Abstract
The Burkitt’s lymphoma cell line Daudi is a potent inducer of human γδ T-cell expansion. Using an in vitro culture system comprised of irradiated Daudi cells as stimulators and normal human lymphocytes as responders, the cellular determinants of this response were investigated. Three of four monoclonal antibodies (mAbs 1-1C4, L243, and 9.3F10) directed against disparate epitopes of human major histocompatibility complex (MHC) class II, as well as a mAb with specificity for CD4 (OKT4), inhibited the expansion of γδ T cells in response to Daudi cell stimulators. mAbs with a specificity for CD74 and CD8 were non-inhibitory. Lymphocyte depletion experiments demonstrated a critical role for the CD4+ T-cell subset in the expansion of γδ T cells. Other data pointed towards requirements for direct cell contact in this system, and the addition of exogenous recombinant interleukin (IL)-2, IL-4, and IL-12 failed to reconstitute γδ T-cell expansion in CD4+ lymphocyte-depleted cultures. These results complement previous findings in murine infectious disease and mycobacterial systems, providing a direct demonstration that CD4+ T cells play a role in γδ T-cell expansion through an interaction with human leucocyte antigen (HLA) class II on Daudi cells. The data point towards important functional links between the acquired and natural immune systems.
INTRODUCTION
γδ T cells are elicited in response to a variety of stimuli, including infectious agents such as mycobacteria, leishmania, malaria, salmonella, and listeria. The precise role of γδ T cells in infections has not been fully defined, though the rapidity and magnitude of their accumulation suggests that they constitute an important first line of immune defence.
The Daudi Burkitt’s lymphoma cell line has been shown to be a potent stimulator of γδ T cells,1–6 and consequently, it has emerged as a useful experimental tool for probing molecular and cellular determinants of γδ T-cell expansion. The γδ T-cell response to Daudi cells is independent of the human leucocyte antigen (HLA) status of the responder cells.1–4,6 Moreover, the expanded γδ T-cell population is confined to a specific T-cell subset (i.e. Vγ9 Vδ2).1,2,4,6 Limiting dilution analysis reveals that some stimuli trigger an unusually high ratio (as high as one in six) of resting γδ T cells.7 These features have led some to suggest that the expansion of γδ T cells in response to Daudi cells, as well as to mycobacterial extracts, is superantigenic in character,6,7 although this view is not universally accepted.8
The role of histocompatibility antigens (HLA) in the expansion of γδ T cells by Daudi cells has not been analysed in depth. While Daudi cells express surface major histocompatibility complex (MHC) class II, they do not express MHC class I at their surfaces because of the absence of β2-microglobulin.9–11 Kaur et al. failed to inhibit γδ T-cell expansion by Daudi cells using a single blocking anti-HLA class II mAb.3 Previous studies of γδ T-cell clones propagated with other stimuli have revealed a requirement for a heterogeneous array of histocompatibility gene products. Data support the existence of γδ T-cell clones (and γδ T-cell subpopulations) with a requirement for gene products from class I,12 class II,13–16 class Ib,16–21 and CD122 loci. Other published reports point toward the lack of requirement for MHC gene products for selected γδ T-cell clones.2,8,23–27 Interestingly, a single γδ T-cell clone reported by Holoshitz et al. recognizes peptide antigens in the context of HLA class II and mycobacterial extracts in an MHC-unrestricted fashion.13 The relationship between these derived γδ T-cell lines to those propagated from Daudi cells is uncertain. Of note, the nature of MHC recognition by γδ T-cells appears to be quite different from that of αβ T cells.16,20 In addition, MHC gene product usage and other antigen-presenting cell requirements for the initiation of γδ T-cell expansion may not be the same as those necessary for the propagation of stable clones or for cellular cytotoxicity.
In order to more fully understand the signals required for γδ T-cell expansion, we have further characterized the proliferative response of resting human peripheral blood mononuclear cells (PBMC) to Daudi cells. In this study, the expansion of γδ T cells to Daudi cells is shown to be significantly inhibited by preincubating the stimulator cells with selected mAbs (1-1C4, L243 and 9.3F10) directed against human HLA class II. Moreover, depletion of CD4+ T cells from the responder PBMC population is shown to result in a complete abrogation of γδ T cell expansion. These data establish that γδ T-cell expansion is, in at least some instances, directly dependent upon CD4+ T cells.
MATERIALS AND METHODS
mAbs and Fab fragments (Table 1)
Table 1.
Monoclonal antibodies
1-1C4, a murine immunoglobulin G2 (IgG2) mAb, was provided F. Sinigaglia (Hoffman-LaRoche, Basel, Switzerland); CerCLIP, a murine IgG1 mAb, was provided by P. Cresswell (Yale University, New Haven, CT). The mAbs BU45 (IgG1) and LN2 (IgG1) were purchased from Accurate Scientific (Westbury, NY) and Pharmingen (San Diego, CA), respectively. The murine hybridomas OKT4 (IgG2b), OKT8 (IgG2), HP6000 (IgG2b), HP6058 (IgG1), L243 (IgG2a), L227 (IgG1), and 9.3F10 (IgG2a) were all purchased from the American Type Culture Collection (Rockville, MD). Purified mAbs were produced by introducing these hybridomas into pristane-primed (Sigma, St. Louis, MO) BALB/C mice (Charles River, Wilmington, MA). After passage over an Affi-gel protein A column (BioRad, Hercules, CA), mAbs were dialysed against phosphate-buffered saline (PBS) and concentrated using Centriprep-10 concentrators (Amicon, Beverly, MA) to 1 mg/ml.
