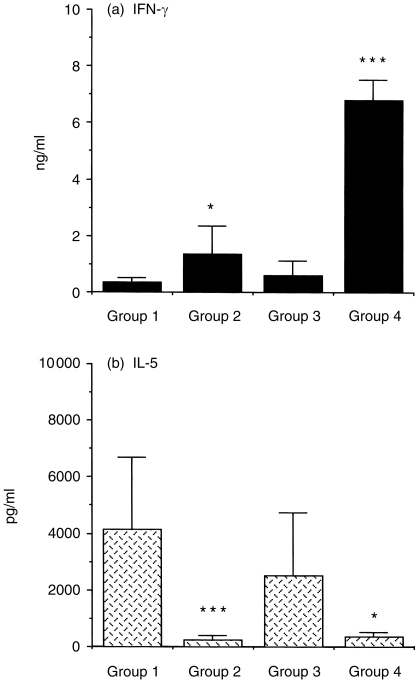
To determine whether the in vivo administration of rIL-12 affects antigen-specific T-cell function and thereby inhibits antigen-induced eosinophil recruitment, the in vitro production of IFN-γ and IL-5 of spleen cells in IL-12-treated mice was examined. In Group 2, IL-12 treatment modestly enhanced IFN-γ secretion about fourfold (Fig. 5a, P < 0·05 versus Group 1) and markedly suppressed IL-5 production by 94% as compared with that of Group 1 control mice (Fig. 5b, P < 0·001). In Group 4, IL-12 treatment significantly elicited IFN-γ secretion about 12-fold (Fig. 5a, P < 0·001 versus Group 3) and inhibited IL-5 production by 85% (Fig. 5b, P < 0·05 versus Group 3).
Figure 5.
The effect of IL-12 on IFN-γ, IL-5 production by splenocytes of mice after the last allergen challenge. Splenocytes from IL-12 and control groups were stimulated with 10 μg/ml Der p I in vitro and culture supernatants were collected after 48 hr, and the levels of cytokine production IFN-γ (a) and IL-5 (b) were measured by ELISA. Values shown are mean±SD of eight mice per group. Significant differences (*P < 0·05, ***P < 0·001) compared with paired control mice, respectively.
Effects of IL-12 treatment on allergen-induced airway inflammation
The results of cellular composition and inflammatory mediators in BAL fluids were shown in Table 2. Consistent with our previous findings, the numbers of macrophages, neutrophils, lymphocytes and eosinophils in the mice of Groups 1 and 3 were significantly higher than in those of Groups 2 and 4, indicating Der p I-induced cell recruitment in these sensitized mice. In Group 4, IL-12 treatment decreased recruitment of allergen-induced eosinophils by 98% compared to that of Group 3 (P < 0·001). Surprisingly, IL-12 treatment in Group 2 did not significantly affect the mean percentage of the eosinophils compared with that of Group 1. Collectively, it seemed that IL-12 treatment was more effective in inhibiting eosinophil recruitment in Group 4 than in Group 2. The percentage of neutrophils was decreased (P < 0·05 versus Group 1) following IL-12 treatment in Group 2, but was unaffected in Group 4 compared with that of Group 3. Compared with the control mice, the percentage of macrophages in both IL-12-treated groups were significantly increased (P < 0·001), whereas the percentage of lymphocytes did not change markedly.
Table 2.
Change in total cell numbers and cellular composition in BAL of sensitized mice exposed to aerosolized allergen
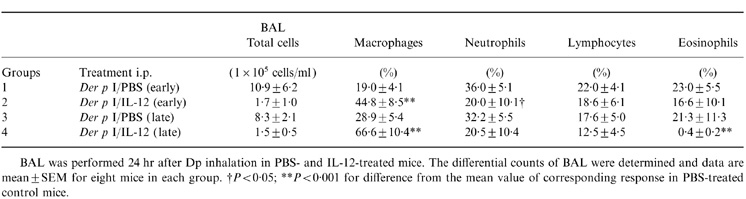
BAL was performed 24 hr after Dp inhalation in PBS- and IL-12-treated mice. The differential counts of BAL were determined and data are mean±SEM for eight mice in each group. †P < 0·05; **P < 0·001 for difference from the mean value of corresponding response in PBS-treated control mice.
