Abstract
BALB/c mice were inoculated with Bartonella henselae by both systemic and mucosal routes. Culture analysis of tissues from mice infected intraperitoneally with a high dose of B. henselae yielded positive results 24 hr after infection. However, culture analysis of blood taken between 6 hr and 7 days after infection from groups receiving live B. henselae were negative. Following intraperitoneal infection, B. henselae was detected by polymerase chain reaction in liver and mesenteric lymph nodes by 6 hr and up to 7 days after infection in liver, kidney and spleen tissue. Enzyme-linked immunosorbent assay (ELISA) of serum samples collected as early as 13 days after infection indicated humoral immune responses to B. henselae. Specific humoral responses remained through week 6. Analysis of faecal samples revealed induction of B. henselae-specific immunoglobulin A by day 28 after infection. In addition, B. henselae-specific cellular responses were indicated by a positive delayed-type hypersensitivity and a T helper 1 (Th1) (CD4+ T cell)-type cytokine response following in vitro stimulation of splenocytes. The significance and implications of these data in relation to B. henselae infections are discussed.
INTRODUCTION
Bartonella henselae is a fastidious, Gram-negative bacterium that has been associated with a variety of human diseases, including cutaneous bacillary angiomatosis, bacillary hepatic peliosis, bacteraemia, relapsing fever, central nervous system disorders and, more commonly, cat scratch disease (CSD).1–9 CSD afflicts an estimated 24 000 persons in the United States annually,10 and is characterized by a broad range of clinical symptoms, manifested in varying degrees of severity, largely dependent upon the immune status of the host. Although B. henselae has emerged as the cause of significant human disease, both in rate of infection as well as severity of disease, little is known regarding pathogenicity and immunity induced during infection.
Historically, human immune responses to CSD have been defined as regional lymphadenopathy in proximity to the inoculation papule (cat scratch or bite). In addition, skin testing based upon the Hanger–Rose diagnostic test indicates cellular immunity to the agent.11 In more recent years, the identification and growth on artificial media have facilitated the use of serological clinical diagnosis. The immunofluorescence assay (IFA) developed by Regnery et al.,12 has provided a routine detection assay for diagnosis of Bartonella-associated human disease. In addition, humoral analysis of human CSD patient sera provide insight into aspects of B. henselae-specific immunity.13,14 However, other than serological analysis of Bartonella exposure, little is known about immunity following exposure to B. henselae. Likewise, serological tests have provided evidence of significant humoral immune responses in domestic cats.15 However, detailed aspects of cat immunity remain elusive. Thus, limited immunological analysis in human or cat hosts has left a gap in our understanding of B. henselae-induced immunity. The characterization of immune responses generated by exposure to Bartonella species is crucial for the understanding of this organism’s ability to cause disease. To advance our understanding of the immune response to B. henselae, a BALB/c mouse model of immunity for the fastidious bacterial pathogen B. henselae has been developed. BALB/c mice were found to be responsive to systemic and mucosal routes of infection as evidenced by the development of B. henselae humoral and cellular immune responses.
MATERIALS AND METHODS
Bacterial strain
B. henselae used in this study originated from the Houston-1 isolate (ATCC #49882). B. henselae stocks were grown on rabbit blood agar plates (BBL Microbiology systems, Cockeysville, MD) at 32° with 5% CO2. Solid agar growth of this stock yields a mixed phenotype of rough and smooth colonies. No clonal selection for phenotype was performed in this study. Bacteria were harvested in a laminar flow hood by gently scraping colonies off the agar surface in brain heart infusion (BHI) broth media. The cells were then collected via centrifugation and suspended in phosphate-buffered saline solution (PBS). Colony-forming units (CFU) of harvested Bartonella cultures were titred on blood agar plates prior to being either frozen for infection studies or inactivated by gamma irradiation (5±105 rads) and stored at −70° until used. A single freeze/thaw of stocks routinely results in less than a one log reduction in viability.