Fab fragments were generated by incubating mAbs at concentrations >2 mg/ml with 250 μl immobilized papain (Pierce, Rockford, IL) in the presence of 3·5 mg/ml cysteine–HCl in a shaking water bath at 37° for 16 hr. Digested Fab fragments were separated over an immobilized protein A column, dialysed against PBS, and concentrated with Centricon-10 concentrators (Amicon).
Immunodepletion of lymphocyte subpopulations
PBMC from healthy donors were enriched by Ficoll–Hypaque (Pharmacia, Piscataway, NJ) density gradient centrifugation. CD4-depleted lymphocytes were prepared by incubating 25×106 PBMC with 100 μg of purified OKT4 suspended in 5 ml OKT4 culture supernatant at 4° for 1 hr. The cells were washed and incubated twice with 1 ml sterile low-tox-H rabbit complement (Accurate Scientific, Westbury, NY) at 37° for 30 min. The cells were washed and suspended in complete media (RPMI-1640 (BioWhittaker, Walkersville, MD) supplemented with 10 mm l-glutamine, penicillin/streptomycin (BioWhittaker), and 10% fetal calf serum (Sigma)). CD8-depleted lymphocytes were prepared in a similar fashion using OKT8. CD4+ T-cell depletion routinely decreased CD4-expressing cells from 50% to less than 5%; CD8+ T-cell depletion routinely decreased CD8-expressing cells from 30% to less than 1%.
Stimulation of PBMC by Daudi cells
Cocultures of Daudi cell stimulators and lymphocyte responders were patterned after those of Fisch et al.1–4,6 In brief, Daudi cells were irradiated with a radioactive cobalt source (100 Gy), resuspended in complete media, and added to a 48-well plate (Costar, Cambridge, MA) at 0·3×106 per well. In some experiments, Daudi cells were preincubated for 1 hr at 37° with 50 μl of the indicated mAb (100 μg/ml). The cells were washed with complete media prior to addition to cultures. The indicated responder population was added at 1×106 cells per well, and exogenous mAb was added to a final concentration of 4 μg/ml. In all cases, the final volume in each well was 1 ml. Plates were incubated in a humidified atmosphere with 5% CO2 for 7–10 days. Cells were harvested and stained with fluoroscein isothiocyanate (FITC)-conjugated anti-T-cell receptor (TCR)-γ/δ-1 (Becton Dickinson, San Jose, CA), anti-CD4–FITC/anti-CD8–phycoerythrin (PE) (Dako, Carpinteria, CA), or isotype-matched negative control mAb (Dako) on ice for 1 hr in 50 μl of phosphate-buffered saline with 0·5% bovine serum albumin and 0·1% sodium azide (Sigma). Flow cytometric analysis was performed on a FACStar (Becton Dickinson). Total cell number was determined by counting the number of trypan blue-excluding cells on a hemocytometer.
RESULTS
Antibodies directed against human MHC class II inhibit Daudi-driven expansion of γδ T cells
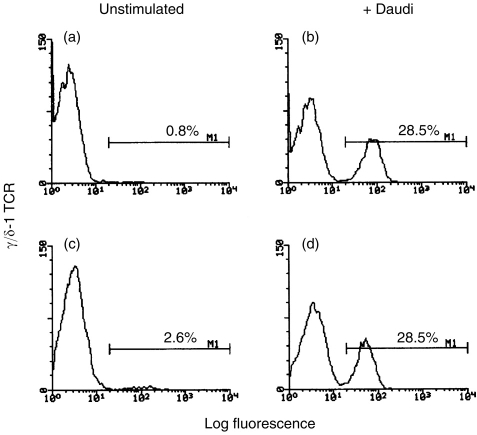
Two-colour flow cytometry can be used effectively to track the marked Daudi-driven expansion of γδ T cells within PBMC populations. In such experiments, others have previously costained the Daudi-stimulated cultures with propidium iodide (PI) in order to exclude dead (PI-staining) cells from the analysis.2–4,6 As an alternative, we determined whether a more simplified approach, based upon selective gating without PI, could be employed. As shown in Fig. 1, identical staining histograms and statistics were obtained by gating either on the lymphocyte population of the stimulated PBMC (Fig. 1a, b) or on PI-excluding cells (Fig. 1c, d). Consequently, all subsequent experiments omit the PI-costaining step and instead show data based upon gating on the stimulated lymphocyte population.
Figure 1.
Two methods of lymphocyte gating generate similar FACS profiles.After a 10-day coculture of 106 PBMC with (or without) irradiated Daudi cells, lymphocytes were stained with FITC-conjugated anti-γ/δ-1 TCR and analysed on a flow cytometer by one of two different methods. In the top two panels, flow cytometric data were collected from 5000 unstimulated (a) or Daudi-stimulated (b) cells fitting flow cytometric characteristics of lymphocytes. In the bottom two panels, flow cytometric data were collected from 5000 unstimulated (c) or Daudi-stimulated (d) cells which excluded propidium iodine. The percentage of γδ TCR+ T cells is indicated in each panel. Similar results were obtained in two additional experiments.
