Abstract
The present study investigated in vitro the regulatory effects of T helper 1 (Th1)-type (interferon-γ, IFN-γ; interleukin-12, IL-12) and Th2-type cytokines (IL-10, IL-13) on Onchocerca volvulus-specific cellular reactivity in onchocerciasis patients, and in exposed endemic control individuals presenting no clinical and parasitological signs of disease. In both patients and controls, addition of IL-10 dose-dependently depressed O. volvulus antigen (OvAg)-specific cellular proliferation, and peripheral blood mononuclear cells (PBMC) from patients who were more sensitive to the suppressive effect of IL-10 than those from endemic controls. However, neutralization of IL-10 by specific antibody did not reverse cellular hyporesponsiveness. In contrast to the inhibitory effects of IL-10, exogenous IL-12 and IL-13 augmented PBMC proliferative responses to OvAg both in patients and controls (P < 0·01) and neutralizing of IL-12 or IL-13 significantly decreased OvAg-specific proliferation in both groups. Exogenous IFN-γ did not activate OvAg-specific proliferative responses in patients, but anti-IFN-γ antibodies abolished cellular reactivity to OvAg. Antibody to IL-10 increased (P < 0·05) OvAg-specific production of IL-5, IL-12 and IFN-γ, and inversely, anti-IFN-γ enhanced IL-10 (in patients only) and IL-5 and IL-13 in both patients and controls. Neutralization of IL-12 activated OvAg-specific production of IL-10, IL-2 and IFN-γ. In conclusion, despite of an overproduction of IL-10, which suppressed cellular reactivity in patients and control individuals, OvAg-specific cellular responses were activated in vitro by exogenous supplementation with IL-12 and IL-13, and cytokine neutralization experiments confirmed that distinct type 1 and type 2 T helper cytokines cross-regulate expression and magnitude of O. volvulus-specific cellular responsiveness in humans.
INTRODUCTION
In onchocerciasis and lymphatic filariasis the manifestation of disease ranges from asymptomatic infection to severe pathology,1,2 and the spectrum of clinical pathology correlates with distinct cellular immune responses. Within communities endemic for onchocerciasis few individuals are observed who must have been continuously exposed to Onchocerca volvulus but who remain apparently uninfected – these are immunologically characterized by dominant T helper 1 (Th1)-type cellular responses to filarial antigens.3 A second category consists of individuals who develop initially a clinically asymptomatic condition during which microfilariae (MF) are detectable in the skin. These individuals are characterized immunologically by a state of cellular anergy or hyporesponsiveness to filaria-derived antigens4 and by an inability to produce Th1-type cytokines: i.e. interferon-γ (IFN-γ) and interleukin-2 (IL-2).5 The third category of patients develop pathogenic immune responses while having no or low parasite loads only. In this subpopulation onchocercal skin disease or in case of lymphytic filariasis chronic lymphatic obstructions are seen together with vigorous cellular reactivity to filarial antigens. These observations point towards a biased or unbalanced cellular immune responsiveness in patients presenting either clinical manifestations or asymptomatic infection and parasite persistence. Several factors have been considered to account for such deviated or modulated immunity with filarial infections: i.e. prenatal or early postnatal tolerance induction,6,7 immune modulation by circulating parasite antigens,8,9 genetic predisposition of the human host10 and unbalanced type 2 versus type 1 T helper cell subpopulations.11 Recent studies have suggested that expression of immunity in filariasis patients and their parasite-specific cellular reactivity are transient, dependent on the state of infection,12,13 in addition, cytokines or cytokine blockage were found to modulate in vitro proliferative reactivity to filarial antigens in human lymphatic filariasis.14 Parasite-specific cellular hyporesponsiveness in lymphatic filariasis patients was found associated with high levels of spontaneous and filarial antigen-induced production of IL-10.11 As Th1-and Th2-type cytokines are mutually inhibitory, elevated IL-10 responses may downregulate Th1-type cytokines (e.g. IFN-γ, IL-2 or IL-12) and, thus, promote cellular unresponsiveness as observed with chronic filarial infections. Thus, cytokine-mediated crossregulation of type 1 and type 2 T helper cell responses may comprise a possible mechanism by which a particular expression of immunity is generated and maintained. The present investigation was aimed to determine the regulatory effects of Th1-and Th2-type cytokines on cellular reactivity in onchocerciasis patients and endemic control individuals. Our investigations support that filaria-specific cellular hyporesponsiveness as well as vigorous reactivity are regulated by the presence or absence of distinct cytokines that cross-regulate, not only magnitude, but also the distinct expression of parasite-specific cellular immunity in onchocerciasis patients.