Immunizations
BALB/c mice (4–6 weeks old) were obtained from Harlan Sprague–Dawley (Indianapolis, IN). B. henselae was prepared as described above, and thawed on ice prior to immunization. Mice were anaesthetized with methoxyflurane (Metofane; Pitman-Moore Inc., Mundelein, IL) and administered live B. henselae at dose of 1±104 or 3±106 CFU/mouse by the routes indicated or 3±108 CFU/mouse intraperitoneally (i.p.) as described below. For oral immunizations, mice were dosed by using a 22-gauge feeding needle attached to a 1 cm3 syringe. For intranasal (i.n.) immunizations, mice were dosed with volumes of 10–20 μl containing ≈3±106 CFU of B. henselae administered into the nostrils using a micropipette fitted with a 200-μl capacity tip. Intravenous (i.v.), i.p. and subcutaneous (s.c.) immunizations utilized a tuberculin syringe and needle. Tail vein injections were performed under anaesthesia and with the use of heat lamp to facilitate venous access. Following immunizations, mice were observed daily for signs of illness. Each immunized group contained at least five mice. None of the challenged mice showed any signs of overt illness during this study.
Detection of B. henselae in vivo
Detection of blood-borne organisms was attempted by using whole blood plating techniques as well as polymerase chain reaction (PCR). Bleeding was performed by using heparinized tubes at 6, 24 and 48 hr and on days 3, 7, 8 and 15 after immunizations. Whole blood was plated on rabbit blood agar plates (BBL) and incubated for up to 30 days at 32° with 5% CO2. Spleen, liver, kidney and mesenteric lymph nodes (MLN) tissues from individual mice were harvested aseptically and homogenized together in 1 ml of BHI broth using disposable pellet pestles (Kontes, Vineland, NJ) before being plated (100 μl per plate) on rabbit blood agar plates. In addition, DNA was extracted from whole blood or tissues using Qiagen DNA extraction kits (Qiagen #29104 and #29306) and subsequently subjected to an ethanol precipitation prior to PCR analysis. Primers A1 (CAGATGATGATCCCAAGC CTTC) and A2 (GTTCTATTGAA-ATTGTGAAGAGAAG) designed by Minnick and Barbian16 from the Intragenic spacer region of the 16 seconds and 23 seconds rRNA gene region of Bartonella were used. For primers A1 and A2, the thermal cycler programme format was as follows: 95° for 1 min, 58° or 1 min, 72° for 1 min for 39 cycles, then 95° for 1 min, 58° for 1 min and 72° for 2 min for one cycle. Spiking of naïve tissue and blood samples with B. henselae bacteria prior to DNA extraction was performed to confirm the detection and sensitivity of this assay. B. henselae DNA was detected down to 15 CFU/ml of whole blood and 3·3 CFU/mg of tissue.
Enzyme-linked immunosorbent assay (ELISA)
Serum and faecal samples were collected from preimmune (naïve) mice at day 0 and throughout the study at the days indicated post immunization. Faecal samples were prepared as previously described.17 Microtitre plates (Immulon II) were coated overnight at 4° with a 1:50 dilution of stock whole cell inactivated (gamma-irradiated) B. henselae (titre 1±106 CFU/ml). Plates were then blocked with 3% skim milk in 1±PBS for 1 hr at 37° and washed three times with PBST (0·05% Tween-20 in PBS). Samples were then added at either a 1:50 dilution (sera) or a 1:1 dilution (faecal extract). Samples were then doubly diluted in PBST and plates incubated for 2 hr at 37°. Plates were washed three times with PBST. Secondary conjugate antibodies were used according to the manufacturer’s recommendations and include goat antimouse immunoglobulin G (IgG)–horseradish peroxidase (HRP) and goat antimouse IgA–HRP conjugates (Kirkegaard & Perry, Gaithersburg, MD). Secondary antibodies were incubated for 1 hr at 37°, washed three times and plates developed using 2,2±-azino-di-3-ethylbenzthiazoline sulphonic acid. Plates were allowed to develop for 30 min before absorbance values at 405 nm were determined.