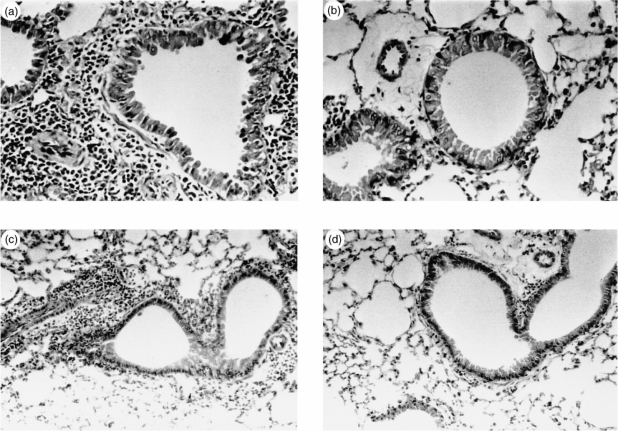
Histopathological examination of lung taken from Groups 1 and 3 control mice after inhalation demonstrated that lung parenchyma was infiltrated with inflammatory cells (Fig. 6a, c). The infiltrates consisted of admixtures of predominantly eosinophils, neutrophils and lymphocytes. In contrast, the IL-12-treated animals were not noted to have histologically significant pulmonary inflammation (Fig. 6b, d).
Figure 6.
Representative light microscopic findings of PBS-treated mice (a, c) and IL-12-treated mice (b, d) (hematoxylin and eosin stain). Lung tissue of mice without IL-12 treatment (a, ×400, and c, ×200) demonstrates extensive cellular infiltration of the periairway regions. In contrast, lung tissue of IL-12-treated mice (b, ×400, and d, ×200) demonstrates complete absence of histologically significant inflammation.
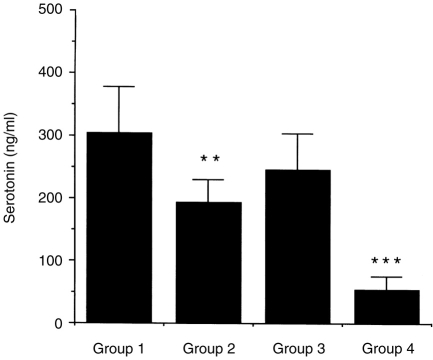
In Fig. 7, the levels of serotonin were markedly increased in PBS-treated mice without IL-12 treatment. In Group 2, IL-12 administration led to a 36% reduction in serotonin levels compared with Group 1. Further, the levels of serotonin were strikingly reduced about 78% in Group 4 compared with that of Group 3.
Figure 7.
The effect of IL-12 on serotonin production in BAL of mice after the last antigen challenge. C57BL/6 mice were treated as described in Fig. 1. Mice were challenged with inhaled Dermatophagoides pteronyssinus and BAL samples were taken from the mice with allergen challenged after 24 hr. BAL fluids from each group of mice were measured by using a specific ELISA. Data represent the mean±SD of eight samples per group. **P < 0·05 compared with Group 1; ***P < 0·001 compared with Group 3.
DISCUSSION
The ability of endogenous IL-12 production to shape developing Th1 cells and their cytokine responses has stimulated much enthusiasm for the potential therapeutic use of IL-12. In several models of infectious diseases, exogenous administration of IL-12 exerts striking effects when given at the time of initial antigen exposure.15,23–26 Similarly, prior studies in the murine model of allergic asthma have shown promise, with marked suppression of allergic responses after the in vivo administration of IL-12.14,21,27 These studies have shown that IL-12 administration at the time of initial antigen sensitization inhibited airway eosinophilia, IL-4 and IL-5 expression, IgE production, and airway hyperresponsiveness in allergen-sensitized mice. However, the vast majority of studies have been short-term systems in which responses are evaluated soon after IL-12 treatment or following a single antigen, parasite, or viral challenge. In addition, the dramatic effects of IL-12 in these diseases are contingent on its administration in a narrow therapeutic window restricted by the time at which it must be administered to exert an effect. However, optimal treatment of allergic diseases requires that the cytokine profile of allergen-specific cells be redirected, with the conversion of Th2 profiles into Th1 cytokine profiles. Thus, this is thought to be the result of the difficulty of reversing a Th2 immune response once it has been established. In the murine model of airway inflammation, we have observed the effects of IL-12 administration to induce immune responses after multiple allergen challenges and to redirect Th1/Th2 balance after established Th2-dominated conditions.
