Abstract
Normal immunological memory is thought to be underpinned by T lymphocytes. However, in rheumatoid arthritis there are indications that T-lymphocyte control has been subverted by self-perpetuating B lymphocytes. Potential mechanisms in other autoimmune states are less clear, but a number of observations suggest that misappropriation of immunological memory by B lymphocytes may be a common feature of human autoantibody-associated disease. Put simply, autoantibodies drive their own production. If so, the availability of safe B-lymphocyte-depleting agents provides a potential means for reversal of autoimmunity.
HOW DO AUTOREACTIVE B LYMPHOCYTES SURVIVE?
Whatever its significance, the presence of autoantibodies remains the one piece of hard evidence about human autoimmunity which has to be built into a pathogenic mechanism. Current dogma states that a B lymphocyte can only avoid early death if it obtains two positive survival signals; cytokines from helper T lymphocytes and, in the follicle centre, antigen, complexed to the complement fragment C3d.1,2 In theory, autoreactive B lymphocytes cannot survive because of T- lymphocyte anergy to self and because any positive survival signal obtained from complexed self antigen is outweighed by a negative signal from uncomplexed antigen. Yet B lymphocytes recognizing one or more of about 50 self proteins do survive in some individuals, and may be associated with disease.3
The prevailing view is that autoreactive B-cell survival is secondary to a failure in the maintenance of T-lymphocyte self-tolerance, induced by an external ‘trigger’ immunogen. This view derives from early ideas about cross-reactivity between bacterial and tissue-specific antigens in rheumatic fever.4 However, this may be a very misleading model for spontaneous autoimmunity.
Enthusiasm for loss of T-lymphocyte tolerance in autoimmunity was bolstered by animal models transferrable by T lymphocytes, such as collagen II arthritis.5 However, there are two problems with these models. The pattern of disease reflects the antigen used and, in virtually every case, fits poorly with the putative human equivalent. Collagen II arthritis affects cartilage structures, including growth plate. Rheumatoid arthritis targets synovium, and also structures such as pericardium and alveoli.6 Cartilage is only affected if adjacent to synovium.
The second problem is that most T-lymphocyte transferrable animal models require immunization with a massive antigen and/or adjuvant load. In human autoantibody-associated diseases, unlike rheumatic fever or post-dysenteric arthritis,7 there is rarely any evidence for recent exposure to a particular foreign antigen. Rheumatoid arthritis occurs at random during adult life, with little or no geographic or temporal clustering. In the absence of evidence of recent antigen exposure, it is difficult to see why a failure of T-lymphocyte tolerance should occur in mid life. What seems more likely is that the random onset of disease reflects a random process in the immune system; we suggest that immunoglobulin gene mutation is the obvious candidate.
AUTOANTIBODIES MAY DRIVE THEIR OWN PRODUCTION
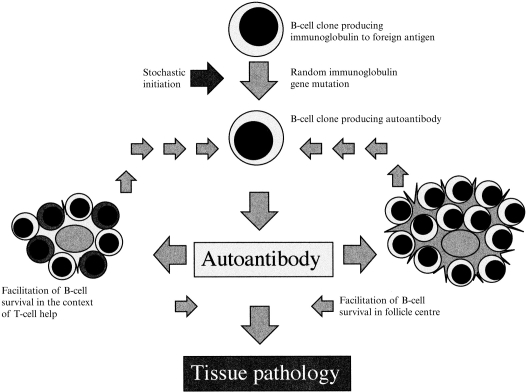
As noted by Pulendran et al.,8 autoantibodies may arise by random mutation during immune responses to any antigen. The autoantigens subsequently recognized need not resemble the original antigen. A single amino acid substitution may cause dramatic changes in the conformation of the antigen-binding site. In most cases dramatic changes in antibody specificity will lead to loss of affinity for foreign antigen and death of the B lymphocyte. However, if the new antigen-binding site interacts with a self antigen in such a way as to generate positive survival signals, the B lymphocyte may survive and proliferate (Fig. 1).
Figure 1.
A generalized mechanism for autoantibody-associated disease.
