Abstract
Elevated levels of the cytokine interleukin-10 (IL-10) have been reported in patients with active systemic lupus erythematosus (SLE). Any role for IL-10 in the pathogenesis of SLE is likely to involve the activation of expression of specific genes within its target cells. We have previously reported elevated levels of the 90 000 MW heat-shock protein (hsp 90) and autoantibodies to hsp 90 in patients with SLE. Recent studies have shown that the cytokine IL-6 activates hsp 90 gene expression via specific transcription factors that include STAT-3 (signal transducer and activator of transcription 3). In view of the known role of STAT proteins in IL-10 signalling pathways, we have investigated the effect of IL-10 on hsp 90 gene expression. Here we report that IL-10 enhances the expression of hsp 90 in both a human hepatoma cell line (HepG2) stably expressing the human IL-10 receptor and peripheral blood mononuclear cells (PBMC). In reporter gene assays IL-10 is able to activate both the hsp 90α and hsp 90β promoters directly. Furthermore, a short region of the hsp 90β promoter which is activated in response to IL-10, contains a STAT-3 binding site. This element but not a mutant derivative unable to bind STAT-3, is able to confer a response to IL-10 on a heterologous promoter. These results may be understood in terms of the shared signalling mechanisms of IL-10 and IL-6 and provide evidence of a role for IL-10 in the overexpression of hsp 90 in SLE, with possible pathological consequences.
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune rheumatic disease mainly confined to women during the childbearing years, that is found in all ethnic groups but is particularly common amongst Afro-Carribeans.1 SLE is characterized by B-lymphocyte hyperactivity, reflected by a broad spectrum of autoantibodies and decreased in vitro and in vivo cellular immune responses. Such immune disturbances may in part reflect abnormalities in the expression of cytokines, which regulate the functions of cells within the normal immune system.
Interleukin-10 (IL-10) is a multifunctional cytokine produced by various cell types including B cells, T cells and monocytes.2 IL-10 is a potent stimulator of B lymphocytes, promoting B-lymphocyte activation, proliferation and differentiation and the stimulation of immunoglobulin and autoantibody production within B cells.3 In contrast, IL-10 has been recognized as an inhibitory cytokine, suppressing the production of pro-inflammatory cytokines as well as inhibiting cell-mediated immunity by antigen-presenting cells and T lymphocytes.4
Elevated circulating levels of IL-10 have been reported in patients with SLE, being highest in patients with active disease.5 In view of the function of IL-10 within the normal immune system, a pathogenic role for IL-10 in SLE has been suggested and is supported by a number of studies. It has been demonstrated that peripheral blood mononuclear cells (PBMC) from untreated lupus patients spontaneously release large amounts of IL-10 in vitro.6 Furthermore, spontaneous immunoglobulin production (particularly DNA autoantibodies) in PBMC from patients with SLE is increased in response to IL-10 and inhibited in response to an anti-IL-10 antibody.7 Defects in the regulation of auto-reactive B and T lymphocytes by a process known as programmed cell death (apoptosis) may play a pathogenic role in SLE. It has been shown that IL-10 increases Bcl-2 expression by germinal centre B lymphocytes,8 preventing their death and suggesting that the stimulating effect of IL-10 on immunoglobulin production may be partly due to increased B-lymphocyte survival.There is also evidence that disease severity in patients with SLE correlates with an increased ratio of IL-10-secreting PBMC.9 A role for IL-10 in murine SLE is supported by the observation that administration of anti-IL-10 antibodies to NZB/W F1 lupus-prone mice, delays the onset of autoimmunity and production of autoantibodies.10
Any role for IL-10 in the pathogenesis of SLE is likely to involve the induction of the expression of specific genes within its target cells, resulting in changes of cellular phenotype. We have previously reported autoantibodies to heat-shock protein 90 (hsp 90)11 and overexpression of hsp 90 in PBMC, within a specific subset of patients with SLE, particularly those with active neuro-psychiatric (NP) and/or cardio-respiratory (CR) disease.12 Elevation of hsp 90 protein levels is dependent on the enhanced transcription of the hsp 90β gene, one of the two genes encoding hsp 90.13 Furthermore, this elevation is not parallelled by elevation of the constitutively expressed heat-shock proteins hsp 73 or hsp 60 and therefore does not represent a general cellular stress response.12 Evidence of a potential role for hsp 90 in the aetiology and pathogenesis of this disease has been supported by studies in autoimmune MRL/lpr mice where elevated levels of hsp 9014 and antibodies to it15 are detectable prior to onset of the disease. However, the precise mechanisms leading to the overexpression of hsp 90 have remained unclear.