MATERIALS AND METHODS
Location of study and study population
This study was conducted in central Togo in West Africa, within the vector controlled area of the Onchocerciasis Control Programme (OCP), where the risk of infection with O. volvulus, however, still remains high.15,16O. volvulus-infected patients and onchocerciasis-free endemic control individuals originated from selected villages where onchocerciasis was mesoendemic. Authorization for this study was given by the Togolese Ministry of Health and informed consent was obtained from all participants before enrolling in this study. Serum samples and peripheral mononuclear blood cells were obtained from all participants (30–50 ml) and the density of O. volvulus MF was determined in skin biopsies taken from the right and left hip.17 In onchocerciasis patients (n = 48, mean age 34 years, range 13–55 years) average density of MF was 54.3 MF/skin biopsy (range 1–357 MF), whereas no MF as well as no clinical signs of onchocerciasis were detected in exposed endemic controls (n = 33, mean age 34 years, range 11–67). Stool samples were collected from all participants and concurrent intestinal helminth or protozoan infections were determined by standard parasitological methodology. Seventy-eight percent of the onchocerciasis patients and 73% of the onchocerciasis-free control individuals were concurrently infected with intestinal helminth and protozoan parasites. None of the participants of this study had received antifilarial treatment previously, and all participants were negative for human immunodeficiency virus-1 (HIV-1) and HIV-2 as determined by enzyme-linked immunosorbent assay (ELISA; Enzygnost, Behring, Marburg, Germany).
O. volvulus antigen-specific ELISA
The O. volvulus antigen-specific (OvAg-specific) total immunoglobulin G (IgG) and IgG isotype reactivity was determined by ELISA,13,17 and preparation of O. volvulus adult worm-derived antigen (OvAg) was effected as described previously.4,5,17
Isolation of peripheral blood mononuclear cells (PBMC) and cell culture experiments
Heparinized venous blood was collected and PBMC were isolated by Ficoll–Paque (Pharmacia, Freiburg, Germany) density gradient centrifugation. Cell culture experiments were conducted as previously described by Soboslay and coworkers.13 Briefly, PBMC were adjusted to 1×107/ml in RPMI-1640 (Gibco, Eggenstein, Germany) supplemented with 25 mm HEPES buffer, 100 U/ml penicillin and 100μg/ml streptomycin, 0·25 μg/ml amphotericin B; they were then immediately used for cytokine secretion assays as described below. For purposes of proliferation assays, cells were seeded at 1×105 cells/well in sterile round-bottomed 96-well microtitre plates (Costar, Fernwald, Germany). Cells were suspended in RPMI (as above) containing 10% fetal calf serum (FCS), and kept in 5% CO2 at 37° and saturated humidity. For purposes of mitogenic stimulation with phytohaemagglutinin (PHA 1:100, Gibco) or concanavalin A (Con A 0·5–5 μg/ml, Gibco), and of antigenic stimulation with O. volvulus antigen (OvAg 40 μg/ml), streptolysin-O (SL-O, 1:50–500, Difco, Augsburg, Germany) and mycobacterial purified protein derivate (PPD 100 μg/ml, Boehringer Mannheim, Mannheim, Germany) cultures were maintained for 3 and 5 days, respectively. For the last 18 hr 1 μCi of 3H-thymidine was added; cells were then harvested on glass fibre filters (Skatron, Lier, Norway) and the incorporated radioactivity determined by scintillation spectroscopy (Beta Plate, LKB-Pharmacia, Freiburg, Germany).
For purposes of exogenous supplementation or neutralization of cytokine activity specific antibodies, control antibodies, or else recombinant cytokines were added at the beginning of the cell culture. For neutralization of cytokine activity, control antibody or else specific antibody for IFN-γ (3 and 5 μg/ml, IC Chemicals, Ismaning, Germany), IL-10, IL-12 and IL-13 (all at 5 μg/ml, Pharmingen, Hamburg, Germany), and for exogenous supplementation recombinant cytokines (Pharmingen, IC Chemicals) were used at concentrations as indicated in Results. Data on proliferative responses represent mean values of triplicate cultures in cpm minus baseline stimulation.
