Abstract
These studies addressed the nature and origin of peptide-receptive class I major histocompatibility complex (MHC-I) molecules used to present exogenous antigens. Peptide-receptive Kb molecules in transporter for antigen presentation (TAP)1−/− and TAP1+/+ macrophages were quantitated by exposing cells to exogenous ovalbumin (OVA)(257–264) peptide and then measuring OVA(257–264):Kb complexes with a T hybridoma assay or flow cytometry (using a complex-specific antibody). Relative to TAP1+/+ cells, TAP1−/− cells had decreased levels of pre-existing cell-surface peptide-receptive MHC-I molecules at 37°. With continued exposure of viable cells to peptide, however, TAP1−/− and TAP1+/+ cells formed similar levels of OVA(257–264):Kb complexes, suggesting that nascent labile MHC-I molecules were captured and stabilized by exogenous peptide. Brefeldin A inhibited generation of OVA(257–264):Kb complexes on TAP1−/− (but not TAP1+/+) cells at 37°, confirming the importance of a flux of unstable nascent MHC-I molecules in TAP1−/− cells at 37°. In contrast, at 26° both TAP1−/− and TAP1+/+ cells expressed brefeldin A-resistant, peptide-receptive MHC-I molecules at similar levels. Alternate MHC-I processing of exogenous particulate antigen correlated with ability to present exogenous peptide. Thus, processing was brefeldin A-sensitive with TAP1−/− macrophages at 37°, but brefeldin A-resistant with TAP1+/+ cells at 37°, as well as with TAP1+/+ or TAP1−/− cells at 26°. We conclude that alternate MHC-I antigen processing normally utilizes pre-existing MHC-I molecules, but TAP1−/− cells at 37° mainly use nascent MHC-I molecules, because of a lack of pre-existing, stable, peptide-receptive MHC-I molecules. The results support a vacuolar processing mechanism with binding of peptides to MHC-I molecules in post-Golgi compartments or on the cell surface.
INTRODUCTION
The conventional class I major histocompatibility complex (MHC-I) antigen-processing pathway involves proteolysis of cytosolic antigens by proteasomes, transport of cytosol-derived peptides into the endoplasmic reticulum (ER) by the transporter for antigen presentation (TAP), and binding of peptides to nascent MHC-I molecules in the ER. Peptide:MHC-I complexes exit the ER and pass via the Golgi to the cell surface, where they are presented to CD8 T cells. This pathway can be blocked by proteasome inhibitors or brefeldin A, an inhibitor of anterograde ER–Golgi transport.
TAP is a heterodimer of TAP1 and TAP2 subunits, and absence of either subunit leads to deficiency of TAP. TAP-deficient cells exhibit decreased delivery of cytosol-derived peptides to nascent MHC-I molecules in the ER and decreased formation of stable peptide:MHC-I complexes, leading to a blockade of the conventional MHC-I antigen-processing pathway. This produces a decrease in the cell-surface expression of MHC-I molecules. Many MHC-I molecules in TAP-deficient cells may remain empty1 or become loaded with low affinity peptides2 and these empty MHC-I molecules or low affinity complexes have a much shorter half life at 37° than peptide:MHC-I complexes on TAP-replete cells. At lower temperatures, such as 26°, however, these thermolabile MHC-I molecules are stable and accumulate to higher levels on the cell surface.1
Some cell-surface MHC-I molecules can bind exogenous peptides and present them to CD8 T cells. Such MHC-I molecules are termed peptide-receptive MHC-I molecules.3 The exact biochemical nature of peptide-receptive MHC-I molecules and the mechanisms by which they are generated on the cell surface are still under investigation. Peptide-receptive MHC-I molecules may be empty of peptide or occupied by some peptides that can subsequently dissociate or be replaced by other peptides. Peptide-receptive MHC-I molecules may also exist in post-Golgi intracellular vacuolar compartments (e.g. endocytic or phagocytic compartments). In addition to their overall deficiency in MHC-I molecules, TAP-deficient cells express decreased levels of peptide-receptive MHC-I molecules on the cell surface.3,4
In addition to the conventional MHC-I pathway for processing of cytosolic antigen, there is much evidence for the existence of other alternate MHC-I antigen processing mechanisms that allow the processing of exogenous antigens in vacuolar compartments for presentation by MHC-I molecules.4–20 However, different studies have supported different alternate MHC-I processing mechanisms, and more than one such mechanism may exist.18 Some studies have suggested that peptides or proteins may exit from vacuolar compartments and enter the cytosol, thereby merging with the TAP-dependent conventional MHC-I antigen-processing pathway.12–14 Other studies have implicated vacuolar processing mechanisms that do not involve cytosolic antigen processing or transport of the presented peptides via TAP.4,10,11,15–17 In some of these cases the processing was found to be TAP independent,15,16 but even vacuolar processing mechanisms may be indirectly influenced by TAP if the supply of post-Golgi peptide-receptive MHC-I molecules used by these mechanisms is influenced by TAP.4 The site where peptides bind to MHC-I molecules during vacuolar alternate MHC-I antigen processing remains unclear, but this may occur either within vacuolar compartments or on the cell surface following regurgitation of peptides from vacuolar compartments at the cell surface.11
We have studied a model of alternate MHC-I antigen processing that involves vacuolar processing of particulate antigens by macrophages. This processing requires phagocytosis and is blocked by cytochalasin D (an inhibitor of phagocytosis) or fixation of the macrophages prior to the addition of antigen.4,10 The results of our prior studies indicate that peptides produced by this processing pathway either bind to peptide-receptive MHC-I molecules residing in post-Golgi vacuolar compartments or bind to MHC-I molecules on the cell surface following peptide recycling and regurgitation.4,10,11 The goals of the current studies are to determine the level of peptide-receptive MHC-I molecules, defined by the ability to bind exogenous peptide, on both TAP1-knockout (TAP1−/−) and wild-type (TAP1+/+) cells under different conditions, and to determine the ability of these MHC-I molecules to contribute to the alternate MHC-I antigen-processing pathway. Brefeldin A was used to block the delivery of newly synthesized MHC-I molecules to post-Golgi compartments and deplete labile pools of peptide-receptive MHC-I molecules in those compartments. In addition, incubation at different temperatures (26° and 37°) was used to control the stability and steady state level of peptide-receptive MHC-I molecules. Using these approaches we were able to determine the relative contributions of newly synthesized (nascent) and stable pre-existing MHC-I molecules to the population of peptide-receptive MHC-I molecules and to alternate MHC-I antigen processing by both TAP1+/+ and TAP1−/− cells under different conditions. The data are most consistent with a vacuolar model for alternate MHC-I antigen processing.
