Abstract
The natural occurrence of Japanese cedar (Cryptomeria japonica; CJ) pollinosis has been reported in Japanese monkeys (Macaca fuscata), an appropriate animal model for developing antipollinosis therapies. However, there has been no study on the incidence of Japanese cedar pollinosis in monkeys. To evaluate the incidence of CJ pollinosis in Japanese monkeys, we investigated the presence of pollinosis symptoms among monkeys in a troop, and the response to CJ allergens in pollinosis monkeys. We examined the presence of pollinosis symptoms in 272 monkeys in a troop throughout the CJ pollination season (February to April). Of the 272 monkeys, 21 (7·7%) showed pollinosis symptoms during the CJ pollen season. Blood samples were taken from the 21 monkeys that showed pollinosis symptoms and were tested for the presence of immunoglobulin E (IgE) antibody for CJ allergens. All 21 monkeys with CJ pollinosis had anti-CJ IgE. Of the 21 monkeys, peripheral blood mononuclear cells (PBMC) could be taken from 12, all of which showed CJ allergen-specific PBMC proliferation. The incidence of CJ pollinosis in a troop was 7·7%. The monkeys with CJ pollinosis demonstrated specific IgE and PBMC proliferation for CJ allergens.
INTRODUCTION
Japanese cedar (Cryptomeria japonica; CJ) pollinosis is one of the most common allergic diseases in Japan.1,2 Our previous seroepidemiological studies showed that ≈30% of the human general population between 20 and 39 years of age who resided in the Tokyo area had anti-CJ immunoglobulin E (IgE) antibody.3,4 In another study of 892 university students, the percentage of carriers of CJ-specific IgE was found to be 27% and the incidence of CJ pollinosis sufferers was 12%.5
The natural occurrence of CJ pollinosis has also been found in Japanese monkeys (Macaca fuscata).6 These monkeys with CJ pollinosis showed symptoms similar to those of human patients: tear production, eye redness, sneezing and rhinorrhea. CJ extract injection into the skin produced a wheal in the monkeys. Furthermore, monkey serum induced a skin allergic reaction to CJ extract in the Prausniz–Küstner test. The presence of anti-CJ IgE in monkeys can be assayed by the CAP system produced by Pharmacia (Uppsala, Sweden) for human IgE.7,8 Japanese monkeys with CJ pollinosis had reactivity to CJ major allergens,7,9 which have been isolated from CJ pollen and characterized as Cry j 1 and Cry j 2.10,11 In addition, leucocytes from these monkeys showed CJ allergen-specific histamine release, as well as significant proliferation in response to CJ allergens.12
Since there is no report that showed the incidence of CJ pollinosis in monkeys, we undertook an investigation of this occurrence. We examined the presence of CJ pollinosis in 272 monkeys in a troop throughout the CJ pollination season, and the immune-response to CJ crude and major allergens in monkeys that showed CJ pollinosis.
MATERIALS AND METHODS
Monkeys
The Japanese monkeys (Macaca fuscata) used in this study are native to Awaji Island, Hyogo Prefecture, Japan. This monkey troop is the only one on this island. The monkeys were in a free-range troop. They daily came to the feeding place and we examined the presence of pollinosis symptoms among 272 Japanese monkeys over 2 years old during the CJ pollination season (February to April) in 1997. All 272 monkeys were named and identified. These monkeys consisted of 159 females (mean age, 8·3 years) and 113 males (mean age, 6·4 years).
Monkeys with CJ pollinosis were captured with the aid of a blowgun and blood samples were taken from them. A mixture of ketamine HCl (Kettaral, Sankyo, Tokyo, Japan) at a concentration of 5 mg/kg body weight and xylazine HCl (Celactar, Bayer, Tokyo, Japan) at about 1 mg/kg body weight was administered intramuscularly by the blowgun to each monkey, based on a rough estimation of the body weight. Each monkey was administered 4 mg/kg of torazoline HCl (T-6886, Sigma Chemicals, St Louis, MO) intravenously or intramuscularly as an antagonist to xylazine HCl after the examinations. The monkeys were released upon regaining consciousness.