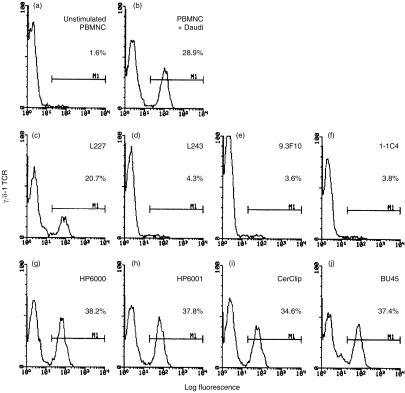
While several studies have argued against a role for MHC in the effector functions of γδ T cells, little data bears upon MHC requirements for γδ T-cell expansion. Daudi and RPMI-8226 cells are the only human cell lines known to function as potent γδ T-cell stimulators.4–6 Kaur et al. previously reported that the generation of γδ T cells by Daudi cells could not be inhibited by a mAb directed against HLA class II.3 Because that study was limited to a single anti-HLA class II mAb, we decided to survey a larger set of mAbs directed against epitopes on both chains of HLA-DR, the most abundant class II heterodimer on Daudi cells. Antibodies directed against either the α (L243) or β (1-1C4 and 9.3F10) chains of HLA-DR completely inhibited the generation of γδ T cells by Daudi cells (Fig. 2). A fourth anticlass II mAb, L227 (with specificity for the β chain of HLA-DR and -DP) was substantially less inhibitory. Control mAbs HP6000 (IgG2) and HP6058 (IgG1) did not significantly alter γδ T-cell expansion.
Figure 2.
Anti-class II mAb inhibit the expansion of γδ T cells.3×105 irradiated Daudi cells were cocultured with 106 responder PBMC and the indicated mAb at 4 μg/ml. Ascites from CerCLIP was used at a final dilution of 1:50. Lymphocytes were harvested, stained with FITC-conjugated anti-γ/δ-1 TCR, and analysed by flow cytometry. Data shown are FACS profiles of 5000 gated lymphocytes. The percentage of γδ TCR+ T cells is indicated in each panel. Three of the four anti-HLA class II mAb (c, d, e, f) inhibited the expansion of γδ T cells induced by cocultivated Daudi cells. Neither mAb directed against the HLA class II-associated glycoprotein CD74/invarient chain (i, j) nor isotype controls (g, h) significantly altered the γδ T cell expansion. Similar FACS histograms were obtained when Daudi cells were preincubated with anti-class II mAb, washed extensively, and then placed in culture. The data shown are representative data from one of 5 experiments.
When added directly to cocultures, anti-HLA class II mAbs can, in principle, bind not just to Daudi cells, but also to other class II-bearing cells within the PBMC pool, e.g. B cells and monocytes. In order to establish that the observed anti-class II mAb-mediated inhibitory activity is a consequence of direct interaction with the Daudi cells, irradiated Daudi cells were preincubated with each mAb for one hour and then washed prior to cocultivation. In these experiments, the same subset of three (out of four) anti-HLA class II mAbs (L243, 1-1C4, and 9.3F10) were again profoundly inhibitory (data not shown). In multiple experiments, individual mAbs were equivalently inhibitory whether they were used to precoat Daudi cells or to continuously block during the cocultivation period.
Univalent Fabs of two of the inhibitory anti-HLA class II mAbs, L243 and 9.3F10, were generated in order to assess their inhibitory capacity. By indirect immunofluorescence and flow cytometry, both of these Fab derivatives were shown to bind class II on Daudi cell surfaces (data not shown). However, neither of these Fab derivatives (at concentrations as high as 10 μg/ml) inhibited the generation of γδ T cells (data not shown; see Discussion). It should be noted that isotype-matched control mAbs (Fig. 2g, h, i, j) did not inhibit γδ T-cell expansion, arguing against Fc-mediated events as the primary inhibitory modality.
The invariant chain (CD74) is a type 2 glycoprotein that associates with nascent HLA class II chains in the endoplasmic reticulum, is subsequently displaced by HLA-DM during loading of antigenic peptides,28 and hence, does not usually appear at the cell surface. However, in some instances, invariant chain can transit to the cell surface,28–30 as is the case for Daudi cells, which express detectable amounts of invariant chain at their surfaces (data not shown). Given the known ability of invariant chain to act as a costimulator on antigen-presenting cells,31 we asked whether it might somehow contribute to the expansion of γδ T cells. However, the mAbs CerCLIP,28 LN2, and BU45, all with specificities for the C-terminal extracellular region of invariant chain29 failed to inhibit the expansion of γδ T cells to Daudi cells (Fig. 2). Hence, while anti-HLA class II mAbs are inhibitory, those directed against the related invariant chain are not.
OKT4 inhibits γδ T-cell expansion in response to Daudi cells
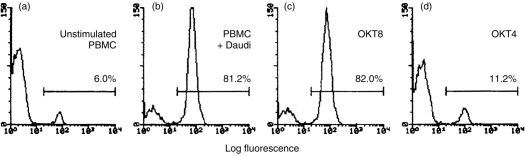
In light of the inhibitory activity of several anticlass II mAbs, along with the well-documented association between MHC class II and the T-cell coreceptor molecule CD4, we next investigated the potential role of CD4+ T cells in Daudi-stimulated γδ T-cell expansion. OKT4, a mAb with specificity for CD4, substantially inhibited the expansion of γδ T cells by Daudi cells (Fig. 3), although in repeat experiments, its inhibitory activity tended to be somewhat lower than that of the anti-HLA class II mAbs (Fig. 2). In contrast, OKT8, a mAb directed against the CD8α coreceptor that is present on a large subset of αβ T cells and a minority of γδ T cells,32 did not significantly inhibit γδ T-cell expansion. Since the CD4 coreceptor is not found on the surface of γδ T cells, the inhibitory activity of OKT4, along with that of the anti-HLA class II mAbs, together suggested that the expansion of Daudi-stimulated γδ T cells may require the participation of CD4+ T cells.