Determination of cytokine production
Freshly isolated PBMC were cultured at a concentration of 3·7×106 cells/ml in RPMI (as above) supplemented with 1% heat inactivated FCS, in the presence of either OvAg (35 μg/ml), PHA (1:100) or SL-O (1:50) in 5% CO2 at 37° and saturated humidity. Cell culture supernatants were collected after 48 hr and stored in liquid nitrogen. Cytokine secretion by stimulated PBMC was quantified by sandwich ELISA using cytokine-specific monoclonal and polyclonal antibodies specific for IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, IL-15 and tumour necrosis factor-α (TNF-α) and IFN-γ (Pharmingen), as previously described.13,17
Statistical data analyses
Results are indicated as mean values ± SD or SEM of different groups. The W-test of Wilk–Shapiro was used to test for normality and the F-test to compare the variance of two groups of data. Significant differences betweeen mean values were identified by either Student’s t-test or the Mann–Whitney test. P < 0·05 was considered significant.
RESULTS
Serological and cellular reactivity in patients and controls
Demographic data on the study groups, O. volvulus antigen(OvAg)-specific IgG1 and IgG4 reactivity, cellular responses to mitogen and antigens, as well as cytokine secretion by PBMC are shown in Table 1. Clearly elevated OvAg-specific reactivity of IgG1 and IgG4 was observed in patients as compared to controls. Cellular reactivity to the mitogens PHA and Con A as well as to O. volvulus-specific antigen was reduced in cases with patent O. volvulus infection, and the pronounced OvAg-specific proliferative reactivity as well as OvAg-induced cytokine secretion by PBMC from endemic controls provided evidence for their exposure to O. volvulus infection.
Table 1.
Demographic data on the study groups, Onchocerca volvulus antigen (OvAg)-specific IgG1 and IgG4 isotype reactivity, proliferation by PBMC to mitogen (PHA 1:100), Streptococcus pyogenes-derived streptolysin O (SL-O, 1:50) and OvAg (40 μg/ml) as well as cytokine secretion by PBMC in response to mitogen and antigens are shown

OvAg-spcific IgG isotype reactivity was determined by ELISA as described in Materials and Methods. Proliferative responses of PBMC are indicated as net counts per minute (▵c.p.m.) of triplicate culture, i.e. [c.p.m. = c.p.m. (induced by PHA, SL-O or OvAg) – c.p.m. medium control ]. Cellular production of cytokines in response to mitogen or antigens is indicated as net values, i.e. [pg/ml (induced by PHA, SL-O or OvAg) – pg/ml (Control)]. The spontaneous cytokine secretion in unstimulated control cultures is indicated as 'Control'. The number of participants is shown in parentheses.*(P < 0·05; **P < 0·01;)
Mitogen- and antigen-induced cytokine production in onchocerciasis patients and endemic controls
Th1-type cytokines
In both study groups, low level secretion of IL-2 by PBMC into medium control cultures was observed, and IL-2 production was not induced when PBMC were stimulated with O. volvulus-specific antigen (Table 1). Bacterial SL-O and mitogen (PHA) stimulated net production of IL-2, with higher amounts being measured in control individuals (Table 1). In the absence of antigen or mitogen as well as when PBMC were stimulated with OvAg, IFN-γ production was higher in controls than in patients. Substantially more IFN-γ was produced when PBMC were stimulated with SL-O or PHA, again with higher levels being secreted by cells from control individuals (Table 1). PBMC from patients and controls secreted low levels of IL-12 (< 50 pg/ml) as well as IL-15 (< 100 pg/ml), and antigenic or mitogenic stimulation of PBMC did not significantly augment cellular production of these cytokines.
Th2-type cytokines
In onchocerciasis patients and endemic controls, spontaneous IL-5 production was low. Pronounced IL-5 levels were detected in both study groups following bacterial antigen (SL-O) or mitogenic stimulation (PHA) of PBMC (Table 1), with higher levels of IL-5 being induced by PHA, SL-O and OvAg in onchocerciasis patients. In medium control cultures, spontaneous cellular production of IL-10 was similar in both groups (Table 1), addition of PHA, SL-O and OvAg to PBMC augment net production of IL-10 with higher amounts of IL-10 being produced by PBMC from patients. In response to OvAg, twice as much of IL-10 was produced by PBMC from patients than by cells from controls. In the absence of antigen spontaneous production of IL-13 into medium control cultures was higher in onchocerciasis patients than in controls but addition of OvAg depressed IL-13 levels in both groups.