Delayed-type hypersensitivity
Immunized mice were subjected to intradermal skin testing to monitor delayed swelling reactions as an indication of specific cellular immune induction. Mice were boosted on day 100 after primary immunization with 1·5±106 CFU using the same route of immunization. One week following the boost, each mouse was challenged in one ear pinna with gamma-irradiated B. henselae(106 CFU) and in the other with 1±PBS as a control. For each mouse, the antigen-injected ear pinna was monitored at 24, 48 and 72 hr for swelling responses and compared to the control ear pinnae as an internal negative control. Swelling was determined from three readings using a ‘General’#142, 6 inch Dial Caliper (General Hardware, Mfg. Co. Inc., New York, NY). Average swelling differences of antigen-injected versus control ears for each group of five to 10 mice were then compared to naïve controls using a one-tailed, two-sample unequal variance t-test. P-values ±0·05 are considered statistically significant.
In vitro stimulation of splenocytes
For each of two experiments, spleens were harvested from two mice from each group immunized either s.c., orally or i.p. with live B. henselae or killed B. henselae at 3±106 CFU s.c. Single cell suspensions were made to 1±106 cells/ml in RPMI medium plus 10% fetal calf serum (FCS). Splenocytes were plated into 96-well microtitre plates at a concentration of 1±105 cells/well. Cells were then stimulated with either killed total B. henselae antigen at 105 CFU/ml per well, RPMI medium plus 10% FCS alone as a negative control or concanavalin A at a final concentration of 1 μg/ml as a positive control. Cells were incubated with antigen at 37° plus 5% CO2 for 5 days. Cell cultures were then harvested and filtered through 0·22 μm filters, diluted and screened for interferon-γ (IFN-γ) and interleukin-4 (IL-4) using ELISA kits as recommended by the manufacturer (Endogen, Woburn, MA).
RESULTS
Experimental infection with B. henselae
Tissues harvested at hours 6, 24, 48 and 72 and at day 7 after i.p. infection with 106 CFU yielded positive PCR results for B. henselae(Table 1). Positive PCR results were observed in tissues as early as 6 hr (liver and MLN) and remained positive up to 7 days (spleen, liver, kidney) after i.p. infection. Despite positive results in tissues, PCR failed to detect B. henselae in whole blood of mice receiving 104 or 106 CFU of live B. henselae. In addition, attempts to culture B. henselae from blood or tissues following i.v., s.c., i.p. or oral challenge with 104 or 106 CFU failed (data not shown). Subsequently, in a further attempt to recover viable B. henselae from challenged mice, 3±108 CFU of live B. henselae was administered i.p. Culture analysis of tissue homogenates from this group indicated positive cultures within a 24-h harvest period, although blood cultures remained negative (data not shown). However, by 48 hr post challenge with 3±108 CFU, cultures from tissue homogenates were negative.
Table 1.
Detection of B. henselae by PCR from tissues following live intraperitoneal immunization
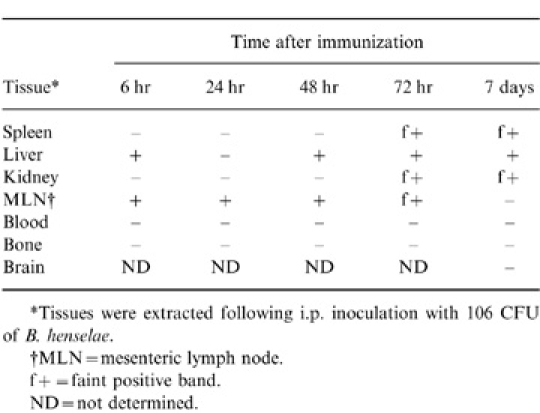
*Tissues were extracted following i.p. inoculation with 106 CFU of B. henselae.
†MLN = mesenteric lymph node.
f+ = faint positive band.
ND = not determined.