Figure 3.
OKT4, but not OKT8, inhibits the expansion of γδ T cells in response to Daudi cells.3×105 irradiated Daudi cells were cocultured with 106 responder PBMC and the either OKT8 (c) or OKT4 (d) at 4 μg/ml. Lymphocytes were harvested, stained with FITC-conjugated anti-γ/δ-1 TCR, and analysed by flow cytometry. Data shown are FACS profiles of 5000 gated lymphocytes. The percentage of γδ TCR+ T cells is indicated in each panel. OKT4 inhibited the expansion of γδ T cells (d), but OKT8 did not (c). Data shown are from a representative data from one of five experiments.
CD4+ T-cell depletion abrogates γδ T-cell expansion in response to Daudi cells
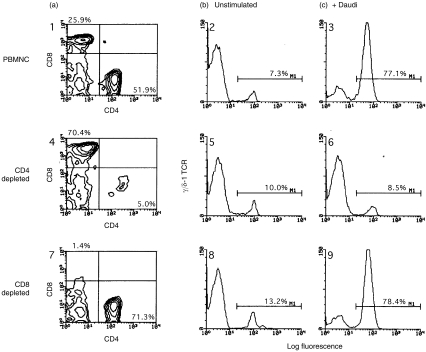
Lymphocyte depletion experiments were performed to identify cell subsets critical for the expansion of γδ T cells, and to ask in a directed way whether CD4+ T cells are needed for this expansion. PBMC were depleted of CD4+ or CD8+ T cells by treating with OKT4 or OKT8, respectively, in conjunction with rabbit complement. Depleted PBMC were then cocultured with irradiated Daudi cells, and the extent of γδ T-cell expansion was determined on days 7–10. A representative experiment is shown in Fig. 4, and a larger set of data, including both the percentage and absolute number of stimulated γδ T cells, are presented in Table 2. Flow cytometric analysis documented the efficacy of the immunodepletion step, with greater than 90% loss of the respective T-cell subsets (Fig. 4, panels 1, 4, 7). Irradiated Daudi cells failed to stimulate the expansion of γδ T cells when cocultured with CD4-depleted lymphocytes (Fig. 4, panel 6). In contrast, coculture of Daudi cells and CD8-depleted lymphocytes resulted in the usual expansion of γδ T cells (Fig. 4, panel 9; Table 2). Thus, the CD4+ T-cell subset is critical for the expansion of γδ T cells in response to Daudi cells.
Figure 4.
Daudi cells fail to induce γδ T-cell expansion in the absence of CD4+ T cells.3×105 irradiated Daudi cells were cocultured with 106 responder lymphocytes for 8 days, harvested, stained, and analysed by flow cytometry. Responder cell populations were total PBMC (panels 1,2,3), CD4-depleted PBMC (panels 4,5,6), and CD8-depleted PBMC (panels 7,8,9). Panels 1, 4, and 7 show 2-color FACS profiles of unstimulated responder lymphocytes stained for CD4 (x-axis) and CD8 ( y-axis). Percentages of CD4+ and CD8+ T cells are shown in the lower right and upper left corners, respectively. Staining for γδ T cells is shown in panels 2, 5, and 8 (unstimulated lymphocytes) and 3, 6, and 9 (Daudi-stimulated lymphocytes). The percentage of γδ TCR+ T cells is indicated in each panel. Irradiated Daudi cells failed to induce γδ T-cell expansion in PBMC populations depleted of CD4+ T cells (panel 6). γδ T cell expansion occurred normally in CD8+ lymphocyte-depleted responder populations (panel 9). There was no significant staining by isotype-matched control antibodies (not shown). Data shown are representative of one of three experiments.
Table 2.
Daudi cells fail to induce γδ T-cell expansion in the absence of CD4+ T cells
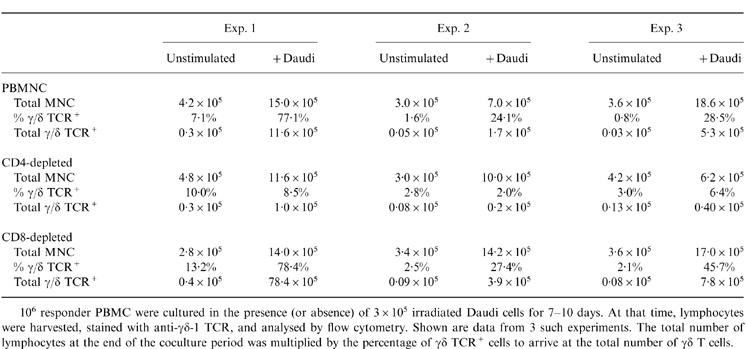
106 responder PBMC were cultured in the presence (or absence) of 3×105 irradiated Daudi cells for 7 – 10 days. At that time, lymphocytes were harvested, stained with anti-γδ-1 TCR, and analysed by flow cytometry. Shown are data from 3 such experiments. The total number of lymphocytes at the end of the coculture period was multiplied by the percentage of γδ TCR+ cells to arrive at the total number of γδ T cells.