Many of the autoantigens associated with rheumatic disease are proteins implicated in survival, differentiation and function of immune cells; notably immunoglobulin G (IgG) Fc, C1q, and nucleoproteins such as topoisomerase-1 and the leucocyte differentation-related oncogene product dek.9–12 Antibodies to these antigens may have a particular opportunity to upset regulatory mechanisms. If an antibody, by modifying the normal function of one of these proteins, stimulated or mimicked T-lymphocyte cytokine activity, whether directly or indirectly, and shifted the balance of unbound and complexed antigen, then its parent clone could become self-perpetuating (Fig. 1). The best characterized example of such a mechanism is in rheumatoid arthritis.13 Subsets of rheumatoid factors may keep their parent B-lymphocyte clones alive by disturbing the control of both T-lymphocyte help and survival signals given by immune complexes in the follicle centre. If rheumatoid factors can, perhaps other autoantibodies can.
RHEUMATOID FACTORS AND THEIR RELATIONSHIP TO DISEASE
Approximately 80% of subjects with rheumatoid arthritis (RA) develop circulating antibodies to IgG Fc, or rheumatoid factors (RF).9 Seronegative cases occur, but in many, RF are present in joints. Seronegative cases probably also comprise conditions which can be clinically indistinguishable from RA, such as spondarthropathies. RF are present in the circulation of a few normal individuals and can also be induced by immunization to foreign antigen or by chronic infection.14 This dictates that if RF are pathogenic in RA, pathogenic potential must be restricted to a subset of RF.
There are recognized differences between RF from normal and RA subjects.9‘Physiological’ RF from normal subjects are chiefly of IgM class and low affinity. RF in RA are of all classes, and are structurally and genetically distinct, as shown by analysis of immunoglobulin VH regions.15 RF from normal individuals tend to come from a common germ line. They show evidence of immunoglobulin gene mutation, but mutations tend to be silent.16 In contrast, RF from RA subjects derive from a wide range of immunoglobulin germ line genes and show a high frequency of substitution mutations, indicating affinity maturation.
It is possible that physiological RF come from the B-1 subset of B lymphocytes,17 associated with ‘natural’ autoantibodies and that pathogenic RF arise from the B-2 subset. B-1 cells show constitutive expression of the nuclear protein STAT3 and distinct regulatory mechanisms in B-1 cells may relate to the blocks to affinity maturation and class switching of physiological RF. However, B-1-derived antibodies can show both these features and the means by which subtypes of RF may persist, discussed later, do not necessarily require either a B-1 or B-2 origin.
Class switching, high affinity and fine specificity may all be important in RF pathogenicity. A critical test of this concept comes from hypergammaglobulinaemic purpura of Waldenström (HPW)18 in which high levels of polyclonal RF occur in the absence of a recognizable stimulus, as in RA, but without arthritis. This raises the issue of RF as inflammatory mediators.
RF AND INFLAMMATION
The pathogenic significance of RF has been doubted because of the difficulty in finding a RF-based effector mechanism which explains the pathology of RA. However, new information on the immunological microenvironment in synovium has led to the identification of a plausible effector pathway. IgG RF exist in the circulation of rheumatoid subjects as oligomers and predominantly dimers.19 These dimers fix complement poorly and, consequently, can escape clearance by red cell complement receptors (CR1). Unlike IgM-based complexes, IgG dimers are small enough to pass out of the circulation and access tissue macrophages. Histological studies indicate that macrophage activation is the initial event in RA synovium, preceding T-lymphocyte accumulation.20
Recent studies indicate that high-level expression of the IgG Fc receptor FcγRIIIa is restricted to macrophages in tissues affected by RA.21 Of the three classes of FcγR, FcγRIII is believed to be particularly important for the binding of small, and specifically dimeric, complexes and the consequent generation of mediators such as tumour necrosis factor-α (TNF-α).22 FcγRI expression is up-regulated by interferon-γ23 and may be chiefly involved in a mature inflammatory response. Its high affinity allows it to bind free, locally synthesized antibody to a specific pathogen and interact with the pathogen subsequently, via multiple receptors. FcγRII is of low affinity and is implicated in binding large complexes, again via multiple receptors.23
A specific role for FcγRIIIa in response to small complexes is supported by the fact that a monoclonal antibody to FcγRIIIa in free soluble form is capable of inducing release of both TNF-α and reactive oxygen species, whereas monoclonal antibodies to FcγRI and FcγRII do not induce TNF-α release under the same conditions.24 TNF-α release has only been achieved via FcγRI and FcγRII by secondary cross-linkage following ligation of the receptors by IgG Fc.25 This suggests that FcγRI and FcγRII may require multiple cross-linking in order to induce signalling but that FcγRIIIa may induce signalling in response to a soluble ligand capable of engaging two, or at most three (two Fab- and one Fc-based interactions), receptors. This would be closely analogous to the situation for an IgG RF dimer with which additional non-RF IgG molecules may be in dynamic association.