To measure the persistence of the Th1 response in mice immunized with IL-12 as adjuvant, the antibody titre and isotype to Der p I have been determined following multiple inhaled allergen exposure in IL-12-treated mice. Despite the lower antigen-specific IFN-γ response from mice of early IL-12 treatment (Group 2), IgG2a antibody persisted for as long as 40 days postimmunization with antigen in the absence of IL-12. The previous data demonstrated the IFN-γ level was much higher in early IL-12 treated mice without further repeated inhaled allergen challenge (data not shown). These allergen-specific Th1 or Th0 cells might be down-regulated by repeated challenge of inhaled allergen. However, allergen-specific IgG2a-secreting memory B cells are less susceptible to this modulation. In fact, Bliss et al. have examined the effects of IL-12 on recall responses to keyhole limpet haemocyanin and showed that antigen-specific IgG2a antibody promoted by IL-12 can last for 6 months.28 However, in Group 2, the impact of IL-12 administration on antibody responses, such as Der p I-specific serum IgG1 and IgE production was transiently suppressed. This finding is consistent with that of German et al. in that they have demonstrated the suppression of IgE by IL-12 to be unstable and have even enhanced the synthesis of IgE antibodies when sensitized mice subsequently received repeated antigen challenge.16
Notably, there is discrepancy between findings regarding the BAL fluid and those regarding cytokine patterns in Group 2 mice. Because the synthesis of IL-5 in the splenocytes recall response to Der p I allergen is inhibited in the same group, the pathophysiological significance of increased eosinophils is questionable. It is possible that the lack of an effect of IL-12 on eosinophilia may have been caused by the long interval between the last dose of IL-12 and BAL in these IL-12-treated mice. IFN-γ is known to inhibit the eosinophilia and to inhibit aspects of mast cell functions, including the release of mediators such as serotonin.29–31 Further, it has been reported that the up-regulating effects of IL-12 on IFN-γ expression last for up to 2 days but are lost by 8 days.32 Therefore, such levels of IFN-γ in these mice were not enough completely to suppress eosinophil recruitment if the inhibitory effects of IL-12 on the antigen-induced eosinophilia and serotonin secretion were mediated by enhanced IFN-γ production. However, Brusselle et al. using IFN-γ receptor-deficient mice have demonstrated that inhibition of the allergen-induced airway eosinophilia by IL-12 is IFN-γ independent during the secondary allergen exposure.33 Thus, it is possible that IL-12 may either directly inhibit eosinophil influx or stimulate the production of mediators other than IFN-γ that have such effects.
To examine the effects of IL-12 on allergen-induced changes when administered after the initial antigen presentation, once the T-cell development has been committed to a Th2 phenotype, mice were sensitized twice to Der p I on day 0 and 14, but were administered IL-12 only during subsequent antigen boosts from day 28. This study showed that IL-12 administration did not suppress the synthesis of Der p I IgG1 antibody, but modestly inhibited IgE response and enhanced the production of IgG2a after the second round of delayed IL-12 treatment in Group 4. Interestingly, the levels of total IgE and IgG2a antibodies were not significantly different between Group 3 and Group 4 during the follow-up. Because more long-term investigation of this immune response is clearly needed, it should not be concluded that IL-12 is unable to reverse the Th2 responses once Th2 effector cells are dominated. However, it was found that administration of IL-12 resulted in up-regulation of IFN-γ production and down-regulation of IL-5 production in the IL-12-treated mice. Notably, delayed IL-12 treatment was effective in inhibiting antigen-induced eosinophilic inflammation after mite inhalation. Whether IL-12 is effective in redirecting immune responses to inhaled antigen when administered after initial antigen sensitization has been controversial. In contrast to these data, Sur et al. observed that IL-12 was not effective when given after the initiation of aerosolized antigen challenge.32 On the other hand, Kips et al. demonstrated that, while IL-12 was given until the time of inhaled antigen challenge, it was effective in inhibiting eosinophil recruitment.21 However, these studies covered only a few weeks of treatment (about 18–21 days) or were examined shortly after cytokine administration. Thus, compared to our results, the discrepancies in these studies are yet to be conclusive.