Measurement of anti-CJ IgE
Specific IgE for CJ pollen allergens were analysed by the CAP system (Pharmacia, Uppsala, Sweden).7,8 All serum samples were stored at −70° until use.
Measurement of specific IgE to two major pollen allergens
Specific IgE to Cry j 1 and Cry j 2 was measured by a fluorometric enzyme-linked immunosorbent assay (ELISA) as previously described.7,8 Briefly, purified protein (1 μg/ml) was used to coat the microplate wells. After incubation of monkey serum (diluted 1:8) with the immobilized antigens, anti-human IgE antibody conjugated with β-d-galactosidase (Pharmacia), diluted 1:25, was added to each well. As the enzyme reaction substrate, 0·2 mm 4-methylumbelliferyl-β-d-galactoside (Sigma) was added and the plates were incubated at 37° for 2 hr. Then, 0·1 m glycine-NaOH (pH 10·3) was added to stop the reaction. Fluorescence units (FU) were measured on a fluorometric microplate reader (Fluoroskan; Flow Laboratories, McLean, VA). The specific IgE level was expressed as arbitrary units (U)/ml.
Proliferative response of peripheral blood mononuclear cells (PBMC) to major allergens
The PBMC proliferative response to major allergens was carried out as previously described.12 Fresh heparinized venous blood was incubated with 1% dextran (T 2000; Pharmacia) solution at room temperature for 1 hr and the supernatants were centrifuged. The pellet was then resuspended and these cells were counted as PBMC and used for proliferative response to allergens. PBMC (5×105) suspended in RPMI-1640 complete medium containing 5% human serum were cultured in 96-well plates in the presence of Cry j 1 or Cry j 2 (2·5 μg/ml), then incubated for 48 hr at 37°, in 5% CO2 and a humidified atmosphere. After being pulsed for 16 hr with 1 μCi/well of [3H]TdR, the cells were harvested and the incorporated radioactivity was measured on a scintillation counter.
RESULTS
The incidence of CJ pollinosis among monkeys in a troop
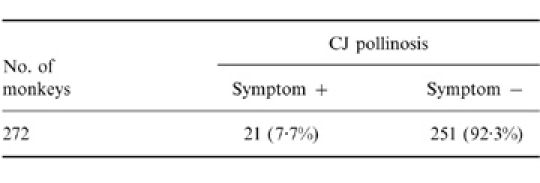
We examined the presence of pollinosis symptoms in 272 monkeys throughout the CJ pollination season. We found that 21 (7·7%) of these monkeys showed pollinosis symptoms (e.g. tear production, eye redness, sneezing) similar to those of human patients during the CJ pollen season (Table 1). The other 251 monkeys had no CJ pollinosis symptoms throughout the CJ pollination season. The 21 monkeys (mean age, 11·8 years) consisted of 16 females and four males. After the pollination season, pollinosis symptoms disappeared in all of the monkeys.
Table 1.
The incidence of CJ pollinosis in monkeys in a troop
The level of IgE to CJ allergens in monkeys with CJ pollinosis
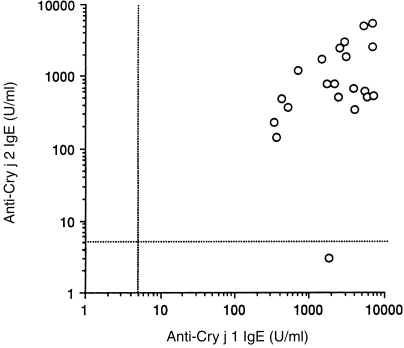
Blood samples from 21 monkeys that showed CJ pollinosis symptoms were collected. We measured anti-CJ IgE in their sera by CAP. All 21 monkeys with CJ pollinosis had anti-CJ IgE (mean value ± SD, 13·7±6·0 U/ml) (Fig. 1). There was no correlation between the level of anti-CJ IgE and their age in the pollinosis monkeys. Furthermore, we measured specific IgE to major allergens (Cry j 1, Cry j 2). Of 21 monkeys, 20 monkeys were found to have both anti-Cry j 1 and Cry j 2 IgE. There was a slight correlation between the levels of anti-Cry j 1 and Cry j 2 IgE (correlation coefficient of 0·44). One monkey demonstrated only anti-Cry j 1 IgE (Fig. 2).