Humoral immunity
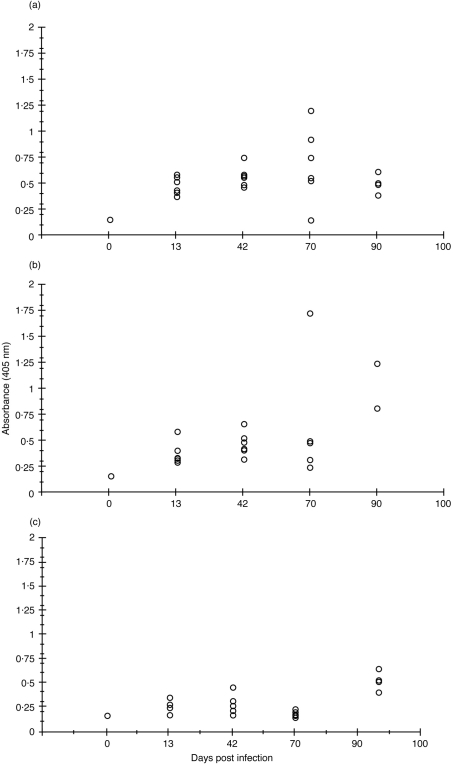
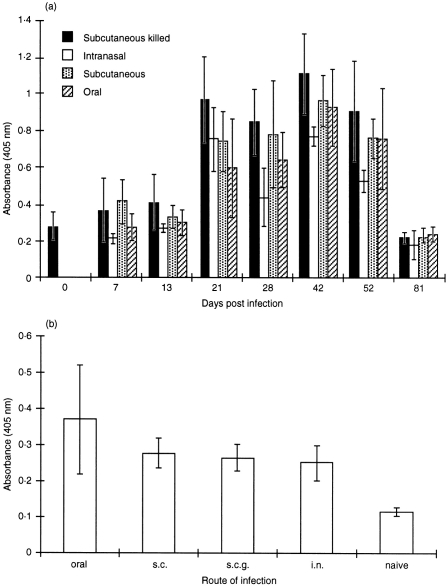
Mice dosed i.v., s.c. or orally with 104 CFU of live B. henselae were monitored for serum IgG responses by ELISA. Serum B. henselae-specific IgG was detected in all groups by day 13 after infection with titres increasing through day 42 (Fig. 1a,b,c). The highest titres were observed at day 70 in the i.v. and s.c. groups following a second dose of 104 CFU on day 50 (Fig. 1a,b), while an oral boost failed to enhance titres at day 70 (Fig. 1c). An additional study using a single dose of 3±106 CFU per mouse dosed either s.c., orally or i.n. with live B. henselae or s.c. with killed B. henselae was performed and B. henselae-specific IgG levels determined. Titres were apparent by day 21 in all four groups, with peak responses occurring at day 42 after immunization. Levels of IgG began to drop by day 52 falling to those of the day 0 (naïve) control by day 81 (Fig. 2a). In addition, faecal samples collected at day 28 after immunization indicated B. henselae-specific IgA induction in mice receiving live B. henselae orally, i.n. or s.c. and killed B. henselae s.c. when compared to naïve animals (Fig. 2b).
Figure 1.
ELISA analysis of B. henselae-specific IgG in serum samples of mice receiving 104 CFU of live B. henselae either(a) intravenously (b) subcutaneously or (c) orally. Data represent the absorbance (405 nm) reading of the 1:100 dilution of each serum tested.
Figure 2.
ELISA analysis of B. henselae-specific(a) serum IgG or (b) faecal IgA in mice receiving 106 CFU of live B. henselae either subcutaneously, orally or intranasally or mice receiving 106 CFU of killed B. hensleae subcutaneously. Data represent the absorbance (405 nm) reading of a 1:100 dilution of each serum and 1:2 dilution of faecal samples tested.