In conclusion, studies suggest that IL-12, as an adjuvant, promotes Th1 cells recall responses but did not suppress the development of Th2 cells after multiple antigen challenges in the absence of IL-12. Thus, the Th1-recall response may be suppressed by a Th2 response after repeated antigen challenges, so that repeated exposure to antigen and IL-12 is necessary to maintain a stable and dominated Th1 response. In addition, the present study indicates that IL-12 can inhibit IL-5 production, increase IFN-γ secretion and suppress antigen-induced airway inflammation in established Th2-type responses of Der p I-sensitized mice, despite the presence of circulating IgE. This supports the idea that IL-12 may be more useful as an immunotherapeutic agent than as a vaccine adjuvant in the treatment of such as atopic asthma.
Acknowledgments
This study was supported by a grant, NSC 88-2314-B-002-194, from the National Science Council of the Republic of China.
Abbreviations
- BAL
bronchoalveolar lavage
- Dp
Dermatophagoides pteronyssinus
- IFN-γ
interferon-γ
- IL-12
interleukin-12
REFERENCES
- 1.Voorhorst R, Spieksma-Boezeman MIA, Spieksma FTM. Is a mite (Dermatophagoides sp) the producer of the house dust allergen? Allergy Asthma. 1964;10:329. [PubMed] [Google Scholar]
- 2.Krillis S, Baldo BA, Basten A. Antigens and allergens from the common house dust mite Dermatophagoides pteronyssinus. Part II. Identification of the major IgE binding antigen by crossed radioimmunoelectrophoresis. J Allergy Clin Immunol. 1984;74:142. doi: 10.1016/0091-6749(84)90277-x. [DOI] [PubMed] [Google Scholar]
- 3.Platts-Mills TA, Snajdr MJ, Ishizaka K, Frankland AW. Measurement of IgE antibody by an antigen-binding assay: correlation with PK activity and IgG and IgA antibodies to allergens. J Immunol. 1978;120:1201. [PubMed] [Google Scholar]
- 4.Desreumaux P, Capron M. Eosinophils in allergic reactions. Curr Opin Immunol. 1996;8:790. doi: 10.1016/s0952-7915(96)80006-9. [DOI] [PubMed] [Google Scholar]
- 5.Busse WW, Calhoun WF, Sedgwick JD. Mechanism of airway inflammation in asthma. Am Rev Respir Dis. 1993;147:20. doi: 10.1164/ajrccm/147.6_Pt_2.S20. [DOI] [PubMed] [Google Scholar]
- 6.Lebman DA, Coffman RL. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J Exp Med. 1988;168:853. doi: 10.1084/jem.168.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkelman FD, Katona IM, Urban JF, et al. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988;141:2335. [PubMed] [Google Scholar]
- 8.Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant human interleukin-5 is a selective activator of human eosinophil function. J Exp Med. 1988;167:219. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JM, Rambaldi A, Biondi A, Chen ZG, Sanderson CJ, Mantovani A. Recombinant human interleukin-5 is a selective eosinophil chemoattractant. Eur J Immunol. 1989;19:701. doi: 10.1002/eji.1830190420. [DOI] [PubMed] [Google Scholar]
- 10.Ricci M, Rossi O, Bertoni M, Matucci A. The importance of Th2-like cells in the pathogenesis of airway allergic inflammation. Clin Exp Allergy. 1993;23:360. doi: 10.1111/j.1365-2222.1993.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of Th1, CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 12.Manetti R, Parronchi P, Giudizi MG, et al. Natural killer cell stimulatory factor (interleukin 12[IL-12]) induces T helper type 1 (Th1) -specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oswald IP, Caspar P, Jankovic D, et al. IL-12 inhibits Th2 cytokine responses induced by egg of Schistosoma mansoni. J Immunol. 1994;153:1707. [PubMed] [Google Scholar]
- 14.Gavett SH, O’Hearn DJ, Xiumin L, et al. Interleukin-12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wynn TA, Jankovic D, Hieny S, Cheever AW, Sher A. IL-12 enhances vaccine-induced immunity to Schistosoma mansoni in mice and decreases T helper 2 cytokine expression, IgE production, and tissue eosinophilia. J Immunol. 1995;154:4701. [PubMed] [Google Scholar]