We have recently shown that the cytokine IL-6, which is also present at elevated levels in patients with active SLE,16 up-regulates hsp 90 expression in the human hepatoma cell line (HepG2) and in peripheral blood mononuclear cells (PBMC) from normal individuals.17 A role for IL-6 in hsp 90 overexpression in SLE has been further supported by the observation that elevation of IL-6 in transgenic mice results in increased levels of hsp 90 and the production of anti-hsp 90 antibodies.18
An investigation of the mechanisms of hsp 90 promoter activation in response to IL-6 has been recently reported.19 In this study, using reporter gene assays, activation of the hsp 90 promoter by IL-6 is mediated by a region of the promoter (from −643 to −623 relative to the transcription start site) which contains a binding site for the IL-6 activated transcription factor STAT-3. It is interesting to note that both IL-6 and IL-10 utilize similar signalling mechanisms for modulating gene expression within their target cells. IL-10 has been shown to mediate intracellular signalling via the Jak/STAT pathway, in which stimulation of receptor-associated Jaks (Jak1 and Tyk2) leads to phosphorylation and activation of the transcription factors STAT-1 and STAT-3.20
The similarity of gene regulatory elements targeted by signalling pathways for both IL-6 and IL-10 through the activation of STATs together with studies supporting a role for IL-10 in the pathogenesis of SLE, has therefore prompted us to investigate the effect of IL-10 on hsp 90 gene expression.
MATERIALS AND METHODS
Reagents and antibodies
Recombinant human IL-10 was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), Levels of hsp 90 were determined by Western blot analysis with the AC88 antibody21 to hsp 90 (a kind gift from Dr D. Toft).
Cell culture
PBMC were isolated by Ficol-Paque (Lymphoprep™; Nycomed, Oslo, Norway). PBMC were cultured at 106 per well in RPMI-1640 plus 3% (v/v) of corresponding human serum in six-well plates (Nunc, Uxbridge, UK) at 37° in a 5% CO2 incubator. PBMC were treated with IL-10 or IL-6 at the concentrations and time intervals indicated in the figure legends. After treatment with IL-10 or IL-6, cells were harvested for Western blot analysis. HepG2 hepatoma cells stably expressing the human IL-10 receptor22 were a kind gift from Dr Heinz Baumann, Roswell Park Cancer Institute, Buffalo, NY and were maintained in monolayer cultures in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) supplemented with 10% (v/v) bovine fetal serum at 37° in a 5% CO2 incubator. For each experiment, cells were trypsinized and re-plated at a density of 106 per well in DMEM plus 10% (v/v) fetal calf serum in six-well plates over night. Cells for treatment with IL-10 were incubated for a further 6 hr in serum-free medium before addition of IL-10 at the concentrations indicated in the figure legends. Following treatment, cells were harvested for Western blot analysis which was performed as described previously17 with the AC88 antibody to hsp 90.
Plasmid constructs
The 5′ hsp 90β promoter chloramphenicol acetyl transferase (CAT) reporter construct hsp 90β–CAT23 was a kind gift from Dr Neil Rebbe (Washington University School of Medicine, St. Louis, MO). Construct hsp 90β–CAT consists of a fragment −1044 to +36 relative to the transcriptional start site, coupled to a CAT vector. The 5′ hsp 90α promoter CAT reporter construct hsp 90α–CAT, was a kind gift from Dr Lee Webber. Construct hsp 90α–CAT consists of a fragment −1050 to +42 relative to the transcriptional start site, coupled to a CAT vector. The hsp 90β promoter wild-type and mutant STAT-3 vectors have been previously described.19 Construct hsp 90–S3E consists of a sequence of the hsp 90β promoter from −643 to −623 relative to the transcriptional start site. Construct hsp 90–MS3E is identical to hsp 90–S3E except for adenine residues at position −641 and −631, replacing the wild-type cytosine residues. Both the wild-type −643 to −623 and mutant hsp 90β promoter STAT-3 sequences are cloned upstream of the thymidine kinase promoter and the CAT reporter gene in the vector pBLCAT2 (see Fig. 1). The wild-type STAT-3 expression vector contains the full coding region for STAT-3 cloned into the mammalian expression vector, pEF-Bos and expressed under control of the elongation factor gene promoter.24
Figure 1.