Figure 1.
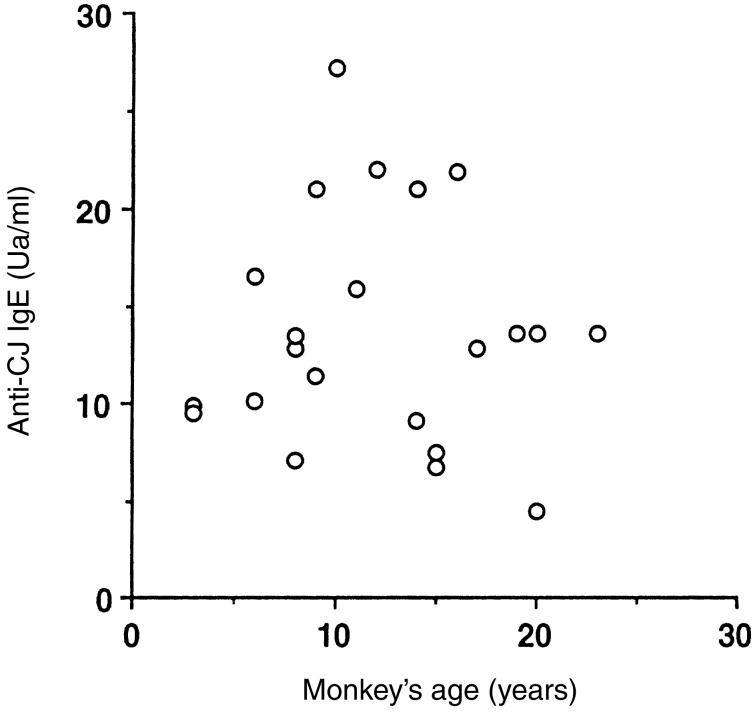
The levels of IgE to Japanese cedar pollen allergens in monkeys with CJ pollinosis by their age.
Figure 2.
The levels of anti-Cry j 1 and Cry j 2 IgE in monkeys with CJ pollinosis.
PBMC proliferation in response to major CJ allergens
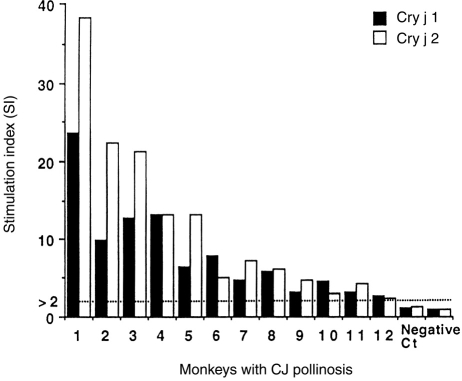
Of 21 monkeys that showed pollinosis symptoms, blood samples were collected from 12 adult monkeys. All 12 monkeys demonstrated specific IgE to Cry j 1 and Cry j 2. We conducted PBMC proliferation tests for CJ major allergens and presented the PBMC proliferation as a stimulation index (SI) (Fig. 3). All 12 monkeys demonstrated PBMC proliferation in response to both Cry j 1 and Cry j 2. As a negative control, two monkeys that had no pollinosis symptoms and no anti-CJ IgE demonstrated no PBMC proliferation in response to Cry j 1 and Cry j 2.