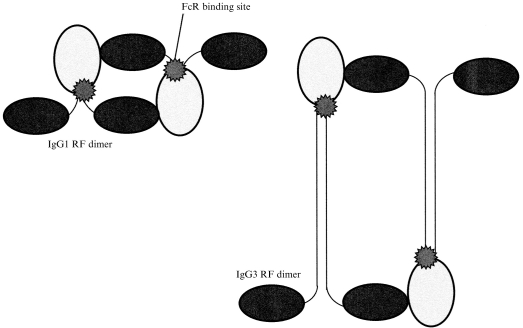
On this basis it can be argued that RA is precisely the inflammatory state that IgG RF dimers should be expected to generate. There are, however, further issues about IgG subclass. Voice and Lachmann26 recently highlighted the importance of subclass and complex size in interactions between complexed IgG and Fc and complement receptors. IgG1 and IgG3 RF dimers may interact differently with FcγRIIIa. Small IgG3-based complexes induce FcγRIIIa-dependent TNF-α production only slightly less well than equivalent IgG1-based complexes in our hands (Abrahams, in preparation). However, self-association may raise specific steric considerations. One possibility relates to the long hinge of IgG3 (Fig. 2). Modelling studies predict that the Fc receptor binding sites on an IgG1 dimer would be approximately 75 Å apart, whereas for IgG3 dimers the distance would be approximately 200 Å with the hinge extended.27,28 There is debate about whether the cysteine-rich segment of the IgG3 hinge is normally extended or compressed. However, steric interference in a self-associated dimer may reduce the likelihood of a compressed configuration. A distance of 75 Å would allow direct apposition of the FcγRIIIa γ-chains responsible for signalling, but 200 Å would not (Fig. 3). This provides one possible explanation for the absence of synovitis in subjects with HPW. ‘Intermediate’ complexes, consisting of self-associated RF, exist in HPW sera, but in patients with no arthritis they were found to be restricted to IgG3.29 This would be consistent with an inability of IgG3 RF dimers to induce signalling via FcγRIIIa.
Figure 2.
Comparison of IgG1 and IgG3 RF dimers showing the difference in hinge length.
Figure 3.
A suggested basis for how the long hinge of IgG3 may inhibit IgG3 RF dimers from bringing the signalling γ-chains of FcγRIIIa into apposition.
The above discussion only relates to the initiation of inflammation in synovium by circulating complexes. The dominant involvement of synovium in RA is likely to involve a series of secondary events. Synovial fibroblasts are unusually responsive to cytokines such as TNF-α in terms of the induction of expression of molecules involved in B-lymphocyte survival: vascular cell adhesion molecule-1 (VCAM-1), decay accelerating factor and complement receptor 2.30 Induction of the expression of these moleculess on synovial subintimal fibroblasts by cytokines released from FcγRIIIa+ macrophages would explain the survival of B lymphocytes in RA synovium, with follicle formation in some cases. A proportion of these B lymphocytes are known to generate RF.9 High concentrations of IgG RFs within the confines of the joint are associated with the formation of larger, complement-fixing complexes, which, together with IgM RF-based complexes, are likely to amplify the inflammatory process.19
RF AND T-LYMPHOCYTE HELP
In order to obtain T-lymphocyte help, B lymphocytes normally take up antigen bound to surface immunoglobulin and present it to a T lymphocyte. It is recognized that B lymphocytes that carry surface antibody to IgG Fc, i.e. RF, can obtain help without requiring T lymphocytes responsive to IgG.15 By taking up IgG attached to a non-self antigen they can present that antigen to a responsive T lymphocyte (Fig. 4).31 RF-producing B cells survive for short periods in everyone, if foreign antigen is available, as after immunization (Fig. 5a). However, the RF produced is mostly IgM. Moreover, there is little evidence of immunoglobulin gene mutations leading to amino acid substitution.16 There seems to be a block to class switching and affinity maturation for RF in normal individuals, suggesting that the acquisition of T-cell help is not enough to allow RF-secreting B lymphocytes to survive long-term. This suggests that there is a protective mechanism operating in the follicle centre.