MATERIALS AND METHODS
Cells
Activated murine peritoneal macrophages were harvested 7–12 days after i.p. inoculation with Listeria monocytogenes.21 TAP1+/+ macrophages were isolated from C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME). TAP1−/− macrophages were isolated from TAP1−/− mice. These mice were derived from 129×B6 TAP1-knockout mice22 that were backcrossed for six generations with C57BL/6 mice and then generously provided to us by Dr Luc Van Kaer (Vanderbilt University, Nashville, TN). These TAP1−/− mice were bred under specific pathogen free conditions at Case Western Reserve University. Macrophages were adhered to 96-well or six-well plates by incubation at 37° for 2 hr, and nonadherent cells were removed by washing. CD8OVA1.3 T hybridoma cells were employed to detect OVA(257–264):Kb complexes. Unless otherwise indicated, all incubations were performed in 5% CO2 atmosphere in standard medium: Dulbecco’s modified Eagle’s minimum essential medium (DMEM; Life Technologies, Grand Island, NY) supplemented with 10% fetal calf serum (FCS; HyClone, Logan, UT), 5×105 m 2-mercaptoethanol (2-ME), l-arginine–HCl (116 mg/l), l-asparagine (36 mg/l), NaHCO3 (2 g/l), sodium pyruvate (1 mm) and antibiotics.
Antigen preparations
OVA(257–264) peptide was dissolved in distilled water at 1 mm concentration and sterile filtered. For experiments, the peptide stock solution was diluted in standard medium. Experiments to assess the processing of exogenous particulate (bacterial) antigen used viable Escherichia coli strain HB101 expressing the Crl–ovalbumin (OVA) fusion protein (HB101.Crl-OVA).10 The Crl–OVA fusion protein contains the OVA(257–277) sequence and is constitutively expressed in the bacterial cytoplasm.10
Antigen processing and presentation
For studies of OVA(257–264) binding to Kb, macrophages were incubated with peptide for 2 hr, washed, fixed, washed and incubated with T cells.23 For thermolability studies, macrophages were first incubated for 19 hr in standard medium at 26° or 37°. Brefeldin A (1 μg/ml final concentration) was added during the last 0·5–1 hr of this incubation. The cells were then incubated for 2 hr with OVA(257–264) peptide or HB101.Crl–OVA bacteria without changing the inhibitor or temperature conditions, washed, fixed in paraformaldehryde, washed and incubated for 24 hr with CD8OVA1.3 T hybridoma cells.23 Antigen presentation was assessed by the amount of interleukin-2 (IL-2) secreted by the T cells, measured using a CTLL-2 bioassay24 with modifications. IL-2-dependent CTLL-2 cells were incubated with T-cell culture supernatants for 24 hr at 37°, and Alamar blue (Alamar Biosciences, Inc., Sacramento, CA) was then added for 18–24 hr. Alamar blue is reduced by metabolically active cells, altering optical density near 600 nm. Both reduced and oxidized forms have high absorbance near 570 nm, but only the oxidized form has high absorbance near 600 nm. Thus, CTLL-2 cell growth and metabolic activity can be judged by production of the reduced form,25 which we measured colorimetrically by subtracting OD595 from OD550 using a dual wavelength microplate reader (Model 550, Bio-Rad, Hercules, CA).25 All antigen presentation assays were done in triplicate, and the results are presented as mean (OD550−OD595)±SD (SD indicated by error bars). Similar IL-2 assays were performed with CTLL-2 cells that were exposed to 3H-thymidine instead of Alamar blue for the last 18–24 hr of the CTLL-2 bioassay. In this case 3H-thymidine incorporation into DNA provided a measure of IL-2-dependent CTLL-2 proliferation that gave virtually identical sensitivity to IL-2 (similar threshold and plateau concentrations of IL-2), validating the use of Alamar blue as an indicator for the IL-2 bioassay.25,26
Flow cytometry
Macrophages were adhered in six-well plates (5–10×106 peritoneal exudate cells/well), and nonadherent cells were removed by washing. The macrophages were then incubated for 2 hr in standard medium with or without 5 μm OVA(257–264) peptide, washed in DMEM three times, removed from the plates by scraping in DMEM and pelleted at 2500 g for 5 min at 4°. The cells were resuspended in fluorescence-activated cell sorting (FACS) buffer (1% rabbit serum, 0·1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS)) at 2×106 cells/ml and plated (100 μl/well) in a 96-well U-bottom plate (Becton Dickenson, Franklin Lakes, NJ). In order to block antigen-non-specific binding of immunoglobulin to murine FcγIII and FcγII receptors, CD16/CD32 Fc block (Pharmingen, San Diego, CA) was diluted to 4 μg/ml in FACS buffer, and 50 μl of this solution was added to each well at 4°. After 10 min incubation with Fc block, the cells were stained for total cell surface Kb expression by adding 50 μl B8-24-3 culture supernatant for 30 min at 4° in the continued presence of CD16/CD32 Fc block. B8-24-3 is a monoclonal mouse immunoglobulin G1 (IgG1) antibody that recognizes Kb, and B8-24-3 hybridoma cells were obtained from the American Type Culture Collection (Rockville, MD). Alternatively, peptide-receptive Kb molecules were detected by incubating fixed or viable macrophages with OVA(257–264) peptide in standard medium at 37° and then staining as described above with 25-D1.16, a monoclonal mouse IgG1 antibody that is specific for OVA(257–264):Kb complexes and was generated by A. Porgador and R. Germain (NIH, Bethesda, MD).27 After incubation with the primary antibody, the cells were washed three times with FACS buffer, incubated for 30 min at 4° in FACS buffer with 5 μg/ml fluorescein isothiocyanate (FITC)-conjugated AffiniPure goat antimouse IgG (F(ab′)2 fragment specific; Jackson ImmunoResearch Laboratories, West Grove, PA), washed again three times with FACS buffer, and analysed with a FACScan flow cytometer (Becton Dickenson, Mansfield, MA). The data were analysed using the WinList 3D program (Verity Software House, Inc., Topsham, ME).