Effects of exogenous IL-10 and neutralization of IL-10 on OvAg-specific cellular responses and cytokine production
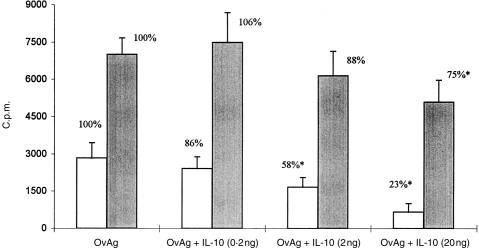
Addition of IL-10 dose-dependently reduced PBMC proliferative responses to OvAg in onchocerciasis patients and in endemic controls; however, reduction was more pronounced in patients than in endemic controls (Fig. 1). IL-10 added exogenously at the beginning of culture depressed cellular reactivity in patients by more than 75% (IL-10 at 20 ng) while in endemic controls responsiveness to OvAg was reduced by exogenous IL-10 at 20 ng by only 25%. In patients, a significant diminution of OvAg-specific reactivity was determined at IL-10 concentrations of 2 ng/ml already, but in endemic controls, PBMC responsiveness diminished significantly only at 10 times higher concentrations of IL-10 (20 ng/ml of IL-10). However, addition of 5 μg/ml of anti-IL-10 monoclonal antibody did not affect cellular responsiveness to OvAg both in endemic controls and onchocerciasis patients, although IL-10 production by PBMC from patients and controls was effectively neutralized, as determined by ELISA (data not shown).
Figure 1.
Proliferative responses by PBMC from onchocerciasis patients (n = 21, open bars) and control individuals (n = 11, shaded bars) to Onchocerca volvulus-derived antigens (OvAg at 40 μg/ml) in the absence or presence of exogenous interleukin-10 (concentrations of IL-10 were 0·2 ng/ml, 2·0 ng/ml, 20 ng/ml). Cellular reactivity is indicated as net counts per minute (▵c.p.m.) of triplicate cultures, i.e. [▵c.p.m. = c.p.m. (OvAg + IL-10) − c.p.m. (medium control)].
In onchocerciasis patients, OvAg-specific cellular production of IFN-γ increased threefold after neutralization of IL-10 by anti-IL-10 treatment (P <0·05), while such effect was not observed in control individuals (Fig. 4). Addition of anti-IL-10 increased OvAg-specific IL-5 secretion in both study groups, which clearly remained higher in onchocerciasis patients as compared to endemic controls (Fig. 3). In the presence of anti-IL-10, otherwise undetectable IL-12 production (178±37 pg/ml) was induced in patients, but when anti-IL-10 and OvAg were combined IL-12 production diminished strongly. Equally, recombinant IL-10 (at 5 ng/ml) spontaneously induced IL-12 secretion by PBMC in patients (n = 10; 43±24 pg/ml) and controls (n = 6; 78 ± 18 pg/ml), but when OvAg and IL-10 were combined IL-12 production by PBMC decreased (patients: 8±8 pg/ml; controls: 10±8 pg/ml).
Figure 4.
Effect of neutralization of IL-10, IL-12, IL-13 and IFN-γ on Onchocerca volvulus-specific cellular production of IFN-γ (a) or IL-10 (b) in onchocerciasis patients (n = 13) and endemic control individuals (n = 18). PBMC were cultured with O. volvulus-derived antigens (OvAg at 40 μg/ml) alone or else with OvAg together with cytokine-neutralizing antibodies for 48 hr as described in Material and Methods. IFN-γ and IL-10 were quantified by ELISA; data represent net production, i.e. baseline production in the absence of OvAg was subtracted.
Figure 3.

Effect of neutralization of IL-10 on cellular production of IL-5 in onchocerciasis patients (n = 13) and endemic control individuals (n = 18). PBMC were stimulated with O. volvulus-derived antigens (OvAg at 40 μg/ml) in the absence or presence of IL-10 neutralizing antibodies (at 5 μg/ml) for 48 hr as described in Materials and Methods; IL-5 concentrations were determined by ELISA. Data represent net IL-5 production, i.e. baseline production (medium control) in the absence of OvAg was subtracted.