Figure 4.
The basis of ‘bystander’ T-cell help to RF-specific B lymphocytes.
Figure 5.
Physiological RF in normal individuals (a); RF in rheumatoid arthritis (b); and RF in HPW (c).
SURVIVAL IN THE FOLLICLE CENTRE
The survival of B lymphocytes in follicle centres depends on the balance between positive and negative signals provided by antigen in different forms (Fig. 6). B-lymphocyte clones recognizing soluble autoantigens should not survive because they should receive a negative survival signal from uncomplexed antigen. However, antigen available in the form of an immune complex can give a positive survival signal. This signal is modulated by the attachment of C3d molecules to the antigen following complement fixation.2 The combined interactions between C3d and its receptor, CR2, and antigen with surface immunoglobulin lead to a positive signal which is amplified tenfold for each C3d molecule attached to the complex. The situation is further modulated by FcγRIIb which binds large immune complexes and provides a negative signal.32
Figure 6.
Relative potency of survival signals for autoantigen-specific and RF-specific B cells. Cross-hatching of elements indicates reduced availability because of competition by other ligands.
Although IgG RF exist in the circulation in rheumatoid subjects largely as non-complement-fixing dimers, this probably reflects both their relatively low concentration in serum and the preferential survival of non-complement-fixing complexes. At sites of IgG RF synthesis, as in rheumatoid synovium, higher concentrations of IgG RF lead to the formation of complement-fixing multimers,18 with the potential to provide RF-specific B lymphocytes with a positive survival signal (Fig. 5b).
In theory, any B-cell clone recognizing a soluble autoantigen could keep itself alive by generating sufficient antibody to provide a supply of complexed antigen. However, it appears that the regulation of positive and negative signals is such that the negative signal from soluble antigen normally dominates. Two aspects of this regulation may fail in the case of RF-secreting clones. An isolated autoreactive B-lymphocyte clone will normally have to compete for the same epitope on an autoantigen with its own secreted immunoglobulin. Thus it may be unable to bind complexed antigen through surface immunoglobulin except if other clones reactive with other epitopes on the same autoantigen are present. This does apply to RF-secreting clones (Fig. 5b) because an IgG RF molecule that masks an antigenic epitope on IgG provides the same epitope on itself. Secondly, surface RF may compete with FcγRIIb for large complexes, reducing the negative signal from this receptor.32 It would appear that, when it comes to preventing survival of IgG RF-secreting B lymphocytes, there are weaknesses in regulatory mechanisms in the follicle centre as well as in the context of T-lymphocyte help.
Multimeric IgG1- and IgG3-based complexes are both capable of fixing complement and RF of both subclasses are likely to enhance RF-specific B-lymphocyte survival. This may contrast with the relative capacity of their dimers to cross-link FcγRIIIa and initiate synovitis and would explain why in HPW RF production is perpetual (multimer-driven), but arthritis (dimer-initiated) does not occur (Fig. 5c).
IgG3 also differs from other IgG subclasses in lacking the Ga epitope on Fc, a common recognition site for RF.33 Even within subclasses, pathogenic potential may depend on the detailed stereochemistry of the CDR–antigen interaction. These considerations may help explain the variable anatomical and temporal patterns seen in different cases of the disease.
The prediction is that IgG RF capable of supporting survival of their parent B-lymphocyte clone would also assist survival of RF-specific clones secreting other isotypes. This would explain why in both RA and HPW large amounts of IgM RF are also produced, to the extent that they have tended to obscure the significance of IgG RF (Fig. 5).
B LYMPHOCYTES AND MEMORY IN OTHER AUTOIMMUNE DISEASES
The ways in which B-lymphocyte clones might become self-perpetuating in other autoimmune states cannot be traced as fully as in rheumatoid arthritis. However, several observations and proposed mechanisms which appear to fit such a framework will be briefly reviewed.