RESULTS
Cell-surface expression of both total and peptide-receptive Kb molecules is lower on TAP1−/− than on TAP1+/+ macrophages at 37°
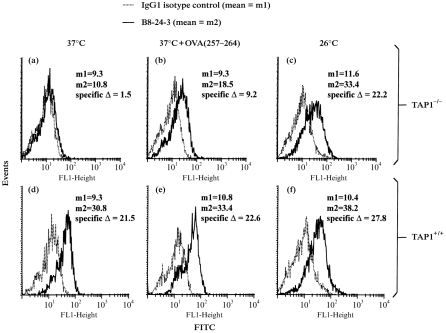
B8-24-3 was used for staining and flow cytometry to measure the total expression of Kb on the surface of murine TAP1−/− and TAP1+/+ macrophages. After culture at 37°, Kb expression on TAP1−/− macrophages was 10% or less of the level found on TAP1+/+ macrophages (Fig. 1). In addition, Kb expression was studied after culture at 26°, since thermolabile MHC-I molecules on TAP-deficient cells are stabilized at this temperature,1 or after exposure to OVA(257–264) peptide, because peptide binding stabilizes MHC-I molecules on TAP-deficient cells. Culture of TAP1−/− macrophages at 26° or at 37° plus OVA(257–264) peptide increased Kb expression (Fig. 1). With TAP1+/+ macrophages, however, incubation with peptide had little or no effect on surface expression of Kb molecules, and incubation at 26° produced only a small increase in surface expression of Kb. These results are consistent with previous results1,28,29 and suggest that peptide-receptive MHC-I molecules can accumulate on the surface of TAP-deficient cells at 26°, but are largely unstable at 37° unless they are rescued by binding of peptide.
Figure 1.
Analysis of total surface expression of Kb on TAP1−/− and TAP1+/+ macrophages by flow cytometry. TAP1−/− and TAP1+/+ macrophages were stained with B8-24-3 to examine surface expression of Kb following various treatments. Isotype controls were included in all experiments. (a–c) TAP1−/− macrophages. (d–f) TAP1+/+ macrophages. The macrophages were incubated overnight at 37° (a, d), overnight at 37° and then for 2 hr at 37° with 5 μm OVA(257–264) (b, e) or overnight at 26° (c, f) prior to staining.
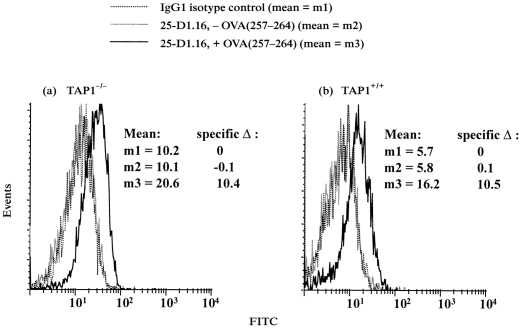
Monoclonal antibody B8-24-3 does not distinguish peptide-receptive and non-receptive Kb molecules. In order to evaluate the level of cell surface peptide-receptive Kb molecules, we employed both T hybridoma assays and a new approach that uses 25-D1.16, a monoclonal antibody specific for OVA(257–264):Kb complexes.27 In the latter approach, macrophages were fixed to prevent the delivery of nascent Kb molecules, incubated with OVA(257–264) to generate OVA(257–264):Kb complexes from peptide-receptive Kb molecules, stained with 25-D1.16 and then analysed by flow cytometry. At 37°, TAP1+/+ macrophages expressed approximately fivefold more surface peptide-receptive Kb molecules than TAP1−/− macrophages (Fig. 2). Alternatively, macrophages were similarly fixed and incubated with peptide, but OVA(257–264):Kb complexes were measured by the response of CD8OVA1.3 T-hybridoma cells, specific for OVA(257–264):Kb. The T-hybridoma assay also indicated that TAP1+/+ macrophages expressed more pre-existing cell surface peptide-receptive Kb molecules than TAP1−/− macrophages (data not shown), consistent with prior results.4
Figure 2.
Quantitation of cell-surface, peptide-receptive Kb molecules on prefixed TAP1−/− and TAP1+/+ macrophages by flow cytometry. Macrophages were fixed with paraformaldehyde, washed, incubated with or without 5 μm OVA(257–264) overnight at 37° and washed extensively. The cells were then stained with 25-D1·16, specific for OVA(257–264):Kb complexes, or isotype-matched control antibody. (a) TAP1−/− macrophages. (b) TAP1+/+ macrophages.