Synthetic oligonucleotides corresponding to the wild-type hsp 90β promoter STAT-3 binding site (a) and a mutant derivative (b), which were cloned upstream of the thymidine kinase promoter and the CAT reporter gene in the vector pBLCAT2.
Transfection, stimulation and CAT assays
Transfection of hsp 90 promoter reporter constructs was performed as described previously17 by the calcium phosphate method of Gorman.25 IL-10-responsive HepG2 cells were plated at a density of 106 cells/well in six-well plates; 5 μg of CAT reporter constructs, 1 μg of a control β-galactosidase expression vector and where indicated, 2 μg of a STAT-3 expression vector, were transfected for 4–6 hr and cells were washed in phosphate-buffered saline (PBS). Cells were maintained in complete medium for 24 hr before a 5-hr period of serum starvation, followed by treatment with 50 ng of IL-10. Cells were then harvested after 15 hr of treatment with IL-10 and lysed by three cycles of repeated freeze–thawing. To normalize for transfection efficiency, CAT activities were corrected for β-galactosidase activities. Assays of CAT activity were performed by the method of Gorman25 on samples that had been equalized for protein content as determined by the method of Bradford.26
RESULTS
IL-10 up-regulates hsp 90 in HepG2 cells and PBMC
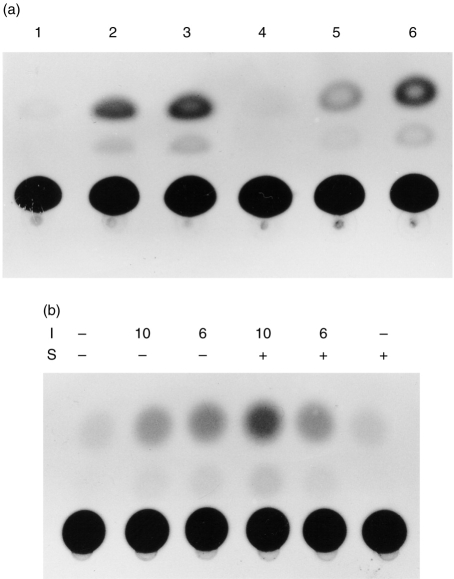
The effect of IL-10 on hsp 90 gene expression was investigated by treatment of both HepG2 cells and PBMC with IL-10 and levels of hsp 90 measured by Western blot analysis (Fig. 2). IL-10 was observed to clearly up-regulate hsp 90 levels within the HepG2 cell line (Fig. 2a) which had been artificially engineered to express the human IL-10 receptor.22 Within the parental cell line (lacking the IL-10 receptor) no elevation of hsp 90 was observed following treatment with IL-10 (result not shown). In cells transfected with an expression vector for STAT-3, hsp 90 levels were also observed to be elevated following IL-10 stimulation, and this elevation was observed to a lesser extent in unstimulated cells transfected with STAT-3 compared to control unstimulated cells. In PBMC, hsp 90 levels are significantly elevated following IL-10 treatment, compared to control untreated cells (Fig. 2b). This effect is also observed in PBMC treated with interferon-γ and IL-6 which has been reported previously.19 Furthermore, an analysis of hsp 90 levels in PBMC following a time–course of IL-10 treatment (Fig. 2c) shows that elevation of hsp 90 levels is rapid. An approximate threefold induction of hsp 90 expression in PBMC is observed after 1 hr of IL-10 treatment compared to control untreated cells, with this increase being sustained for 48 hr of treatment.
Figure 2.