Contact between Daudi cells and responding PBMC is necessary for the expansion of γδ T cells
We tested the possibility that cytokines derived from CD4+ T cells may account for the observed role of the latter in γδ T-cell expansion. Interleukin-2 (IL-2) and, in some reports, IL-12, have previously been reported to stimulate proliferation of γδ T cells.33,34 However, when added to cocultures of Daudi cells and CD4-depleted PBMC, exogenous recombinantcytokines with T-cell stimulatory capacity (IL-2, IL-4, IL-12 and granulocyte–macrophage colony-stimulating factor (GM-CSF)) failed to compensate for the loss of CD4+ T cells and reconstitute γδ T-cell expansion (data not shown).
To more broadly look for a soluble factor(s) secreted either by irradiated Daudi cells or by Daudi-stimulated CD4+ T cells that might be responsible for the expansion of γδ T cells, we set up experiments in which different cellular components were cultured in adjacent compartments separated by a semipermeable membrane. PBMC responders were cultured in one compartment, and the combination of irradiated Daudi cell stimulators plus PBMC were placed in the second compartment. After 10 days, there was no significant expansion of γδ T cells in the isolated PBMC population (data not shown). A second type of experiment was configured in which CD4-depleted T-cell responders plus Daudi cells were cultured in one compartment, while whole PBMC plus stimulatory Daudi cells were in the adjacent compartment. Again, there was no significant expansion of γδ T cells in the CD4-depleted compartment under these conditions (data not shown). Thus, γδ T cells require physical contact with stimulatory Daudi cells, and there is no evidence pointing to a soluble CD4+ T-cell product that induces γδ T-cell expansion.
DISCUSSION
The present study provides direct evidence for a role for CD4+ T cells in γδ T-cell expansion, using Daudi stimulation of γδ T cells as an experimental model. Three of four mAbs directed against HLA-DR, as well as a mAb directed against CD4, inhibited the expansion of γδ T cells in the Daudi stimulation system. Moreover, immunodepletion of CD4+, but not CD8+, T cells eliminated γδ T-cell expansion. In addition, the evidence in hand points away from a role for secreted Daudi or T-cell products and instead favour a requirement for direct cellular contact as the critical stimulatory mode in this system.
The finding that multiple (three out of four) anti-HLA class II mAbs substantially inhibit the expansion of γδ T cells contrasts with the findings of Kaur et al. who reported no such inhibition with a single anti-HLA class II mAb (distinct from the ones used here).3 Our data fit nicely with reports that the anti-HLA class II mAb L243 has been reported to inhibit γδ T-cell proliferation in response to allogeneic dendritic cells35 and the activation of γδ T cells by mycobacterial sonicates.36 The inhibitory activity of anti-HLA class II mAbs for γδ T-cell stimulation is thus not limited to those situations in which Daudi cells are the relevant APCs. Of note, the inhibitory mAbs used in this study have specificities for both the α and β chains of HLA class II. While CD4’s predominant interaction is thought to be with the MHC class II β chain, there is evidence supporting an interaction with the HLA class II α chain as well. Of course, there remains the possibility that HLA class II engages yet another (non-CD4) molecule on the T cell in promoting γδ T-cell expansion.
Despite retention of binding to surface class II, univalent Fab fragments generated from two of the inhibitory anti-class II HLA mAbs (L243 and 9.3F10) failed to significantly inhibit the expansion of γδ T cells. The inability of these Fabs to inhibit may reflect loss of blocking activity because of their reduced size, as has been documented for other receptor–ligand pairs.37,38 It should be noted, however, that mAbs directed against HLA class II have been shown to alter homotypic aggregation and other functional properties of a variety of B-cell lines, including Daudi cells.39–42 Hence, there remains the formal possibility that intact bivalent mAbs, but not univalent Fab derivatives, can induce phenotypic changes in Daudi cells that alter their ability to effectively stimulate γδ T cells. Nonetheless, the inhibitory activity of an anti-CD4 mAb (OKT4), in addition to the CD4+ T-cell immunodepletion findings, together suggest that class II:CD4 interaction is an important functional component in this system. This does not preclude other parallel activities for the anti-HLA class II mAbs
The immunodepletion data provide direct evidence for the contribution of CD4+ T cells to γδ T-cell expansion. Interestingly, two studies have independently suggested that CD4+ T cells are critical for γδ T-cell expansion in response to infectious agents in mouse models. Van der Heyde et al. demonstrated the importance of γδ T cells in clearing acute Plasmodium chabaudi adami infection in B57BL/6 mice.43 Clearing of parasitaemia was dependent upon the γδ T cell expansion that accompanied infection. Significantly, when mice were immunodepleted of CD4+ T cells, parasitaemia persisted and γδ T-cell expansion failed to occur, suggesting a potential link between these lymphocyte subpopulations. Rosat et al. reported a similar connection between CD4+ and γδ T cells in a mouse model of leishmaniasis.44 In that system, mice depleted of CD4+ T cells showed substantially less expansion of γδ T cells in response to an experimental infection with Leishmania major. Moreover, they further demonstrated a complete absence of γδ T-cell expansion in knockout mice lacking αβ T cells. This is an expansion deficit only, as γδ T cells otherwise appear to develop normally in these αβ T-cell receptor knockout animals.45
There are yet other reports, extending beyond murine infectious disease models, that bear upon the role of CD4+ T cells in γδ T-cell expansion. These deal with γδ T-cell expansion in response to mycobacterial antigens.7,8,13,46–48 By depleting lymphocyte subpopulations, Vila et al. identified a subset of CD4+ CD45RO+ CD7−αβ TCR-bearing T cells which are critical for the in vitro proliferation of γδ T cell in response to non-peptide phospholigand antigens derived from mycobacteria.46 Pechhold et al. demonstrated that both OKT4 and L243 completely inhibit the expansion of γδ T cells in reponse to Mycobacterium tuberculosis.36 The CD4+ T-cell secretory products, IL-2 and, to a lesser extent, IL-4 and interferon-γ, were found to augment the expansion of Vγ9 T cells, contrasting with our negative findings in this regard. It should be noted, however, that the augmentation of γδ T-cell expansion mediated by soluble factors that they reported was relatively low (two- to fivefold). Similarly, others have determined that the γδ T-cell expansion stimulated by non-peptide phospholigand antigens derived from mycobacteria was critically dependent upon HLA class II, especially HLA-DR.7,47,48 Thus, the expansion of γδ T cells in response to M. tuberculosis shares many of the characteristics of γδ T-cell expansion in response to irradiated Daudi cells. The present study complements these various earlier ones by directly solidifying the CD4+ T cell:γδ T-cell link in a simpler in vitro system that combines both Ab blocking and cellular depletion in a single experimental model.