After incubation of viable cells with OVA(257–264) peptide at 37°, OVA(257–264):Kb complexes accumulate to similar levels on TAP1−/− and TAP1+/+ macrophages
The previous studies examined cell surface levels of peptide-receptive Kb molecules on fixed cells to prevent the delivery of nascent Kb molecules. However, under physiological conditions, nascent MHC-I molecules are delivered to the cell surface and may contribute significantly to the population of peptide-receptive MHC-I molecules. To test this hypothesis, viable macrophages were incubated with OVA(257–264) peptide for 2 hr at 37° to generate complexes from peptide-receptive Kb molecules, and the macrophages were then washed and fixed. In this case, both flow cytometry (Fig. 3) and T-cell assays (data not shown) demonstrated that the levels of OVA(257–264):Kb complexes achieved on TAP1−/− macrophages were similar to the levels achieved on TAP1+/+ macrophages. This suggests that nascent MHC-I molecules are continuously delivered to the cell surface at 37° and contribute to the level of peptide-receptive MHC-I molecules, especially on TAP1−/− cells. Moreover, capture of exogenous peptide and stabilization of nascent MHC-I molecules appears to proceed at similar rates in viable TAP1+/+ and TAP1−/− macrophages at 37°. This suggests a hypothesis that the delivery of peptide-receptive molecules (or their precursors) to the cell surface proceeds at similar rates in both TAP1+/+ and TAP1−/− macrophages, but that TAP1−/− macrophages have lower steady state levels of surface peptide-receptive MHC-I molecules because of lower stability of the peptide-receptive MHC-I molecules (or their precursors) that are generated on TAP1−/− cells.
Figure 3.
Analysis of OVA(257–264):Kb complexes formed on viable TAP1−/− and TAP1+/+ macrophages by flow cytometry. Viable macrophages were pulsed with or without 5 μm OVA(257–264) at 37° for 2 hr. The cells were then stained with 25-D1.16 or isotype-matched control antibody. (a) TAP1−/− macrophages. (b) TAP1+/+ macrophages.
Brefeldin A inhibits exogenous peptide presentation by TAP1−/− but not TAP1+/+ macrophages at 37°, whereas brefeldin A has no effect on peptide presentation by either cell type at 26°
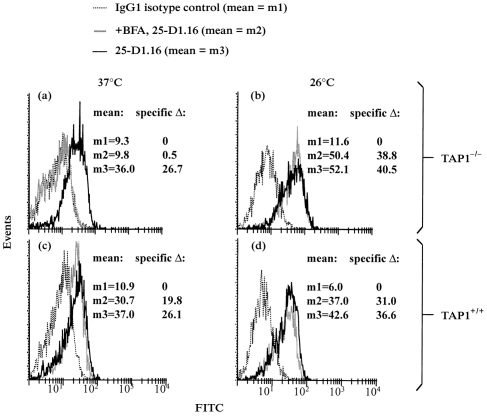
In order to test the hypothesis that peptide-receptive molecules on TAP1+/+ versus TAP1−/− macrophages have different nature and stability, the delivery of nascent MHC-I molecules to the cell surface was blocked, and the subsequent survival of peptide-receptive MHC-I molecules was monitored. For these purposes, brefeldin A was used to block the transport of nascent MHC-I molecules from the ER to the cell surface. In addition, the survival of peptide-receptive MHC-I molecules was examined at both 37° and 26°, as the latter temperature is known to prolong the survival of unstable, peptide-receptive MHC-I molecules.1 Thus, macrophages were incubated overnight at 26° or 37°, and brefeldin A was added to the cells for 1 hr under the same conditions. The macrophages were then exposed to OVA(257–264) for an additional 2 hr at the original temperature and in the continued presence or absence of brefeldin A, and the cells were then washed and fixed. OVA(257–264):Kb complexes were assessed by flow cytometry with 25-D1.16 (Fig. 4) and by the CD8OVA1.3 T-hybridoma assay (Fig. 5). At 26°, brefeldin A produced only minor shifts in the level of peptide-receptive Kb molecules, indicating that peptide-receptive Kb molecules on both TAP1+/+ and TAP1−/− macrophages were stable at this temperature (Figs 4 and 5). At 37°, in contrast, treatment with brefeldin A greatly decreased the level of peptide-receptive Kb molecules on TAP1−/− macrophages but produced only a slight decrease or no significant change in the level of peptide-receptive Kb molecules on TAP1+/+ macrophages (Figs 4 and 5). Thus, at 37° peptide-receptive MHC-I molecules are less stable on TAP1−/− macrophages than on TAP1+/+ macrophages, and nascent labile Kb molecules contribute to the presentation of exogenous peptide by TAP1−/− macrophages.
Figure 4.
Effect of brefeldin A on formation of OVA(257–264):Kb complexes by viable TAP1−/− and TAP1+/+ macrophages as assessed by flow cytometry. TAP1−/− and TAP1+/+ macrophages were cultured for 18 hr at 26° or 37° and then treated with or without brefeldin A (1 μg/ml) for 1 hr at the same temperature. The cells were then incubated with 5 μm OVA(257–264) for 2 hr at the same temperature in the continued presence or absence of brefeldin A. The cells were then stained with 25-D1.16 or isotype-matched control antibody. (a) TAP1−/− macrophages at 37°. (b) TAP1−/−macrophages at 26°. (c) TAP1+/+ macrophages at 37°. (d) TAP1+/+ macrophages at 26°.
Figure 5.
Effect of brefeldin A on formation of OVA(257–264):Kb complexes by viable TAP1−/− and TAP1+/+ macrophages as assessed by T-cell assay. TAP1−/− and TAP1+/+ macrophages were cultured for 18 hr at 26° or 37° and then treated with or without brefeldin A (1 μg/ml) for 1 hr at the same temperature. The cells were then incubated with various concentrations of OVA(257–264) for 2 hr at the same temperature in the continued presence or absence of brefeldin A, washed, fixed, washed, and incubated with CD8OVA1.3 T-hybridoma cells, specific for OVA(257–264):Kb complexes. (a) TAP1−/− macrophages. (b) TAP1+/+ macrophages.