(a) Western blot with antibody AC88 To hsp 90. HepG2 cells were treated with either 50 ng/ml (50) or 100 ng/ml (100) of IL-10 for 12 hr or untreated (0). Cells transfected with 2 μg of a STAT-3 expression vector are shown (+ 3). (b) Western blot with antibody AC88 To hsp 90. PBMC were untreated (lanes 1 and 2), treated with interferon-γ (lane 3), IL-6 (lane 4), or treated for 16 hr with 50 ng/ml of either IL-6 (lane 5) or IL-10 (lane 6). (c) Time–course of hsp 90 elevation in PBMC treated with IL-10. PBMC were untreated or treated with 50 ng/ml of IL-10 for the times indicated in the Figure. Values for hsp 90 levels represent fold induction relative to control untreated cells.
IL-10 activates The hsp 90α and -β promoters
To investigate the mechanisms by which IL-10 induces enhanced hsp 90 levels in HepG2 cells, a direct effect on the hsp 90 promoter was studied using plasmid constructs in which the hsp 90α and -β promoters are coupled to a CAT reporter gene. As shown in Fig. 3(a) and Table 1, IL-10 significantly activates both the hsp 90α and -β promoters, as measured by CAT activity in IL-10-treated and untreated cells. Cells cotransfected with a STAT-3 expression vector and treated with IL-10, exhibit slightly higher CAT activity than control IL-10-treated cells. In view of the known effects of IL-6 on hsp 90 gene regulation, IL-6 treatment of cells transfected with hsp 90 promoter constructs was used to compare the responsiveness of the reporter constructs to IL-10 and IL-6. Figure 3(b) illustrates responsiveness of the hsp 90β promoter construct to both IL-6 and IL-10 and indicates that the two effects are comparable.
Figure 3.
Assay of CAT activity in HepG2 cells transfected with hsp 90α and -β promoter–CAT constructs. (a) HepG2 cells were transfected with 2 μg of either hsp 90α–CAT (lanes 1–3) or hsp 90β–CAT (lanes 4–6). Cells were left untreated (lanes 1, 4) or treated with 100 ng/ml IL-10 for 12 hr (lanes 2, 3 and 5, 6). Lanes 3 and 6 correspond to cells cotransfected with1 μg of a STAT-3 expression vector. (b) Assay of CAT activity in HepG2 cells transfected with hsp 90β–CAT and treated with IL-6 or IL-10. HepG2 cells were transfected with 2 μg of hsp 90β–CAT and treated (I) with either 50 ng/ml IL-6 (6) or 100 ng/ml IL-10 (10) for 12 hr or untreated (−). Cells cotransfected (S) with 1 μg of a STAT-3 expression vector are denoted (+).
Table 1.
Effect of IL-10 on the activity of hsp 90 promoter CAT constructs

Values represent the CAT activity of each construct either alone or cotransfected with a STAT-3 expression vector (+ STAT-3) in HepG2 cells treated (+ IL-10) or untreated (- IL-10) with IL-10 for 12 hr before harvesting. Values were obtained by scanning densitometry of assays of the type shown in Fig. 2,Fig. 3,Fig. 4
IL-10 activates the hsp 90β promoter via a STAT-3 binding site
In view of previous studies which have shown that a region of the hsp 90β promoter (−643 to −623 relative to the transcriptional start site) can confer responsiveness to IL-6 when linked to a heterologous promoter, we have analysed the responsiveness of this region to IL-10. HepG2 cells were transfected with construct hsp 90-S3E, corresponding to this region linked to the heterologous thymidine kinase promoter and also with a construct hsp 90-MS3E, in which the wild-type STAT-3 site has been mutated and CAT activity of the constructs was then measured following treatment of cells with IL-10. Figure 4(a) and Table 1 show that the wild-type STAT-3 sequence gave high levels of CAT activity in response to IL-10 and activation was enhanced by cotransfection with a STAT-3 expression vector. Conversely the mutant construct (Fig. 4b and Table 1) was not responsive to IL-10 and no significant increase in CAT activity was observed following cotransfection with STAT-3.
Figure 4.

Assay of CAT activity in HepG2 cells transfected with hsp 90-S3E (a) or hsp 90-MS3E (b). HepG2 cells were transfected with 2 μg of either CAT reporter construct and treated (I) with 100 ng/ml IL-10 (10) for 12 hr or untreated (−). Cells cotransfected (S) with 1 μg of a STAT-3 expression vector are denoted (+).