Davies and colleagues have suggested that the formation of antibodies to C1q is a crucial early step in the common sporadic form of systemic lupus.10 Anti-C1q antibodies can trigger complement consumption. In rare genetically determined cases complement components are primarily deficient. In either case, limited availability of complement components may both impair clearance of immune complexes and nuclear material normally cleared via complement and, by limiting generation of C3d, reduce the efficiency of B-lymphocyte clonal selection, allowing the genesis of autoantibodies.
Anti-C1q antibodies have the potential to interfere with the rules of antibody–antigen interactions in a way similar to RF. An anti-C1q-specific B lymphocyte can endocytose any antigen complexed with C1q and present it to T cells. C1q–anti-C1q complexes could disturb regulation in the follicle centre in a similar way to RF polymers. The kinetics of self-perpetuation of anti-C1q-specific B lymphocytes would differ from RF-specific B lymphocytes because the clone would not generate both antigen and antibody locally, e.g. in a joint. C1q–anti-C1q complexes would probably also be too big to cross vessel walls, suggesting that the synovitis of lupus may be due to immune complexes present as a result of mechanisms downstream of complement depletion.
In myasthenia gravis B lymphocytes generating antibodies to nicotinic acetyl choline receptors (AChR) survive in the thymus.34 The interesting question may be not why B lymphocytes of this specificity arise, but why, once generated, they persist. In what way does their surface or secreted immunoglobulin make them able to survive within the thymic environment? AChR are present on myoid cells which are found in close contact with professional antigen-presenting cells in myasthenic, but not normal, thymus.34 Could anti-AChR modulate myoid cell function in such a way that the thymic environment becomes favourable to B-lymphocyte survival? There have to be answers to these questions; answers likely to make additional reasons for loss of T-lymphocyte tolerance to AChR redundant.
In multiple sclerosis T-cell responses to myelin antigens are often invoked. However, in contrast to experimental allergic encephalomyelitis, the pathological domain is not that of myelin, but of the blood–brain barrier.35 The only established immunological abnormality is the persistent survival of a few B-lymphocyte clones in the central nervous system (CNS). The key question is how these clones live within the CNS, generating the same oligoclonal immunoglobulin bands over many years. CNS damage may simply reflect intolerance of high levels of immunoglobulin, which induce macrophage phagocytosis of myelin in vitro. Recent immunohistochemical studies indicate that initial activation of microglia occurs in the absence of T lymphocytes.36 B-lymphocyte survival in the CNS should be precluded by the absence of stromal cell ligands such as VCAM-1. However, if a B lymphocyte were to generate surface or secreted immunoglobulin which overcame the need for such ligands it might survive in the CNS, without being exposed to the competitive pressures of a lymphoid organ.
Two other aspects of autoimmunity of recent interest may be relevant to the potential for autoreactive B lymphocytes to self-perpetuate. It is now accepted that a proportion of autoantibodies can penetrate cells, alter cell function and even cause cell death.37 During cell death, availability of autoantigen is altered by changes in cell compartmentalization.38 This raises the possibility that in conditions such as systemic sclerosis B lymphocytes generating antibodies to antigens such as topoisomerase-1 perpetuate their existence by causing cell death during division, with release of autoantigen in a form suitable both for induction of a T-cell response and for providing a positive signal to follicular B cells. Mechanisms of this sort may be relevant to the wide variety of syndromes associated with antibodies to nucleic acid-binding proteins.39
In other autoantibody-associated disorders mechanisms involving autoreactive B-lymphocyte self-perpetuation become more difficult to identify. They are likely to be diverse, since the most plausible mechanism is different in each condition so far analysed. In sarcoidosis, for instance, autoantibodies may mimic lectins.40 However, there are almost certainly some conditions, probably including type I diabetes, in which autoantibodies are secondary to a truly T-lymphocyte-driven mechanism.
A DIGITAL ANALOGY
The case has been made for autoimmune disease being driven, not by a primary failure of T-lymphocyte tolerance but by antibody. As for the genome or computer software, the true danger to the immune system may be a chance mutation in an information string which converts ‘data’ to ‘command’. As a transcribed sequence of DNA may become a lethal stop codon and a misread floppy disk may lead to a system crash, if an antibody becomes a B-lymphocyte growth promoter it may be very difficult for the immune system to re-establish control.