Brefeldin A inhibits alternate MHC-I antigen processing by TAP1−/− but not TAP1+/+ macrophages at 37°, whereas processing by both TAP1−/− and TAP1+/+ macrophages is brefeldin A resistant at 26°
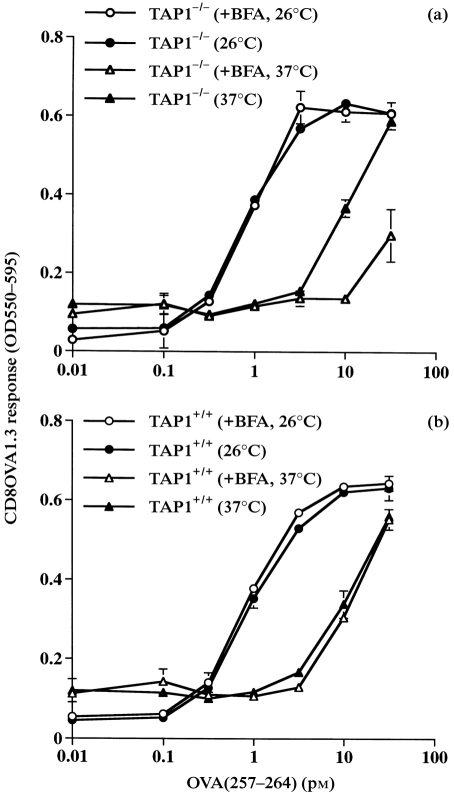
Prior experiments suggested the existence of a vacuolar alternate MHC-I antigen-processing pathway involving the binding of peptide to post-Golgi, peptide-receptive MHC-I molecules within vacuolar compartments or on the cell surface.4,10,11,18 Despite indications that this vacuolar alternate MHC-I antigen-processing pathway did not require transport of the presented peptide via TAP, TAP1−/− macrophages showed lower levels of processing via this pathway.4 The experiments described above suggest that the limited longevity of peptide-receptive MHC-I molecules on TAP1−/− macrophages may contribute to this deficiency. To test this hypothesis, we assessed the effects of brefeldin A on alternate MHC-I antigen processing in TAP1−/− and TAP1+/+ macrophages at 26° and 37°. The protocol was similar to the procedure described above, except that OVA(257–264) peptide was not added, and HB101.Crl–OVA was used as an extracellular particulate antigen that required processing via the alternate MHC-I pathway. As previously observed,4 brefeldin A had no effect on alternate MHC-I processing by TAP1+/+ cells at 37° (Fig. 6b). In contrast, exposure of TAP1−/− cells to brefeldin A at 37° caused a strong inhibition of alternate MHC-I processing (Fig. 6a). This indicates that the supply of peptide-receptive MHC-I molecules used for alternate MHC-I processing is brefeldin A sensitive at 37° in TAP1−/− cells but not in TAP1+/+ cells. Consistent with the studies of exogenous peptide binding to receptive MHC-I molecules, brefeldin A had no effect on alternate MHC-I processing at 26° by either TAP1+/+ or TAP1−/− macrophages (Fig. 6).
Figure 6.
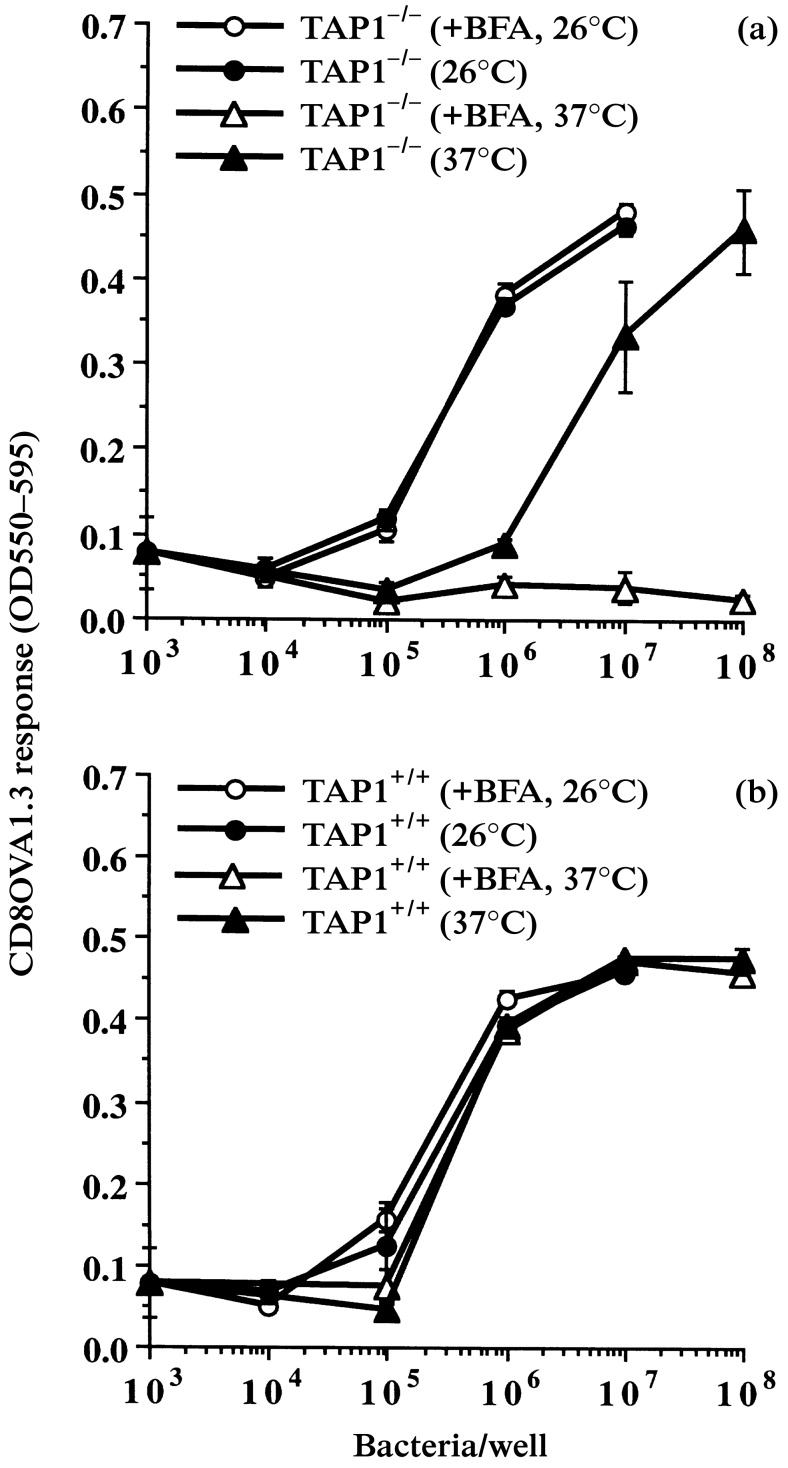
Effect of brefeldin A on alternate MHC-I antigen processing and presentation. TAP1−/− and TAP1+/+ macrophages were cultured for 18 hr at 26° or 37° and then treated with or without brefeldin A (1 μg/ml) for 30 min at the same temperature. HB101.Crl–OVA was added for 2 hr at the same temperature in the continued presence or absence of brefeldin A. The macrophages were washed, fixed, washed and incubated with CD8OVA1.3 T-hybridoma cells. (a) TAP1−/− macrophages. (b) TAP1+/+ macrophages.