Figure 3.
PBMC proliferative response to Cry j 1 and Cry j 2 in monkeys with CJ pollinosis.
DISCUSSION
In our previous study, we reported that in 276 unselected monkeys in nine troops throughout Japan, 45 (16%) had anti-CJ IgE.8 The positive rate of anti-CJ IgE among nine troops ranged from 0% to 31%.8 We found that there was a variation of reactivity to CJ allergens among different troops. However, the causes of variations of reactivity among troops have not yet been clearly established. At all events, we found that many monkeys had been sensitized with CJ allergens. However, we had no information about the incidence of CJ pollinosis in monkeys in these troops.
In this study, we evaluated the incidence of CJ pollinosis among monkeys of more than 2 years old in one of the nine troops. In our previous study, we collected sera from 51 monkeys (mean age±SD, 9·1±3·6 years) in this troop and measured the anti-CJ IgE in the serum samples.8 We reported that the positive rate of anti-CJ IgE (25·5%) in this troop was the third highest positive rate in the nine troops.8 In the present study, we found that 21 monkeys (7·7%) showed pollinosis symptoms during the CJ pollination season and no symptoms after the pollen season had ended. We confirm that there are many monkeys with CJ pollinosis in this troop.
In this study, fewer male monkeys had CJ pollinosis than female monkeys. Most male Japanese monkeys leave their troop when they grow to adulthood (5–7 years old).13 Consequently, there were fewer adult male moneys in this troop than female monkeys. We think that the low incidence of CJ pollinosis in male monkeys might depend on the smaller number of adult male monkeys.
In this study, those monkeys that showed pollinosis symptoms demonstrated anti-CJ IgE. Furthermore, all monkeys had specific IgE to Cry j 1 or Cry j 2, which are major allergens in CJ pollen. Of 21 monkeys, 20 demonstrated both anti-Cry j 1 and anti-Cry j 2 IgE. In humans, ≈90% of CJ pollinosis patients have both anti-Cry j 1 and Cry j 2 IgE.14 The IgE-reactivity to the two major allergens in monkeys is similar to human patients.
In this study, pollinosis monkeys with CJ pollinosis showed a significant PBMC proliferation in response to CJ major allergens. In our previous study, we found that two monkeys with CJ pollinosis showed PBMC proliferation in response to the same major allergens. Analysis of T-cell epitopes in Cry j 1 and Cry j 2 for antipollinosis therapies in humans was reported.15,16 We reconfirmed that many monkeys showed PBMC proliferation to these allergens. In the future, these monkeys may be useful for the study of T-cell epitopes in these allergens.
Finally, we found that many monkeys have sensitivity to CJ pollen allergens and show symptoms similar to those of human patients with this pollinosis. We believe that monkeys with this pollinosis can serve as a suitable animal model for developing such antipollinosis therapies as desensitization treatments.
Acknowledgments
We are grateful to the staff of Awaji Monkey Park for their assistance in our observation of CJ pollinosis symptom among monkeys.