THERAPEUTIC IMPLICATIONS
If self-perpetuating B lymphocytes exist, their therapeutic implications are enormous. It is now possible to ablate B lymphocytes with an anti-CD20 antibody. If enough self-perpetuating autoreactive cells could be destroyed the implication is that autoimmunity would collapse and lymphoid tissue be repopulated by B lymphocytes innocent of any autoimmune tendency. Anti-CD20 therapy is licenced for lymphoma and, apart from transient symptoms due to cytolysis, is well tolerated.41 Up to 95% of B lymphocytes can be cleared with repopulation in 100 days. This is in stark contrast to anti-T-lymphocyte therapy, for which the justification is unclear and which, even if successful, carries a risk of long-term immunodeficiency. Anti-T-lymphocyte strategies have been disappointing. Interestingly, pan-lymphocyte depletion in RA with anti-CD52 produced benefit limited approximately to the period of B lymphopenia42 suggesting that autoreactive B-lymphocyte depletion did not reach the putative threshold. Anecdotal reports suggest that RA is about as amenable to long-term remission as lymphoma. Significant long-term remission rates in lymphoma probably require maximal doses of depleting antibody (2–3 g is recommended for anti-CD20) combined with conventional cytotoxic agents.43 Specific anti-B-cell therapy of this type has not been explored. It might just produce long-term cure.
REFERENCES
- 1.Lindhout E, Koopman G, Pals ST, de Groot C. Triple check for antigen specificity of B cells during germinal centre reactions. Immunol Today. 1997;18:573. doi: 10.1016/s0167-5699(97)01160-2. [DOI] [PubMed] [Google Scholar]
- 2.Fearon DT, Carter RH. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 3.Roitt IM. Principles of autoimmunity. In: Brostoff J, Scadding GK, Male M, Roitt IM, editors. Clinical Immunology. London: Gower Medical Publishing; 1991. 4, 4 1 10. [Google Scholar]
- 4.Glynn LE, editor. Clinical Aspects of Immunology. Vol. 38. Oxford: Blackwell Scientific Publications; 1975. Rheumatic Fever; p. 1079. [Google Scholar]
- 5.Chiocchia G, Boissier MC, Manoury B, Fournier C. T cell regulation of collagen-induced arthritis in mice. Immunomodulation of arthritis by cytotoxic T cell hybridomas specific for type II collagen. Eur J Immunol. 1993;23:327. doi: 10.1002/eji.1830230204. [DOI] [PubMed] [Google Scholar]
- 6.Matteson EL, Cohen MD, Conn DL. Rheumatoid arthritis; clinical features and systemic involvement. In: Klippel JH, Dieppe PA, editors. Rheumatology. 5.4.1.4.8. London: Mosby; 1998. [Google Scholar]
- 7.Toivanen A. Reactive arthritis and Reiter’s syndrome; history and clinical features. In: Klippel JH, Dieppe PA, editors. Rheumatology. 6.11.1.11.8. London: Mosby; 1998. [Google Scholar]
- 8.Pulendran B, Van Driel R, Nossal GJ. Immunological tolerance in germinal centres. Immunol Today. 1997;18:27. doi: 10.1016/s0167-5699(97)80011-4. [DOI] [PubMed] [Google Scholar]
- 9.Carson DA. Rheumatoid factor. In: Kelley WN, Harris ED, Ruddy S, Sledge CB, editors. Textbook of Rheumatology. Philadelphia: W. B. Saunders; 1993. p. 155. [Google Scholar]
- 10.Davies KA. Complement, immune complexes and systemic lupus erythematosus. Br J Rheumatol. 1996;35:5. doi: 10.1093/rheumatology/35.1.5. [DOI] [PubMed] [Google Scholar]
- 11.Guldner HH, Szostecki C, Vosberg HP, Lakomek HJ, Penner E, Bautz FA. Scl 70 autoantibodies from scleroderma patients recognize a 95 kDa protein identified as DNA topoisomerase I. Chromosome. 1986;94:132. doi: 10.1007/BF00286991. [DOI] [PubMed] [Google Scholar]