Effects of addition or neutralization of IL-12 and IL-13 on O. volvulus-specific responses
In contrast to the inhibitory effects of IL-10 on PBMC proliferative responses, addition of IL-12 augmented responsiveness to OvAg both in patients and controls by 100% (P < 0·01) (Fig. 2). Neutralizing antibody to IL-12 (at 5 μg/ml) significantly abolished OvAg-specific cellular reactivity in both groups. Similarly, IL-13 (at 5 ng/ml) profoundly increased cellular reactivity to OvAg in patients and controls by 89% and 153%, respectively, and addition of anti-IL-13 (at 5 μg/ml) reduced responsiveness to OvAg dramatically in both groups (Fig. 2). Neutralization of IL-12 substantially enhanced OvAg-specific cellular IL-10 production in patients, while no such effect was observed in control individuals (Fig. 4b). However, anti-IL-12 treatment in the presence of OvAg also augmented otherwise undetectable IL-2 production (728±238 pg/ml), and tripled OvAg-specific production of IFN-γ by PBMC from onchocerciasis patients (Fig. 4a). The combination of neutralizing antibody to IL-13 and stimulation with OvAg had no effect on IFN-γ secretion by PBMC, but anti-IL-13 and OvAg substantially augmented IL-10 production in onchocerciasis patients only (Fig. 4a, b).
Figure 2.
Cellular reactivity of PBMC from onchocerciasis patients (PAT, n = 22) and endemic control individuals (CONTR, n = 29) to Onchocerca volvulus-derived antigens (OvAg at 40 μg/ml) in the presence of interleukin-12 (IL-12 at 5 ng/ml), IL-12 neutralizing antibody (anti-IL-12 at 5 μg/ml), interleukin-13 (IL-13 at 5 ng/ml) or IL-13 neutralizing antibody (anti-IL-13 at 5 μg/ml). Cellular reactivity is indicated as net counts per minute (▵c.p.m.) of triplicate cultures, i.e. [▵c.p.m. = cpm (OvAg + cytokine or cytokine neutralizing antibody) − c.p.m. (medium control containing control antibody)] (**P < 0·01).
Effects of addition or neutralization of IFN-γ on O. volvulus-specific cellular responses
In patients and controls, PBMC proliferative reactivity to OvAg was not influenced significantly by exogenous addition of IFN-γ (1000 U/ml), however, neutralization of IFN-γ by specific antibody diminished responsiveness in both study groups (P < 0·05) (data not shown). In control individuals, a significant diminution of PBMC reactivity to OvAg was observed only when IFN-γ neutralizing antibody was added at higher concentration of 5 μg/ml. When recombinant IFN-γ and anti-IL-10 neutralizing antibodies were combined cellular reactivity decreased in patients (P < 0·05) while it remained unaffected in control individuals. OvAg (at 40 μg/ml) together with anti-IFN-γ increased IL-10 levels in PBMC culture supernatants from patients, but no such effect was observed in controls (Fig. 4b). In both study groups, anti-IFN-γ and OvAg weakly heightened net cellular production of IL-5, but strongly augmented net production of IL-13 (PAT (n = 8): from 0 pg/ml to 397±255 pg/ml; CONTR (n = 8): from 0 pg/ml to 506±326 pg/ml). Also, anti-IFN-γ treatment induced spontaneous IL-12 production in patients only, but the combination of anti-IFN-γ and OvAg strongly diminished IL-12 secretion by PBMC from patients. Exogenous IFN-γ together with anti-IL-10 antibody did not induce production of IL-2 or IL-12 by PBMC in both groups (not shown).
DISCUSSION
Parasite-specific cellular unresponsiveness and a T helper type 2 cytokine profile predominate in onchocerciasis and lymphatic filariasis patients with persisting microfilariae in the skin or circulating blood.8,18 One mechanism by which such cellular unresponsiveness to filarial antigens is thought to be mediated is a spontaneous cellular overproduction of IL-10 which downregulates cytokine production in general and such prevents activation of appropriate and protective immune mechanisms.11 Our observations in onchocerciasis patients have confirmed that IL-10 dose-dependently suppressed OvAg-specific cellular proliferation; however, neutralization of IL-10 by specific antibody could not reverse this cellular unresponsiveness. Furthermore, the extent by which IL-10 suppressed filaria-specific responses showed that PBMC from patients were more sensitive to the inhibitory effects of IL-10 than cells isolated from control individuals. In humans, IL-10 is produced by both type 1 and type 2 T helper cells and IL-10 is equally suppressing proliferative responses and cytokine secretion of both Th1- and Th2-type cell subpopulations.19 The differential sensibility of PBMC from controls and patients to IL-10 observed in our study, supports the notion of Mahanty et al.11 of unbalanced T helper cell subpopulations in chronically infected lymphatic filariasis patients, in whom a reduced number of Th1 type helper cells is more susceptible to the suppressive effects of exogenously added IL-10.