Our data demonstrate that the Daudi-stimulated expansion of γδ T cells requires the participation of CD4+ T cells. The findings of Krensky et al.49 are of interest in this regard, showing that T-cell lines obtained from long-term cultures of irradiated Daudi cells and IL-2 consist exclusively of CD4+ T cells. The surface molecules on Daudi cells that are recognized by these T cells were not identified.
Our data support a model of γδ T-cell expansion which incorporates CD4+ T-cell interaction and direct contact with the stimulator (Daudi) cells. Hence, in agreement with the conclusion of Holoshitz et al.8 and in contrast to the suggestion of others,6,7γδ T-cell expansion, at least in the Daudi system, does not appear to have the characteristics of a superantigen-driven response. Whether CD4+ T-cell-dependent interaction is necessary during further maturation (e.g. acquisition of cytotoxic effector function) of the immune response remains to be determined. It has been previously demonstrated that γδ T cells (and other cells of the natural immune system) secrete lymphokines capable of modifying the responses of CD4+ T cells.50,51 Our results point toward a reciprocal interaction in which CD4+ T cells and γδ T cells are critical partners, suggesting a more intimate interaction between T cells of the natural and acquired immune systems.
Acknowledgments
We thank Dr H. Meyerson, Dr J.-H. Huang, and D. Geho for helpful discussions, as well as Dr M. Lamm for his support throughout the years. We also thank Dr R. Miller and G. Riely for their critical review of the manuscript, as well as S. Brill for her expert secretarial assistance. This work was supported by NIH RO1 CA74958 and NIH RO1 AI31044.
REFERENCES
- 1.Sturm E, Braakman E, Fisch P, Vreugdenhil R, Sondel P, Bolhuis RL.H. Human vγ9-Vδ2 T cell receptor γδ lymphocytes show specificity to Daudi Burkitt’s lymphoma cells. J Immunol. 1990;145:3202. [PubMed] [Google Scholar]
- 2.Fisch P, Oettel K, Fudim N, Surfus JE, Malkovsky M, Sondel PM. MHC-unrestricted cytotoxic and proliferative responses of two distinct human γ/δ T cell subsets to Daudi cells. J Immunol. 1992;148:2315. [PubMed] [Google Scholar]
- 3.Kaur I, Voss SD, Gupta RS, Schell K, Fisch P, Sondel PM. Human peripheral γδ T cells recognize hsp60 molecules on Daudi Burkitt’s lymphoma cells. J Immunol. 1993;150:2046. [PubMed] [Google Scholar]
- 4.Kaur I, Jong JD, Schell K, Hank J, Fisch P, Sondel PM. Human peripheral γδ T cells are stimulated by Daudi Burkitt’s lymphoma and not by any other Burkitt’s lymphoma tested. Cell Immunol. 1994;156:54. doi: 10.1006/cimm.1994.1152. [DOI] [PubMed] [Google Scholar]
- 5.Selin LK, Stewart S, Shen C, Mao HQ, Wilkins JA. Reactivity of γδ T cells induced by the tumour cell line RPMI 8226: Functional heterogeneity of clonal populations and role of GroEL heat shock proteins. Scand J Immunol. 1992;36:107. doi: 10.1111/j.1365-3083.1992.tb02946.x. [DOI] [PubMed] [Google Scholar]
- 6.Fisch P, Malkovsky M, Kovats S, et al. Recognition of human Vγ9/Vδ2 T cells of a GroEL homolog on Daudi Burkitt’s lymphoma cells. Science. 1990;250:1269. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer K, Schoel B, Plesnila N, et al. A lectin-binding, protease-resistant mycobacterial ligand specifically activates vγ9+ human γδ cells. J Immunol. 1992;148:575. [PubMed] [Google Scholar]
- 8.Holoshitz J, Romzek NC, Jia Y, et al. MHC-independent presentation of mycobacteria to human γδ T cells. Int Immunol. 1993;5:1437. doi: 10.1093/intimm/5.11.1437. [DOI] [PubMed] [Google Scholar]
- 9.Arce-Gomez B, Jones EA, Barnstable CJ, Solomon E, Bodmer WF. The genetic control of HLA-A and B antigens in somatic cell hybrids: requirement for β2 microglobulin. Tiss Antigens. 1978;11:96. doi: 10.1111/j.1399-0039.1978.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosa F, Berissi H, Weissenbach J, Maroteaux L, Fellous M, Revel M. The β2-microglobulin mRNA in human Daudi cells in has a mutated initiation codon but is still inducible by interferon. EMBO J. 1983;2:239. doi: 10.1002/j.1460-2075.1983.tb01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seong RH, Clayberger CA, Krensky AM, Parnes JR. Rescue of Daudi cell HLA expression by transfection of the mouse β2-microglobulin gene. J Exp Med. 1988;167:288. doi: 10.1084/jem.167.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spits H, Paliard X, Engelhard VH, Vries JE, D Cytotoxic activity and lymphokine production of T cell receptor (TCR) -αβ+ and TCR-γδ+ cytotoxic T lymphocyte (CTL) clones recognizing HLA-A2 and HLA-A2 mutants. J Immunol. 1990;144:4156. [PubMed] [Google Scholar]