DISCUSSION
Although the regulation of the heat-shock proteins by stressful stimuli has been intensively studied, much less attention has been given to their regulation by non-stressful stimuli, such as cytokines. This is of particular importance, since hsp expression has been observed to be regulated during a number of normal cellular events such as T-cell activation and the differentiation of monocytes to macrophages.27,28 Hence such regulatory mechanisms may play a key role in determining the level of hsp expression in unstressed cells as well as being important in their overexpression in specific disease states.
We have previously demonstrated that treatment of cells with IL-6 can up-regulate hsp 90 levels in vitro17 and that such elevation of hsp 90 levels is also observed in vivo in transgenic mice overexpressing IL-6·18 This effect is due to the IL-6-induced activation of the transcription factors NF-IL6 and STAT-3 and is mediated via a short specific region of the hsp 90 promoter which contains binding sites for these factors.17,19 Here we have extended these observations by showing that IL-10 can also enhance hsp 90 levels, activating the hsp 90β gene promoter via the same short element which is targeted by IL-6. As IL-10 activates STAT-3 but not NF-IL620 it is likely that this effect is mediated via the IL-10-induced activation of STAT-3 and in agreement with this the ability of IL-10 to activate the hsp 90α and -β gene promoters is enhanced by cotransfection of a STAT-3 expression vector (see Fig. 3). Hence the activation of hsp 90 by IL-10 is likely to reflect the similarity of its signalling pathway to that of IL-6 and indicates that such activation can occur in the absence of activation of NF-IL6 which is achieved only by IL-6. This is in accordance with our previous observation that enhanced hsp 90 levels are observed both in transgenic mice containing the IL-6 gene and in transgenic mice in which the gene encoding NF-IL6 has been inactivated resulting in enhanced levels of IL-6.18
The induction of hsp 90 expression by both IL-6 and IL-10 infers a role for hsp 90 expression both within the normal functioning of the immune system and as part of an immune response. It is likely that both IL-6 and IL-10 regulate the expression of hsp 90 ensuring appropriate levels of chaperoning functions of hsp 90 within immune cells. More importantly, these results have important implications for our understanding of the mechanisms of hsp 90 elevation in SLE. Thus, since both IL-616 and IL-105,6 are elevated in SLE, it will be necessary to investigate the relative effects of these two factors in producing the hsp 90 elevation observed in this disease. Elevation of hsp 90 in patients with SLE and in MRL/lpr mice may be responsible for the production of autoantibodies to this protein. Interestingly this situation is also observed in human breast cancer patients where elevated hsp 90 levels in tumour tissue29 are parallelled by the development of autoantibodies to hsp 90.30 The generation of an immune response to elevated hsp 90 levels in SLE is likely to require the surface localization of this protein, providing accessibility to the immune system. We have previously detected the surface localization of hsp 9031 on peripheral blood monocytes and lymphoctes. Furthermore this observation was specific to hsp 90 and correlated with the degree of overexpression of hsp 90 in these patients. Evolutionary conservation of the heat-shock proteins at the amino acid level, results in homologues of human heat-shock proteins in bacteria and other organisms which can infect man. These heat-shock proteins constitute major targets for an immune response during infection. Antibodies against Candica albicans hsp 90 are observed in systemic candidal infections, and some of these react with human hsp 90.32,33 Reactivity of antibodies and T cells induced by exposure to the heat-shock proteins of micro-organisms with corresponding cell surface localized human heat-shock proteins, provides a possible model for a pathological consequence of heat-shock protein overexpression.