Similarly as observed with anti-IL-10 treatment, OvAg-specific proliferative reactivity in onchocerciasis patients and controls was not affected by IFN-γ alone, and remained also low when exogenous IFN-γ and anti-IL-10 antibodies were combined. Thus, despite its downregulatory capacity, IL-10 appeared not to be the sole factor responsible for depression of cellular responsiveness, and equally IFN-γ alone was not capable of restoring OvAg-specific proliferation. However, anti-IFN-γ treatment significantly diminished cellular proliferation – such suppression of reactivity most likely being mediated by increased IL-10 secretion by PBMC following anti IFN-γ treatment (see Fig. 4b).
These observations suggested additional regulatory mechanisms which might modulate expression and magnitude of OvAg-specific cellular responsiveness. We have found that both IL-12 and IL-13 augmented OvAg-specific proliferation in patients and control individuals – for IL-12 such effects have previously been described in Brugia malayi patients and, in addition, IL-12 was found to act in an antigen non-specific manner.20 Furthermore, activation of PBMC by OvAg in the presence of anti-IL-10 antibody increased cellular production of the T helper type 1 cytokine IFN-γ confirming the cross-regulatory interaction betweeen the Th1-type cytokines IFN-γ and IL-12 and the Th2-type cytokine IL-10.21 Interestingly, anti-IFN-γ treatment augmented spontaneous Th1 type IL-12 production which, however, diminished upon addition of O. volvulus-derived antigens. Also, neutralization of IL-12 augmented IL-2 production by PBMC. These observations add evidence that during chronic O. volvulus infection regulatory immune mechanisms stabilize the delicately balanced host–parasite interaction such that filarial persistence may not inevitably induce inflammatory and potentially immunopathogenetic responses. As previously shown, IL-12 will induce IL-10 in both CD4+ and CD8+T-cell clones,22 but the modulatory capacity of IL-12 on antifilarial immunity has yet to be investigated in thorough detail. In murine intestinal nematode infections, injection of IL-12 in vivo generated both Th1- as well as Th2-type cytokine responses and promoted parasite survival during primary infection.23,24
In our investigation, O. volvulus-specific cellular anergy of onchocerciasis patients converted in vitro into a vigorous reactivity when exogenous IL-12 and IL-13 were added to PBMC cultures, while neutralization of these cytokines by specific antibody dramatically reduced OvAg-specific responses. Interestingly, and similarly as for IL-10, PBMC from onchocerciasis patients secreted spontaneously twice as much of IL-13 as controls. Interleukin-13 is mainly produced by type 2 T helper cells25 and its biological activity is widely overlapping with IL-4, which is crucial for the development of Th2-type immune responses.21 Both cytokines induce immunoglobulin isotype switching to IgE and IgG4,26 regulate the cytotoxic activity and cytokine production of monocytes,25 and IL-13 inhibited production of the Th1-type cytokines IL-12 and IFN-γ. Interleukin-13 is considered as a promoter for development of Th2-type immunity,27 and IL-13 was shown to be critically important for resistance to intestinal Trichuris muris infection in mice.28 The spontaneous production of IL-10 and its inhibitory effects on cellular responsiveness and cytokine production, and the continuous presence of IL-13, which will induce IgG4 and IgE, fit well into the concept of a predisposition of individuals in filaria-endemic areas for a Th2-type dominated immunity: i.e. a predisposition which may bias primary responses upon encounter with filarial and other helminth parasites.
Equally important, the intrinsic molecular nature of the O. volvulus-derived antigens may have contributed to deviate cellular responsiveness by modulating or suppressing production of Th1- and Th2-type cytokines. In previous investigations, recombinant, as well as purified, proteins of filarial parasites induced IgE and IgG4,29 preferentially stimulated lymphocyte IL-4 and IL-5 secretion,30 inhibited B-cell activation31 and polyclonal antibody production, or else enhanced IL-10·32 In addition, generation of human Th2-type T-cell clones was successful by using excretory–secretory products derived from helminth parasites.19 Such evidence is supportive to the notion that molecular components contained in helminth-derived antigen extracts will promote development of Th2-type immune responses. Furthermore, the inhibitory and modulatory capacity of filarial antigens on human T-cell function may also be mediated by its effects on antigen presentation and on costimulatory signal pathways. Next to the capacity of IL-10 to inhibit major histocompatibility complex (MHC) class II antigen expression on antigen-presenting cells (APC),33 we observed an inhibitory effect of OvAg on CD28 costimulatory molecule expression on T cells, and a diminished CD40 and CD80 (B7·1) expression on APC from onchocerciasis patients (our unpublished observations).