In addition, this experiment indicates that TAP1−/− and TAP1+/+ macrophages are equally efficient at alternate MHC-I processing at 26° (compare 26° results in Fig. 6a and Fig. 6b), i.e. under conditions where post-Golgi, peptide-receptive MHC-I molecules are similarly present on both TAP1−/− and TAP1+/+ cells. This confirms that OVA peptide produced by the alternate MHC-I processing mechanism does not require transport by TAP. Thus, this alternate MHC-I processing mechanism is not dependent on TAP when adequate levels of post-Golgi peptide-receptive MHC-I molecules are present.
DISCUSSION
The goals of this study were to investigate the origin, nature and stability of cell-surface, peptide-receptive MHC-I molecules, and to determine their contribution to the alternate MHC-I antigen-processing pathway. We defined peptide-receptive MHC-I molecules by their ability to bind to exogenous peptides (or peptides derived from exogenous antigens) to generate specific peptide:MHC-I complexes. For experiments with exogenous peptide, the macrophages were incubated with OVA(257–264), and 25-D1.16 was used to detect resulting OVA(257–264):Kb complexes by flow cytometry, or CD8OVA1.3 T-hybridoma cells were used to detect the same complexes by T-cell assay. These two assays have different advantages. The flow cytometry assay provides a more quantitative measure of specific complexes, whereas the T-cell assay is much more sensitive than the flow cytometry assay.
OVA(257–264):Kb complexes can be detected by T-cell assay after exposing TAP1+/+ macrophages to as little as 1–10 pm OVA(257–264) peptide. In contrast, these complexes are detected by flow cytometry with 25-D1.16 only after exposure of macrophages to 10–100 nm OVA(257–264) peptide (data not shown), although this approach may be more sensitive with different cell systems.27 Thus, cells must be incubated with very different concentrations of peptide in order to use these two different assays to detect peptide-receptive MHC-I molecules. It is important to note that all of our observations regarding expression levels of peptide-receptive MHC-I molecules (based on the relative ability of TAP1+/+ and TAP1−/− macrophages to bind exogenous peptides) were confirmed using both assays and, hence, over a wide range of peptide concentrations. On the other hand, the levels of specific peptide–MHC-I complexes generated after exposing cells to particulate protein antigen (that requires processing) are much lower than the levels achieved with high concentrations of exogenous peptide, and the sensitivity of the flow cytometry assay is inadequate to detect these lower levels. Therefore, protein processing experiments that were designed to investigate the mechanisms of the alternate MHC-I antigen-processing pathway were evaluated using the T-cell assay (Fig. 6), not the flow cytometry assay.
Our studies of exogenous peptide binding to peptide-receptive MHC-I molecules established several important points. First, we confirmed recent observations3,4 that TAP-deficient cells express lower levels of pre-existing, cell-surface, peptide-receptive MHC-I molecules, as well as the long-standing observation that TAP-deficient cells have lower total MHC-I expression than TAP-replete cells.30 Second, we observed that viable cells at 37° can bind similar levels of exogenous peptide, regardless of TAP expression, and we proposed that this results from peptide capture and stabilization of nascent (i.e. newly synthesized) MHC-I molecules that are otherwise unstable on TAP1−/− cells. Third, we confirmed this hypothesis by demonstrating that at 37° the binding of exogenous peptide by TAP1−/− cells is inhibited by brefeldin A, indicating dependence on a flux of nascent MHC-I molecules from the ER/Golgi. In contrast, the binding of exogenous peptide by TAP1+/+ cells is brefeldin A resistant, implying that there is a stable pool of pre-existing cell-surface (or post-Golgi), peptide-receptive MHC-I molecules (or precursors thereof via peptide dissociation or exchange, see below).
Although peptide–MHC-I assembly and surface expression is decreased in TAP1−/− cells because of lack of appropriate peptides in the ER,22,30–32 some MHC-I molecules do escape ER retention and reach the cell surface in TAP-deficient cells. While they have a shorter half life than the majority of MHC-I molecules on TAP-replete cells (which may be loaded with higher affinity peptides), these MHC-I molecules were previously found to be stabilized at 26° or by binding of high affinity peptides.1,28,29 Our results with TAP1−/− macrophages also showed that Kb molecules accumulated to higher levels at 26° than at 37°, and incubation with OVA(257–264) also enhanced cell surface expression of Kb in TAP1−/− macrophages. Incubation at 26° also enhanced surface expression of MHC-I molecules in TAP1+/+ macrophages, but the proportional enhancement of Kb expression was less dramatic on TAP1+/+ cells because of their higher baseline levels of pre-existing cell surface Kb molecules.