We are currently correlating the levels of hsp 90 expression in PBMC in a large series of patients with SLE, with their levels of circulating IL-6 and IL-10 in order to investigate this effect further. Such studies are of importance since we have previously shown that the elevated levels of hsp 90 in transgenic mice overexpressing IL-6 can provoke an autoimmune response to this protein18 whilst the elevation of hsp 90 levels and the autoimmune response to it which is observed in MRL/lpr mice both precede the onset of disease symptoms.14,15
REFERENCES
- 1.Johnson AE, Gordon C, Palmer RG, Bacon PA. The prevalence and incidence of systemic lupus erythematosus in Birmingham, England. Relationship to ethnicity and country of birth. Arthritis Rheum. 1995;38:551. doi: 10.1002/art.1780380415. [DOI] [PubMed] [Google Scholar]
- 2.Moore KW, O’Garra A, De Waal Malefyt R, Viera P, Mossman TR. Interleukin-10. Annu Rev Immunol. 1993;11:165. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 3.Rousset F, Garcia E, Thierry D. Interleukin-10 is a potent growth and differentiation factor for activated human B Lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Waal Malefyt R, Abrams J, Bennet B, Figdor CG, Vries JE. Interleukin-10 inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park YB, Lee SK, Kim DS, Lee J, Lee CH, Song CH. Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin Exp Rheum. 1998;16:283. [PubMed] [Google Scholar]
- 6.Linker-Israeli M. Cytokine abnormalities in human lupus. Clin Immunol Immunopathol. 1992;63:10. doi: 10.1016/0090-1229(92)90084-2. [DOI] [PubMed] [Google Scholar]
- 7.Llorente L, Zou W, Levy Y, Richard-Patin Y. Role of IL-10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehrian R, Quismorio FP, Strassmann G, et al. Synergistic effect between IL-10 and bcl-2 genotypes in determining susceptibility to systemic lupus erythematosus. Arthritis Rheum. 1998;41(4):596. doi: 10.1002/1529-0131(199804)41:4<596::AID-ART6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Hagiwara E, Gourley MF, Lee S, Klinman DM. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin-10: interferon-γ-secreting cells in the peripheral blood. Arthritis Rheum. 1996;39:397. doi: 10.1002/art.1780390305. [DOI] [PubMed] [Google Scholar]
- 10.Ishida H, Muchamuel T, Sakaguchi S. Continuous administration of anti-interleukin-10 antibodies delays onset of autoimmunity in NZB/W F1 mice. J Exp Med. 1994;179:305. doi: 10.1084/jem.179.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conroy SE, Faulds GB, Williams W, Latchman DS, Isenberg DA. Detection of autoantibodies to the 90kD heat shock protein in SLE and other autoimmune diseases. Br J Rheumatol. 1994;33:923. doi: 10.1093/rheumatology/33.10.923. [DOI] [PubMed] [Google Scholar]
- 12.Dhillon VB, McCallum S, Norton PM, et al. Differential heat shock protein over-expression and its clinical relevance in systemic lupus erythematosus. Ann Rheum Dis. 1993;52:436. doi: 10.1136/ard.52.6.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Twomey B, Dhillon V, McCallum S, Isenberg D, Latchman D. Elevated levels of the 90 kD heat shock protein in patients with systemic lupus erythematosus are dependent upon enhanced transcription of the hsp90β gene. J Autoimm. 1993;6:495. doi: 10.1006/jaut.1993.1041. [DOI] [PubMed] [Google Scholar]
- 14.Faulds GB, Isenberg DA, Latchman DS. The tissue-specific elevation in synthesis of the 90kD heat shock protein precedes the onset of disease in lupus prone MRL/lpr mice. J Rheumatol. 1994;21:234. [PubMed] [Google Scholar]
- 15.Faulds GB, Conroy SE, Mahadeo M, Isenberg DA, Latchman DS. Increased levels of the autoantibodies to HSPs with increasing age in MRL/lpr mice. Br J Rheum. 1995;34:610. doi: 10.1093/rheumatology/34.7.610. [DOI] [PubMed] [Google Scholar]
- 16.Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Kilinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. Aputative role in pathogenesis. J Immunol. 1997;147:117. [PubMed] [Google Scholar]