Abbreviations
- CJ
Cryptomeria japonica
- PBMC
peripheral blood mononuclear cells
- SI
simulation index
REFERENCES
- 1.Ishizaki T, Koizumi K, Ikemori R, Ishiyama Y, Kushibiki E. Studies of prevalence of Japanese cedar pollinosis among the residents in a densely cultivated area. Ann Allergy. 1987;58:265. [PubMed] [Google Scholar]
- 2.Platts-Mills TAE, Solomon WR. Aerobiology and inhalant allergens. In: Middleton E, Reed CE, Ellis EF, Adkinson NF, Yunginger JW, Busse WW, editors. Allergy: Principles and Practice. 4. Vol. 1. St. Louis: CV Mosby; 1993. p. 469. [Google Scholar]
- 3.Inouye S, Sakaguchi M, Mori H, et al. Seroepidemiology of Sugi (Japanese cedar) pollinosis: increase of IgE antibody positive rate in recent years. Igaku no Ayumi. 1986;138:285. (in Japanese with English summary) [Google Scholar]
- 4.Inouye S, Sakaguchi M, Hori T, Watanabe T. A seroepidemiological study of Cryptomeria japonica (Japanese cedar) pollinosis: measurement of IgE antibody. Igaku No Ayumi. 1986;136:959. (in Japanese) [Google Scholar]
- 5.Nakamura S. Investigation into the frequency of cases with Japanese cedar pollinosis among the students and staff members in our university. Jpn J Allergol. 1990;39:476. (in Japanese with English summary) [PubMed] [Google Scholar]
- 6.Yokota A, Minezawa M, Nakamura S, Kanaizuka T, Gotoh S, Baba S. Naturally occurring Japanese cedar (Cryptomeria japonica) pollenosis in Japanese monkeys (Macaca fuscata) inhabiting Miyajima Island. Primate Res. 1987;3:112. (in Japanese with English summary) [Google Scholar]
- 7.Sakaguchi M, Inouye S, Imaoka K, et al. Measurement of serum IgE antibodies against Japanese cedar pollen (Cryptomeria japonica) in Japanese monkeys (Macaca fuscata) with pollinosis. J Med Primatol. 1992;21:323. [PubMed] [Google Scholar]
- 8.Hashimoto M, Sakaguchi M, Inouye S, et al. Prevalence of IgE antibody to crude and purified allergens of Japanese cedar pollen among different troops of Japanese monkeys (Macaca fuscata) J Med Primatol. 1994;23:393. doi: 10.1111/j.1600-0684.1994.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi M, Hashimoto M, Nigi H, et al. Comparison of specific IgE antibody to B cell epitopes of the major allergen (Cry j 1) of Japanese cedar (Cryptomeria japonica) pollen in humans and monkeys with pollinosis. Immunology. 1997;91:161. doi: 10.1046/j.1365-2567.1997.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasueda H, Yui Y, Shimizu T, Shida T. Isolation and partial characterization of the major allergen from Japanese cedar (Cryptomeria japonica) pollen. J Allergy Clin Immunol. 1983;71:77. doi: 10.1016/0091-6749(83)90550-x. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi M, Inouye S, Taniai M, Ando S, Usui M, Matuhasi T. Identification of the second major allergen of Japanese cedar pollen. Allergy. 1990;45:309. doi: 10.1111/j.1398-9995.1990.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto M, Kobayashi T, Nigi H, et al. Response of monkeys with pollinosis to two major allergens of Japanese cedar pollen. Int Arch Allergy Immunol. 1997;112:88. doi: 10.1159/000237436. [DOI] [PubMed] [Google Scholar]
- 13.Sugiyama Y. Life history of male Japanese monkeys. Adv Stud Behavior. 1976;7:255. [Google Scholar]
- 14.Hashimoto M, Nigi H, Sakaguchi M, et al. Sensitivity to two major allergens (Cry j 1 and Cry j 2) in patients with Japanese cedar (Cryptomeria japonica) pollinosis. Clin Exp Allergy. 1995;25:848. doi: 10.1111/j.1365-2222.1995.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 15.Hashiguchi S, Hino K, Taniguchi Y, et al. Immunodominance of seven regions of a major allergen, Cry j 2, of Japanese cedar pollen for T cell immunity. Allergy. 1996;51:621. doi: 10.1111/j.1398-9995.1996.tb04682.x. [DOI] [PubMed] [Google Scholar]
- 16.Sone T, Morikawa K, Miyahara M, et al. T cell epitopes in Japanese cedar pollen allergens: Choice of major T cell epitopes in Cry j 1 and Cry j 2 toward design of the peptide-based immunotherapeutics for the management of Japanese cedar pollinosis. J Immunol. 1998;161:448. [PubMed] [Google Scholar]