What then is the composition of the peptide-receptive MHC-I molecules? Do they contain peptides (perhaps with low affinity for the MHC-I allele) that can dissociate or be exchanged for another peptide, or are they truly empty? Our data indicate that a large proportion of peptide-receptive MHC-I molecules (or their precursors) on TAP1+/+ cells are stable (i.e. brefeldin A resistant) at 37° (Figs 4 and 5), suggesting that they are peptide stabilized. It is also possible that peptide-receptive MHC-I molecules on TAP1−/− cells are associated with peptides (perhaps of lower affinity) that are sufficient to stabilize these molecules at 26° but not at 37°.2 This implies that the binding of exogenous peptides and the alternate MHC-I processing mechanisms may both involve a process of peptide exchange, wherein previously bound peptides, which may stabilize the MHC-I molecules, either dissociate to create empty peptide-receptive MHC-I molecules or else undergo some type of peptide exchange reaction.
After defining differences in the origin and stability of the peptide-receptive MHC-I molecules on TAP1+/+ and TAP1−/− macrophages, we determined the correlation of these differences with the abilities of these cells to process exogenous particulate antigen by the alternate MHC-I antigen-processing pathway. Under various conditions, processing activity correlated with the level of peptide-receptive MHC-I molecules on the cell surface (which may also reflect the levels in post-Golgi vacuolar compartments, such as phagosomes, see below). At 37°, TAP1−/− macrophages processed exogenous antigen less efficiently than TAP1+/+ macrophages, consistent with their lower cell-surface levels of peptide-receptive MHC-I molecules. Significantly, these experiments revealed that treatment with brefeldin A blocked processing at 37° by TAP1−/− macrophages, but not TAP1+/+ macrophages, consistent with the unique brefeldin A-sensitivity of peptide-receptive MHC-I expression on these cells at 37°. Thus, the absence of a stable pool of pre-existing peptide-receptive MHC-I molecules necessitated the delivery of nascent peptide-receptive MHC-I molecules, while nascent molecules were not necessary if such a stable pool was present. These data are consistent with a vacuolar mechanism of alternate MHC-I antigen processing, wherein exogenous particulate antigens are processed within post-Golgi vacuolar compartments and peptides bind to MHC-I molecules either within these vacuolar compartments, or on the cell surface following peptide regurgitation.10,18 In this model the presented peptides do not transit through the cytosol and are not themselves transported via TAP.
These protocols show that the alternate MHC-I antigen processing pathway is brefeldin A-resistant in TAP1+/+ cells, indicating that a post-Golgi pool of peptide-receptive MHC-I molecules is utilized. However, longer preincubations with brefeldin A (e.g. 1·5–3 hr) do partially inhibit this pathway, even in TAP1+/+ cells (data not shown). In addition to other potential effects, such prolonged treatment may deplete even the relatively stable pool of post-Golgi, peptide-receptive MHC-I molecules in TAP1+/+ cells. This is analogous to the observation that class II MHC antigen processing is brefeldin A-resistant for short periods, but can be inhibited by prolonged (3–4 hr) pretreatment of macrophages with brefeldin A, which eventually depletes the post-Golgi pool of nascent class II MHC molecules.33 Thus, kinetic parameters must be carefully considered for experiments with brefeldin A.
The 26° antigen-processing experiment provides additional clear evidence that TAP is not an intrinsic component of the alternate MHC-I antigen processing pathway. While other 26° experiments have been reported, these experiments are the first to examine the alternate MHC-I pathway with the processing step done at 26°. At 26°, both TAP1−/− and TAP1+/+ macrophages showed similar, efficient processing of exogenous particulate antigen for presentation by MHC-I molecules. This demonstrates that the presented peptide, OVA(257–264), did not require TAP-dependent transport, and this implies that TAP is not necessary for the alternate MHC-I pathway as long as there is an adequate supply of post-Golgi, peptide-receptive MHC-I molecules. However, when the supply of post-Golgi, peptide-receptive MHC-I molecules is limited by the absence of TAP at 37°, this can indirectly inhibit the alternate MHC-I pathway.
While our data indicate an alternate MHC-I antigen processing mechanism that involves peptide binding to MHC-I molecules in a post-Golgi compartment, it is still not clear whether this occurs within vacuolar compartments (e.g. phagosomes) or on the cell surface following peptide regurgitation (i.e. release of peptides by recycling from vacuolar compartments).10,18 Our current experiments have evaluated cell surface peptide-receptive MHC-I molecules, but the results may well also apply to peptide-receptive MHC-I molecules within vacuolar compartments, as these may derive from, or mingle with, the cell-surface pool via endocytosis, phagocytosis and recycling.
In conclusion, these results provide increased understanding concerning the nature and genesis of post-Golgi peptide-receptive MHC-I molecules, the relationship between TAP expression and the expression of post-Golgi peptide-receptive MHC-I molecules, the stability and function of peptide-receptive MHC-I molecules in TAP-deficient cells, and the nature and source of the peptide-receptive MHC-I molecules that are used to present exogenous antigen. We demonstrate that the functional level of post-Golgi peptide-receptive MHC-I molecules determines the efficiency of alternate MHC-I antigen processing, supporting a vacuolar mechanism for this pathway. These mechanisms define the function of a pathway that allows exogenous antigens to elicit MHC-I-restricted CD8 T-cell responses that may be important in immunity to microbes and tumours.
Acknowledgments
C57BL/6 backcrossed TAP1 knockout mice were generously provided by Dr Luc Van Kaer (Vanderbilt University, Nashville, TN). We thank Dr Ron Germain (NIH, Bethesda, MD) for generously providing much helpful advice and the 25-D1.16 monoclonal antibody. We appreciate helpful comments and advice from Dr Man-Sun Sy (Case Western Reserve University, Cleveland, OH). Michael Sramkoski provided valuable assistance with the flow cytometry analyses. This work was supported by NIH grants AI34343, CA70149 and AI35726 to CH.