- 17.Stephanou A, Amin V, Isenberg DA, Akira S, Kishimoto T, Latchman DS. Interleukin-6 activates heat-shock protein 90β gene expression. Biochem J. 1997;321:103. doi: 10.1042/bj3210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephanou A, Conroy S, Isenberg DA, et al. Elevation of IL-6 in transgenic mice results in increased levels of the 90 kDa heat shock protein (hsp90) and the production of anti- hsp90 antibodies. J Autoimm. 1998;11:249. doi: 10.1006/jaut.1998.0194. [DOI] [PubMed] [Google Scholar]
- 19.Stephanou A, Isenberg DA, Akira S, Kishimoto T, Latchman DS. The NF-IL6 and STAT-3 signalling pathways co-operate to mediate the activation of the hsp90β gene by IL-6 but have opposite effects on its inducibility by heat shock. Biochem J. 1998;330:189. doi: 10.1042/bj3300189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and jak1 and the differential asembly of STAT1α and STAT3 complexes in human T cells and monocytes. J Immunol. 1995;155:1079. [PubMed] [Google Scholar]
- 21.Riehl RM, Sullivan WP, Vroman BT, Baver VJ, Pearson GR, Toft DO. Immunological evidence that the non-hormone binding component of avian steroid receptors exists in a wide range of tissues and species. Biochemistry. 1985;24:6586. doi: 10.1021/bi00344a042. [DOI] [PubMed] [Google Scholar]
- 22.Lai C-F, Ripperger J, Morella KK, et al. Receptors for interleukin (IL) -10 and IL-6-type cytokines use similar signaling mechanisms for inducing transcription through IL-6 response elements. J Biol Chem. 1996;271:13968. doi: 10.1074/jbc.271.24.13968. [DOI] [PubMed] [Google Scholar]
- 23.Rebbe NF, Ware J, Bertina RM, Modrich P, Stafford DW. Nucleotide sequence of a cDNA for a member of the human 90-kDa heat shock protein family. Gene. 1987;53:235. doi: 10.1016/0378-1119(87)90012-6. [DOI] [PubMed] [Google Scholar]
- 24.Minami M, Inoue M, Wei S, et al. STAT3 activation is a critical step in gp130-mediated terminal differentiation and growth arrest of a myeloid cell line. Proc Natl Acad Sci USA. 1996;93:3963. doi: 10.1073/pnas.93.9.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorman CM. DNA Cloning: a Practical Approach. Vol. 2. Oxford: IRL Press; 1985. High efficiency gene transfer into mammalian cells. [Google Scholar]
- 26.Bradford M. A rapid and sensitive method for the quantitation of micro-gram quantities of proteins utilising the principle of protein-dye binding. Anal Biochem. 1976;72:248. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 27.Twomey BM, McCallum S, Isenberg DA, Latchman DS. Elevation of heat shock protein synthesis and hsp gene transcription during monocyte to macrophage differentiation of U937 cells. Clin Exp Immunol. 1993;93:178. doi: 10.1111/j.1365-2249.1993.tb07962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haire RN, Peterson MS, O’Leary JJ. Mitogen activation induces the enhanced synthesis of two heat shock proteins in human lymphocytes. J Cell Biol. 1988;106:883. doi: 10.1083/jcb.106.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jameel A, Skilton RA, Campbell TA, Chander SK, Coombes RC, Luqmani YA. Clinical and biological significance of hsp89 alpha in human breast cancer. Int J Cancer. 1992;50:409. doi: 10.1002/ijc.2910500315. [DOI] [PubMed] [Google Scholar]
- 30.Conroy SE, Gibson SL, Brunstrom G, Isenberg DA, Luqmani Y, Latchman DS. Autoantibodies to the 90kD heat shock protein in sera of breast cancer patients. Lancet. 1995;345:126. doi: 10.1016/s0140-6736(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 31.Erkeller-Yuksel FM, Isenberg DA, Dhillon VB, Latchman DS, Lydyard PM. Surface expression of heat shock protein 90 by blood mononuclear cells from patients with systemic lupus erythematosis. J Autoimmun. 1992;5:803. doi: 10.1016/0896-8411(92)90194-u. [DOI] [PubMed] [Google Scholar]
- 32.Matthews R, Burnie J. The role of hsp90 in fungal infection. Immunology Today. 1992;13:345. doi: 10.1016/0167-5699(92)90169-8. [DOI] [PubMed] [Google Scholar]
- 33.Matthews R. Candida albicans hsp 90: Link between protective and autoimmunity. J Med Microbiol. 1992;36:367. doi: 10.1099/00222615-36-6-367. [DOI] [PubMed] [Google Scholar]