Taken together, multiple effector pathways act on development and expression of O. volvulus specific immune responsiveness in infected humans. Despite that, IL-10 will enforce cellular hyporesponsiveness, as observed in microfilariae-positive onchocerciasis patients, such cellular anergy remains reversible in vitro by supplementation with distinct cytokines: i.e. IL-12 and IL-13. Our investigation also support that in human onchocerciasis not only IL-10 is involved in dampening ongoing parasite-specific T-cell activation but, in addition, the intrinsic molecular nature of O. volvulus-derived antigens selectively inhibited cytokine secretion necessary for an appropriate and potentially protective immune response.
Acknowledgments
This study was supported by the Togolese Ministry of Health. The authors wish to thank medical assistants at village dispensaries and the laboratory staff at the Centre Hospitalier de la Région Centrale in Sokodé, Togo for their expert technical support. This work was supported by the Edna McConnell Clark Foundation (Grant 98025), the Deutsche Forschungsgemeinschaft (Grant So367/1) and the Dr Stingl Afrika Fonds.
REFERENCES
- 1.Murdoch ME. The skin and the immune response in onchocerciasis. Trop Doctor. 1992;22(Suppl.1):44. doi: 10.1177/00494755920220S109. [DOI] [PubMed] [Google Scholar]
- 2.King CL, Nutman TB. Regulation of the immune response in lymphatic filariasis and onchocerciasis. Immunol Today. 1992;12:A54. doi: 10.1016/S0167-5699(05)80016-7. [DOI] [PubMed] [Google Scholar]
- 3.Elson LH, Calvopina MH, Paredes WY, et al. Immunity in onchocerciasis: Putative immune persons produce a Th1-like response to Onchocerca volvulus. Immunol Today. 1995;171:652. doi: 10.1093/infdis/171.3.652. [DOI] [PubMed] [Google Scholar]
- 4.Greene GM, Fanning MM, Ellner JJ. Non-specific suppression of antigen-induced lymphocyte blastogenesis in Onchocerca volvulus infection. Clin Exp Immunol. 1983;96:259. [PMC free article] [PubMed] [Google Scholar]
- 5.Gallin MY, Edmonds K, Ellner JJ, et al. Cell-mediated immune responses in human infection with Onchocerca volvulus. J Immunol. 1988;140:1999. [PubMed] [Google Scholar]
- 6.Steel C, Guinea A, Mccarthy J, et al. Long-term effect of prenatal exposure to maternal micro filaraemia on immune responsiveness to filarial parasite antigens. Lancet. 1994;343:890. doi: 10.1016/s0140-6736(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 7.Elson LH, Days A, Calvopina M, et al. In utero exposure to Onchocerca volvulus: relationship to subsequent infection intensity and cellular immune responsiveness. Infect Immun. 1996;64:5061. doi: 10.1128/iai.64.12.5061-5065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ottesen EA. Infection and disease in lymphatic filariasis: an immunological perspective. Parasitology. 1992;104:S71. doi: 10.1017/s0031182000075259. [DOI] [PubMed] [Google Scholar]
- 9.Almeida De AB, Maia E, Silva MC, Braga C, Freedman DO. Differences in the frequency of cytokine-producing cells in antigenemic and nonantigenemic individuals with bancroftian filariasis. Infect Immun. 1998;66:1377. doi: 10.1128/iai.66.4.1377-1383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer CG, Gallin MY, Erttman KD, et al. HLA-D alleles associated with generalized disease, localized disease, and putative immunity in Onchocerca volvulus infection. Proc Natl Acad Sci USA. 1994;91:7515. doi: 10.1073/pnas.91.16.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahanty S, Nutman TB. Immunoregulation in human lymphatic filariasis: the role of interleukin-10. Parasite Immunol. 1995;17:385. doi: 10.1111/j.1365-3024.1995.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 12.Freedman DO. Immune dynamics in the pathogenesis of human lymphytic filariasis. Parasitol Today. 1998;14:229. doi: 10.1016/s0169-4758(98)01244-7. [DOI] [PubMed] [Google Scholar]