- 16.Germann T, Guckes S, Bongartz M, et al. Administration of IL-12 during ongoing immune responses fails to permanently suppress and can even enhance the synthesis of antigen-specific IgE. Int Immunol. 1995;7:1649. doi: 10.1093/intimm/7.10.1649. [DOI] [PubMed] [Google Scholar]
- 17.McKnight AJ, Zimmer GJ, Fogelman I, Wolf SF, Abbas AK. Effects of IL-12 on helper T cell-dependent immune responses in vivo. J Immunol. 1994;152:2172. [PubMed] [Google Scholar]
- 18.Afonso LCC, Scharton TM, Vieira LQ, et al. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 19.Finkelman FD, Madden KB, Cheever AW, et al. Effects of interleukin-12 on immune responses and host protection in mice infected with intestinal nematode parasites. J Exp Med. 1994;179:1563. doi: 10.1084/jem.179.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bliss J, Van Cleave V, Murray K, et al. IL-12, as an adjuvant, promotes a T helper 1 cell, but does not suppress a T helper 2 cell recall response. J Immunol. 1996;156:887. [PubMed] [Google Scholar]
- 21.Kips JC, Brusselle GJ, Joos GF, Sigounas A, Holbert D, Metzger WJ. Interleukin-12 inhibits antigen-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med. 1996;153:535. doi: 10.1164/ajrccm.153.2.8564093. [DOI] [PubMed] [Google Scholar]
- 22.Chiang B-L, Ding H-J, Chou C-C, Hsieh K-H. Isolation of type 1 and type 2 cloned mite allergen-specific T cells from an asthmatic child. Pediatr Allergy Immunol. 1996;7:193. doi: 10.1111/j.1399-3038.1996.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 23.Heinzel FP, Schoenhaut DS, Rerko RM, Rosser LE, Gately MK. Recombinant interleukin-12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sypek JP, Chung CL, Mayor SEH, et al. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou P, Sieve MC, Bennett J, et al. IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-γ. J Immunol. 1995;155:785. [PubMed] [Google Scholar]
- 26.Schijns VE, Haagmans BL, Horzinek MC. IL-12 stimulates an antiviral type 1 cytokine response but lacks adjuvant activity in IFN-γ-receptor-deficient mice. J Immunol. 1995;155:2525. [PubMed] [Google Scholar]
- 27.Iwamoto I, Kumano K, Kasai M, Kurasawa K, Nakao A. Interleukin-12 prevents antigen-induced eosinophil recruitment into mouse airways. Am J Respir Crit Care Med. 1996;154:1257. doi: 10.1164/ajrccm.154.5.8912732. [DOI] [PubMed] [Google Scholar]
- 28.Bliss J, Maylor R, Stokes K, Murray KS, Ketchem MA, Wolf SF. Interleukin-12 as vaccine adjuvant. Ann N Y Acad Sci. 1996;795:26. doi: 10.1111/j.1749-6632.1996.tb52652.x. [DOI] [PubMed] [Google Scholar]
- 29.Iwamoto I, Nakajima H, Endo H, Yoshida S. Interferon γ regulates antigen-induced eosinophil recruitment into the mouse airways by inhibiting the infiltration of CD4+ T cells. J Exp Med. 1993;177:573. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X-M, Chopra RK, Chou T-Y, Schofield BH, Wills-Karp M, Huang S-K. Mucosal IFN-γ gene transfer inhibits pulmonary allergic responses in mice. J Immunol. 1996;157:3216. [PubMed] [Google Scholar]
- 31.Coleman JW, Buckley MG, Holliday MR, Morris AG. Interferon-gamma inhibits serotonin release from mouse peritoneal mast cells. Eur J Immunol. 1991;21:2559. doi: 10.1002/eji.1830211037. [DOI] [PubMed] [Google Scholar]
- 32.Sur S, Lam J, Bouchard P, Sigounas A, Holbert D, Metzger WJ. Immunomodulatory effects of IL-12 on allergic lung inflammation depend on timing of doses. J Immunol. 1996;157:4173. [PubMed] [Google Scholar]
- 33.Brusselle GG, Kips JC, Peleman RA, et al. Role of IFN-γ in the inhibition of the allergic airway inflammation caused by IL-12. Am J Respir Cell Mol Biol. 1997;17:767. doi: 10.1165/ajrcmb.17.6.2820. [DOI] [PubMed] [Google Scholar]