- 13.Holoshitz J, Vila LM, Keroack BJ, McKinley DR, Bayne NK. Dual antigenic recognition by cloned human γδ T cells. J Clin Invest. 1992;89:308. doi: 10.1172/JCI115577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozbor D, Trinchieri G, Monos DS, et al. Human TCR-γ+/δ+, CD8:+ T lymphocytes recognize tetanus toxoid in an MHC-restricted fashion. J Exp Med. 1989;169:1847. doi: 10.1084/jem.169.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefrancois L, Lecorre R, Mayo J, Bluestone JA, Goodman T. Extrathymic selection of TCR γδ+ T cells by class II major histocompatibility complex molecules. Cell. 1990;63:333. doi: 10.1016/0092-8674(90)90166-c. [DOI] [PubMed] [Google Scholar]
- 16.Schild H, Mavaddat N, Litzenberger C, et al. The nature of major histocompatibility complex recognition by γδ T cells. Cell. 1994;76:29. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 17.Ito K, Kaer LV, Bonneville M, Hsu S, Murphy D, Tonegawa S. Recognition of the product of a novel MHC TL region gene (27b) by a mouse γδ T cell receptor. Cell. 1990;62:549. doi: 10.1016/0092-8674(90)90019-b. [DOI] [PubMed] [Google Scholar]
- 18.Kaliyaperumal A, Falchetto R, Cox A, et al. Functional expression and recognition of nonclassical MHC class I T10b is not peptide dependent. J Immunol. 1995;155:2379. [PubMed] [Google Scholar]
- 19.Vidovic D, Rogliic M, McKune K, Guerder S, MacKay C, Dembic Z. Qa-1restricted recognition of foreign antigen by a γδ T-cell hybridoma. Nature. 1989;340:646. doi: 10.1038/340646a0. [DOI] [PubMed] [Google Scholar]
- 20.Crowley MP, Reich Z, Mavaddat N, Altman JD, Chien Y-H. The recognition of the nonclassical major Histocompatibility complex (MHC) class I molecule, T10, by the γδ T cell G8. J Exp Med. 1997;185:1223. doi: 10.1084/jem.185.7.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279:1737. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 22.Porcelli S, Brenner MB, Greenstein JL, Balk SP, Terhorst C, Bleicher PA. Recognition of cluster of differentiation 1 antigens by human CD4− CD8− cytolytic T lymphocytes. Nature. 1989;341:447. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- 23.Kim HT, Nelson EL, Calyberger C, Sanjanwala M, Sklar J, Krensky AM. γδ T cell recognition of tumor Ig peptide. J Immunol. 1995;154:1614. [PubMed] [Google Scholar]
- 24.Lam V, Demars R, Chen BP, et al. Human T cell receptor γδ expressing T cell lines recognize MHC-controlled elements on autologous EBV-LCL that are not HLA-1, B, C, -DR, -DQ, or -DP. J Immunol. 1990;145:36. [PubMed] [Google Scholar]
- 25.Wright A, Lee JE, Link MP, et al. Cytotoxic T lymphocytes specific for self tumor immunoglobulin express T cell receptor γ chain. J Exp Med. 1989;169:1557. doi: 10.1084/jem.169.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sano Y, Dudley E, Carding S, Lin R-H, Hayday AC, Janeway CA. γδ T-cell lines isolated from intestinal epithelium respond to a B-cell lymphoma. Immunology. 1993;80:388. [PMC free article] [PubMed] [Google Scholar]
- 27.Sciammas R, Johnson RM, Sperling AI, et al. Unique antigen recognition by a herpesvirus-specific TCR-γδ cell. J Immunol. 1994;152:5392. [PubMed] [Google Scholar]
- 28.Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II αβ dimers and facilitates peptide loading. Cell. 1995;82:155. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 29.Wraight CJ, Endert PV, Moller P, et al. Human major histocompatibility complex class II invariant chain is expressed on the cell surface. J Biol Chem. 1990;265:5787. [PubMed] [Google Scholar]
- 30.Henne C, Schwenk F, Koch N, Moller P. Surface expression of the invariant chain (CD74) is independent of concomitant expression of major histocompatibility complex class II antigens. Immunology. 1995;84:177. [PMC free article] [PubMed] [Google Scholar]
- 31.Naujokas MF, Morin M, Anderson MS, Peterson M, Miller J. The chondroitin sulfate form of invariant chain can enhance stimulation of T cell responses through interaction with CD44. Cell. 1993;74:257. doi: 10.1016/0092-8674(93)90417-o. [DOI] [PubMed] [Google Scholar]