- 13.Soboslay PT, Geiger SM, Weiss N, et al. The diverse expression of immunity in humans at distinct states of Onchocerca volvulus infection. Immunology. 1997;90:592. doi: 10.1046/j.1365-2567.1997.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King CL, Mahanty S, Kumaraswami V, et al. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J Clin Invest. 1993;92:1667. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Sole G, Accorsi S, Cresveaux J, et al. Distribution and severity of onchocerciasis in southern Benin, Ghana and Togo. Acta Tropica. 1992;52:87. doi: 10.1016/0001-706x(92)90024-r. [DOI] [PubMed] [Google Scholar]
- 16.Remme JHF, De Sole G, Oortmarsen Van GJ. The predicted and observed decline in onchocerciasis infection during 14 years of successful control of Simulium spp. in West Africa. Bull WHO. 1990;69:331. doi: 10.1089/ten.2005.11.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soboslay PTL, Lüder CGK, Hoffmann WH, et al. Ivermectin-facilitated immunity in onchocerciasis. Activation of parasite-specific Th1 type responses with subclinical Onchocerca volvulus infection. Clin Exp Immunol. 1994;96:238. doi: 10.1111/j.1365-2249.1994.tb06548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ottesen EA. Immune responsiveness and pathogenesis of human onchocerciasis. J Infect Dis. 1995;171:659. doi: 10.1093/infdis/171.3.659. [DOI] [PubMed] [Google Scholar]
- 19.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 (IL-10) is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353. [PubMed] [Google Scholar]
- 20.Mahanty S, Ravichandran M, Raman U, Jayaraman K, Kumaraswami V, Nutman TB. Regulation of parasite antigen-driven immune responses by interleukin-10 (IL-10) and IL-12 in lymphatic filariasis. Infect Immun. 1997;65:1742. doi: 10.1128/iai.65.5.1742-1747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosman TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 22.Gerosa F, Paganin C, Peritt D, et al. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-γ and interleukin-10. J Exp Med. 1996;183:2559. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bancroft AJ, Else KJ, Sypek JP, Grencis RK. Interleukin-12 promotes a chronic intestinal nematode infection. Eur J Immunol. 1997;27:866. doi: 10.1002/eji.1830270410. [DOI] [PubMed] [Google Scholar]
- 24.Finkelman FD, Madden KB, Cheever AW, et al. Effects of IL-12 on immune responses and host protection in mice infected with intestinal nematode parasites. J Exp Med. 1994;179:1563. doi: 10.1084/jem.179.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Waal-Malefyt R, Figdor CG, Hujibens R, et al. Effects of IL-13 on phenotype, cytokine production and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-γ and IL-10. J Immunol. 1993;151:6370. [PubMed] [Google Scholar]
- 26.Punnonen J, Devries JE. IL-13 induces proliferation, Ig isotype switching, and Ig synthesis in immature human fetal B cells. J Immunol. 1994;152:1094. [PubMed] [Google Scholar]
- 27.De Vries JE, Zurawski G. Immunoregulatory properties of IL-13: its potential role in atopic diseases. Int Arch Allergy Immunol. 1995;106:175. doi: 10.1159/000236842. [DOI] [PubMed] [Google Scholar]
- 28.Bancroft AJ, Mckenzie ANJ, Grencis RK. A critical role for IL-13 in resistance to intestinal nematode infection. Eur J Immunol. 1998;160:3453. [PubMed] [Google Scholar]
- 29.Garraud O, Nkenfou C, Bradley JE, Nutman TB. Identification of recombinant filarial proteins capable of inducing polyclonal and antigen-specific IgE and IgG4 antibodies. J Immunol. 1995;155:1316. [PubMed] [Google Scholar]
- 30.Mahanty S, Abrams JS, King CL, Limaye AP, Nutman TB. Parallel regulation of IL-4 and IL-5 in human helminth infedctions. J Immunol. 1993;148:3567. [PubMed] [Google Scholar]
- 31.Harnett W, Harnett MM. Inhibition of murine B cell proliferation and down-regulation of protein kinase C levels by a phosphorylcholine-containing filarial excretory-secretory product. J Immunol. 1993;151:4829. [PubMed] [Google Scholar]
- 32.Hartmann S, Kyewski B, Sonnenburg B, Lucius R. A filarial cystein protease inhibitor downregulates T cell proliferation and enhances interleukin-10 production. Eur J Immunol. 1997;27:2253. doi: 10.1002/eji.1830270920. [DOI] [PubMed] [Google Scholar]
- 33.De Waal-Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacitiy of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]