- 12.Murray KJ, Szer W, Grom AA, et al. Antibodies to the 45 kDa DEK nuclear antigen in pauciarticular onset juvenile rheumatoid arthritis and iridocyclitis: selective association with MHC gene. J Rheumatol. 1997;24:560. [PubMed] [Google Scholar]
- 13.Edwards JCW, editor. Rheumatoid Arthritis; Questions & Uncertainties. Oxford: Blackwell Scientific; 1999. Is rheumatoid factor relevant. [Google Scholar]
- 14.Welch MJ, Fong S, Vaughan J, Carson D. Increased frequency of rheumatoid factor precursor B lymphocytes after immunization of normal adults with tetanus toxoid. Clin Exp Immunol. 1983;51:299. [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson KM, Randen I, Børretzen M, Forre O, Natvig JB. Variable region gene useage of human monoclonal rheumatoid factors derived from healthy donors following immunization. Eur J Immunol. 1994;24:165. doi: 10.1002/eji.1830240808. [DOI] [PubMed] [Google Scholar]
- 16.Børretzen M, Randen I, Zdarsky E, Forre O, Natvig JB, Thompson KM. Control of autoantibody affinity by selection against amino acid replacements in the complementarity-determining regions. Proc Natl Acad Sci USA. 1994;91:129171. doi: 10.1073/pnas.91.26.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karras JG, Wang Z, Huo L, Howard RG, Frank DA, Rothstein TL. Signal transducer and activator of transcription-3 (STAT3) is constitutively activated in normal, self-renewing B-1 cells but only inducibly expressed in conventional B lymphocytes. J Exp Med. 1997;185:1035. doi: 10.1084/jem.185.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capra JD, Winchester RJ, Kunkel HG. Hypergamma-globulinemic purpura. Studies on the unusual anti-γ-globulins characteristic of the sera of these patients. Medicine. 1971;50:125. [PubMed] [Google Scholar]
- 19.Mannik M, Nardella FA. IgG rheumatoid factors and self association of these antibodies. Clin Rheumatic Dis. 1985;11:551. [PubMed] [Google Scholar]
- 20.Versendaal H, Jonker M, Bresnihan B, et al. Arthritis Rheum. 1999. Asymptomatic synovitis precedes clinically manifest arthritis. in press. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia A, Blades S, Cambridge G, Edwards JC.W. Differential distribution of FcγRIIIa in normal human tissues and co-localization with DAF and fibrillin-1: implications for immunological microenviroments. Immunology. 1998;94:65. doi: 10.1046/j.1365-2567.1998.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klassen RJL, Goldschemeding R, Tetteroo PAT, Von Dem Borne AE.G.K. The Fc Valency of an immune complex is the decisive factor for binding to low-affinity Fcγ receptors. Eur J Immunol. 1988;18:1373. doi: 10.1002/eji.1830180911. [DOI] [PubMed] [Google Scholar]
- 23.van De Winkel JGJ, Capel PJA. Human IgG Fc receptor heterogenity: molecular aspects and clinical implications. Immunol Today. 1993;14:215. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 24.Abrahams VM, Cambridge G, Edwards JCW. FcγRIIIa mediates TNFα secretion by human monocytes/macrophages. Br J Rheumatol 37 (Abstracts Suppl) 1998;1:90. [Google Scholar]
- 25.Debets JMH, Van De Winkel JGJ, Ceuppens JL, Dieteren IE.M, Burman WA. Cross-linking of both FcγRI and FcγII induces secretion of tumor necrosis factor by human monocytes, requiring high affinity Fc–FcγR interactions. J Immunol. 1990;144:1304. [PubMed] [Google Scholar]
- 26.Voice J, Lachmann PJ. Neutrophil Fc gamma and complement receptors involved in binding soluble IgG immune complexes and in specific granule release induced by soluble IgG immune complexes. Eur J Immunol. 1997;27:2514. doi: 10.1002/eji.1830271008. [DOI] [PubMed] [Google Scholar]