- 32.Terry LA, Disanto JP, Small TN, Flomenberg N. Differential expression and regulation of the human CD8α and CD8β chains. Tiss Antigens. 1990;35:82. doi: 10.1111/j.1399-0039.1990.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 33.Nishimura H, Hiromatsu K, Kobayashi N, et al. IL-15 is a novel growth factor for murine γδ T cells induced by infection. J Immunol. 1996;156:663. [PubMed] [Google Scholar]
- 34.Marx S, Welsch D, Kabelitz D. Activation of human γδ T cells by mycobacterium tuberculosis and Daudi lymphoma cells: differential regulatory effect of IL-10 and IL-12. J Immunol. 1997;158:2842. [PubMed] [Google Scholar]
- 35.Takamizawa M, Fagnoni F, Mehta-Damani A, Rivas A, Engleman EG. Cellular and molecular basis of human γδ T cell activation: role of accessory molecules in alloactivation. J Clin Invest. 1995;95:296. doi: 10.1172/JCI117654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pechhold K, Wesch D, Schondelmaier S, Kabelitz D. Primary activation of Vγ9-expressing γδ T cells by. J Immunol. 1994;152:4984. [PubMed] [Google Scholar]
- 37.Kumar A, Moreau JL, Gilbert M, Theze J. Internalization of interleukin 2 (IL-2) by high affinity IL-2 receptors is required for the growth of IL-2-dependent T cell lines. J Immunol. 1987;139:3680. [PubMed] [Google Scholar]
- 38.Ledbetter JA, Rose LM, Spooner CE, Beatty PG, Martin PJ, Clark EA. Antibodies to common leukocyte antigen p220 influence human T cell proliferation by modifying IL-2 receptor expression. J Immunol. 1985;135:1819. [PubMed] [Google Scholar]
- 39.Taylor DS, Nowell PC, Kornbluth J. Anti-class I antibodies inhibit the T cell-independent proliferation of human B lymphocytes. J Immunol. 1987;139:1792. [PubMed] [Google Scholar]
- 40.Kansas GS, Tedder TF. Transmembrane signals generated through MHC class II, CD19, CD20, CD39, and CD40 antigens induce LFA-dependent and independent adhesion in human B cells through a tyrosine kinase-dependent pathway. J Immunol. 1991;147:4094. [PubMed] [Google Scholar]
- 41.Holtrop S, Rijke-Schilder GP.M, Koene RAP, Tax WJ.M. The human Fc receptor for mouse IgG2b on monocytes and EBV-B cells is functionally inhibited by anti-HLA class II antibodies. Scand J Immunol. 1993;37:195. doi: 10.1111/j.1365-3083.1993.tb01756.x. [DOI] [PubMed] [Google Scholar]
- 42.Wagner N, Engel P, Vega M, Tedder TF. Ligation of MHC class I and class II molecules can lead to heterologous desensitization of signal transduction pathways that regulate homotypic adhesion in human lymphocytes. J Immunol. 1994;152:5275. [PubMed] [Google Scholar]
- 43.van der Heyde HC, Elloso MM, Chang W-L, Kaplan M, Manning DD, Weidanz WP. γδ T cells function in cell-mediated immunity to acute blood-stage malaria. J Immunol. 1995;154:3985. [PubMed] [Google Scholar]
- 44.Rosat J-P, Canceicao-Silva F, Waanders GA, et al. Expansion of γδ+T cells in BALB/c mice infected with is dependent upon Th2-type CD4+ T cells. Infect Immun. 1995;63:3000. doi: 10.1128/iai.63.8.3000-3004.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Philpott KL, Viney JL, Kay G, et al. Lymphoid development in mice congenitally lacking T cell receptor αβ-expressing cells. Science. 1992;256:1448. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 46.Vila LM, Haftel HM, Park H-S, et al. Expansion of Mycobacterium-reactive γδ T cells by a subset of memory helper T cells. Infect Immun. 1995;63:1211. doi: 10.1128/iai.63.4.1211-1217.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boom WH, Chervenak KA, Mincek MA, Ellner JJ. Role of the mononuclear phagocyte as an antigen-presenting cell for human γδ T cells activated by live Mycobacterium tuberculosis. Infect Immun. 1992;60:3480. doi: 10.1128/iai.60.9.3480-3488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haregewoin A, Soman G, Hom RC, Finberg RW. Human γδ+ T cells respond to mycobacterial heat-shock protein. Nature. 1989;340:309. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- 49.Krensky AM, Reiss CS, Mier JW, Strominger JL, Burakoff SJ. Long-term human cytolytic T-cell lines allospecific for HLA-DR6 antigen are OKT4+ Proc Natl Acad Sci USA. 1982;79:2365. doi: 10.1073/pnas.79.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morita C, Beckman EM, Bukowski JF, et al. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunology. 1995;93:495. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 51.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1998;272:50. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]