Abbreviations
- MHC-I
class I MHC
- TAP
transporter for antigen presentation
- ER
endoplasmic reticulum
REFERENCES
- 1.Ljunggren H-G, Stam NJ, Ohlen C, et al. Empty MHC class I molecules come out in the cold. Nature. 1990;346:476. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- 2.De Silva AD, Boestaenu A, Song R, Harhaj E, Harding CV, Joyce S. 1998. Peptide receptive Kb molecules are thermolabile because they assemble with low affinity peptides in TAP deficient RMA-S cells. [PubMed] [Google Scholar]
- 3.Day PM, Esquivel F, Lukszo J, Bennink JR, Yewdell JW. Effect of TAP on the generation and intracellular trafficking of peptide-receptive major histocompatibility complex class I molecules. Immunity. 1995;2:137. doi: 10.1016/s1074-7613(95)80014-x. [DOI] [PubMed] [Google Scholar]
- 4.Song R, Harding CV. Roles of proteasomes, TAP and β2-microglobulin in the processing of bacterial or particulate antigens via an alternate class I MHC processing pathway. J Immunol. 1996;156:4182. [PubMed] [Google Scholar]
- 5.Bevan MJ. Cross-priming for a secondary response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone FR, Bevan MJ. Class I-restricted processing and presentation of exogenous cell-associated antigen in vivo. J Exp Med. 1990;171:377. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gooding LR, Edwards CB. H-2 antigen requirements in the in vitro induction of SV40-specific cytotoxic T lymphocytes. J Immunol. 1980;124:1258. [PubMed] [Google Scholar]
- 8.Jin Y, Shih WK, Berkower I. Human T cell response to the surface antigen of hepatitis B virus (HBsAg). Endosomal and nonendosomal processing pathways are accessible to both endogenous and exogenous antigen. J Exp Med. 1988;168:293. doi: 10.1084/jem.168.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins DS, Findlay K, Harding CV. Processing of exogenous liposome-encapsulated antigens in vivo generates class I MHC-restricted T cell responses. J Immunol. 1992;148:3336. [PubMed] [Google Scholar]
- 10.Pfeifer JD, Wick MJ, Roberts RL, Findlay KF, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 11.Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;53:4925. [PubMed] [Google Scholar]
- 12.Reis e Sousa C, Germain R. Major histocompatibility complex class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis. J Exp Med. 1995;182:841. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 14.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 15.Bachmann MF, Oxenius A, Pircher H, et al. TAP1-independent loading of class I molecules by exogenous viral proteins. Eur J Immunol. 1995;25:1739. doi: 10.1002/eji.1830250637. [DOI] [PubMed] [Google Scholar]
- 16.Liu T, Zhou X, Orvell C, Lederer E, Ljunggren HG, Jondal M. Heat-inactivated Sendai virus can enter multiple MHC class I processing pathways and generate cytotoxic T lymphocyte responses in vivo. J Immunol. 1995;154:3147. [PubMed] [Google Scholar]
- 17.Schirmbeck R, Melber K, Reimann J. Hepatitis B virus small surface antigen particles are processed in a novel endosomal pathway for major histocompatibility complex class I-restricted epitope presentation. Eur J Immunol. 1995;25:1063. doi: 10.1002/eji.1830250431. [DOI] [PubMed] [Google Scholar]
- 18.Harding CV. Phagocytic processing of antigens for presentation by MHC molecules. Trends Cell Biol. 1995;5:105. doi: 10.1016/s0962-8924(00)88959-x. [DOI] [PubMed] [Google Scholar]
- 19.Bevan MJ. Antigen presentation to cytotoxic T lymphocytes in vivo. J Exp Med. 1995;182:639. doi: 10.1084/jem.182.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rock KL. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996;17:131. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 21.Harding C. Choosing and preparing antigen-presenting cells. In: Coligan JE, Margulies DH, Shevach EM, Strober W, Kruisbeek AM, editors. Current Protocols in Immunology. Wiley, New York, NY: 1997. p. 16.1. [Google Scholar]
- 22.Van Kaer L, Aston-Rickardt PG, Ploegh HL, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules and CD4+ T cells. Cell. 1992;71:1205. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 23.Harding C. Presenting exogenous antigens to T cells. In: Coligan JE, Margulies DH, Shevach EM, Strober W, Kruisbeek AM, editors. Current Protocols in Immunology. Wiley, New York, NY: 1997. p. 16.2. [Google Scholar]
- 24.Harding CV. Techniques for studying phagocytic processing of bacteria for class I or II MHC-restricted antigen recognition by T lymphocytes. Methods Cell Biol. 1994;45:307. doi: 10.1016/s0091-679x(08)61859-2. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed SA, Gogal RM, Jr, Walsh JE. A new rapid, and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to H3-thymidine incorporation assay. J Immunol Methods. 1994;170:211. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 26.Matousek MP, Nedrud JN, Cieplak W, Harding CV. Inhibition of class II MHC antigen processing and presentation by Escherichia coli heat-labile enterotoxin requires an enzymatically active A subunit. Infection Immunity. 1998;66:3480. doi: 10.1128/iai.66.7.3480-3484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 28.Rock KL, Gramm C, Benacerraf B. Low temperature and peptides favor the formation of class I heterodimers on RMA-S cells at the cell surface. Proc Natl Acad Sci USA. 1991;88:4200. doi: 10.1073/pnas.88.10.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elliott T, Cerundolo V, Elvin J, Townsend A. Peptide-induced conformational change of the class I heavy chain. Nature. 1991;351:402. doi: 10.1038/351402a0. [DOI] [PubMed] [Google Scholar]
- 30.Townsend A, Öhlén C, Bastin H, Ljunggren H-G, Foster L, Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989;340:443. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- 31.Spies T, Demars R. Restored expression of major histocompatibiltiy class I molecules by gene transfer of a putative peptide transporter. Nature. 1991;351:323. doi: 10.1038/351323a0. [DOI] [PubMed] [Google Scholar]
- 32.Attaya M, Jameson S, Martinex CK, et al. Ham-2 corrects the class I antigen-processing defect in RMA-S cells. Nature. 1992;355:647. doi: 10.1038/355647a0. [DOI] [PubMed] [Google Scholar]
- 33.Griffin JP, Chu R, Harding CV. Early endosomes and a late endocytic compartment generate different peptide-class II MHC complexes via distinct processing mechanisms. J Immunol. 1997;158:1523. [PubMed] [Google Scholar]