- 27.Morgan A, Jones ND, Nesbitt AM, Chaplin L, Bodmer MW, Emtage JS. The N terminal end of the CH2 domain of chimeric human IgG1 anti-HLA-DR is necessary for C1q, FcgRI and FcgRIII binding. Immunology. 1995;86:319. [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards JCW, Sutton BJ. What does IgG look like? Br J Rheumatol 37 (Abstracts Suppl) 1998;1:7. [Google Scholar]
- 29.Serino G, Pitingolo F, Granatieri C, et al. Hypergammaglobulinemic purpura of Waldenström: characterisation of circulating immune complexes. Acta Haemat. 1983;69:152. doi: 10.1159/000206881. [DOI] [PubMed] [Google Scholar]
- 30.Edwards JCW, Leigh RD, Cambridge G. Expression of molecules involved in B lymphocyte survival and differentiation by synovial fibroblasts. Clin Exp Immunol. 1997;108:407. doi: 10.1046/j.1365-2249.1997.4061306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roosnek E, Lanzavecchia A. Efficient and selective presentation of antigen-antibody complexes by rheumatoid factor B cells. J Exp Med. 1991;173:487. doi: 10.1084/jem.173.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarmay G, Konez G, Pecint I, Gergerly J. Fc gamma receptor type IIb induced recruitment of inositol and protein phosphatases to the signal transductory complex of human B cells. Immunol Lett. 1997;57:159. doi: 10.1016/s0165-2478(97)00055-2. [DOI] [PubMed] [Google Scholar]
- 33.Jefferis R. Rheumatoid factors, B cells and immunoglobulin genes. Br Med Bull. 1995;51:312. doi: 10.1093/oxfordjournals.bmb.a072963. [DOI] [PubMed] [Google Scholar]
- 34.Kirchner T, Hoppe F, Schalke B, Muller-Hermelink HK. Microenvironment of thymic myoid cells in myasthenia gravis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;54:295. doi: 10.1007/BF02899226. [DOI] [PubMed] [Google Scholar]
- 35.Tourtellotte WW, Walsh MJ, Baumhefner RW, Staugaitis SM, Shapshak P. The current status of multiple sclerosis intra-blood–brain-barrier IgG synthesis. Ann N Y Acad Sci. 1984;436:52. doi: 10.1111/j.1749-6632.1984.tb14775.x. [DOI] [PubMed] [Google Scholar]
- 36.Gay FW, Drye TJ, Dick GW, Esiri MM. The application of multifactorial cluster analysis in the staging of plaques in early multiple sclerosis. Identification and characterization of the primary demyelinating lesion. Brain. 1997;120:1461. doi: 10.1093/brain/120.8.1461. [DOI] [PubMed] [Google Scholar]
- 37.Alarcon-Segovia D, Ruiz-Arguelles A, Llorente L. Broken dogma: penetration of autoantibodies into living cells. Immunol Today. 1996;17:163. doi: 10.1016/s0167-5699(96)90258-3. [DOI] [PubMed] [Google Scholar]
- 38.Vaishnaw AK, McNally JD, Elkon KB. Apoptosis in the rheumatic diseases. Arthritis Rheum. 1997;40:1917. doi: 10.1002/art.1780401102. [DOI] [PubMed] [Google Scholar]
- 39.Craft J, Hardin JA, Kelley WN, Harris ED, Ruddy S, Sledge CB, editors. Textbook of Rheumatology. Philadelphia: W. B. Saunders; 1993. Antinuclear antibodies; p. 164. [Google Scholar]
- 40.Pilatte Y, Tisserand EM, Greffard A, Bignon J, Lambre CR. Anticarbohydrate autoantibodies to sialidase-treated erythrocytes and thymocytes in serum from patients with pulmonary sarcoidosis. Am J Med. 1990;88:486. doi: 10.1016/0002-9343(90)90427-f. [DOI] [PubMed] [Google Scholar]
- 41.Maloney DG, Grillo-Lopez AJ, Bodkin DJ, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. J Clin Oncol. 1997;15:3266. doi: 10.1200/JCO.1997.15.10.3266. [DOI] [PubMed] [Google Scholar]
- 42.Isaacs JD, Watts RA, Hazleman BL, et al. Humanised monoclonal antibody therapy for rheumatoid arthritis. Lancet. 1992;340:748. doi: 10.1016/0140-6736(92)92294-p. [DOI] [PubMed] [Google Scholar]
- 43.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825. doi: 10.1200/JCO.1998.16.8.2825. Additional data on file, Roche Products, Welwyn, UK. [DOI] [PubMed] [Google Scholar]