Delayed-type hypersensitivity
To assess the induction of Bartonella-specific cellular immune responses, the presence of delayed-type hypersensitivity (DTH) was tested for in mice receiving 106 CFU of live B. henselae by the s.c., i.n. or oral routes of immunization or 106 CFU of killed B. henselae s.c. Inactivated B. henselae was injected into one ear pinna of immunized mice and ear swelling measured 48 hr later. The only statistically significant DTH response occurred in the s.c. immunized mice receiving live B. henselae (Table 2). In addition, although below statistically significant levels, orally and i.n. immunized mice receiving live B. henselae and mice receiving gamma-irradiated B. henselae s.c. developed some degree of ear swelling in response to B. henselae compared to non-immune animals (Table 2).
Table 2.
Measurement of DTH responses in mice following administration of B. henselae
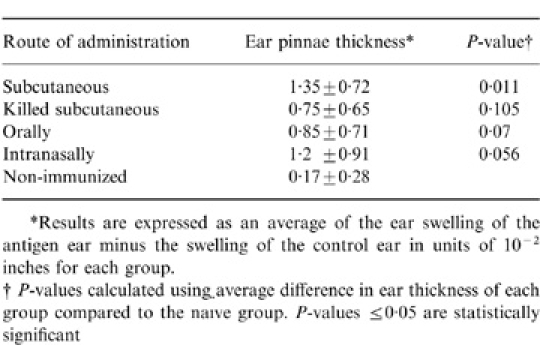
*Results are expressed as an average of the ear swelling of the antigen ear minus the swelling of the control ear in units of 10− 2 inches for each group.
†P-values calculated using average difference in ear thickness of each group compared to the naïve group. P-values ≤ 0·05 are statistically significant
Induction of cellular cytokine expression
In addition to DTH as an indicator of cellular immune induction, in vitro stimulation of splenocytes with B. henselae antigen was performed to assess the production of IFN-γ and IL-4 in response to antigen stimulation. Splenocytes were harvested from mice previously immunized with live B. henselae via the s.c., i.p. and oral routes or s.c. with killed B. henselae. The results shown in Table 3 indicate induction of both IL-4 and IFN-γ expression in splenocytes from immunized animals following in vitro antigen stimulation. In fact, IFN-γ expression in immunized as well as non immunized groups exceeded levels obtained with ConA positive control stimulation. In contrast, levels of IL-4 production increased only in the group immunized with live B. henselae subcutaneously while remaining below or near the detectable limits (<15 pg/ml) in all other groups (Table 3).
Cytokine expression from splenocytes following in vitro stimulation with whole cell B. henselae antigen
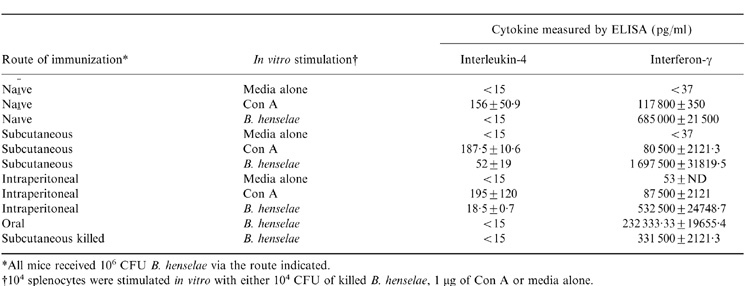
*All mice received 106 CFU B. henselae via the route indicated.
†104 splenocytes were stimulated in vitro with either 104 CFU of killed B. henselae, 1 µg of Con A or media alone.
DISCUSSION
This report provides novel information regarding the immunity induced by B. henselae, the aetiological agent of cat scratch disease. In an attempt to provide data that would advance our understanding of B. henselae-induced immunity, BALB/c mice were infected with B. henselae and assessed for the development of bacteraemia, organ dissemination and the induction of B. henselae-specific immunity. By establishing a murine model of immunity, a basis for future immune studies of this organism is provided.
B. henselae was cultured from organ homogenates 24 hr following a 108 CFU dose. However, attempts at recovery of viable bacteria from blood and tissues failed in mice receiving 104 or 106 CFU, and by 48 hr, no organisms were recovered from animals receiving 108 CFU. Thus, it is likely that recovery of viable bacteria at 24 hr after high dose challenge (108 CFU) is representative of residual organisms from the original inoculum rather than in vivo growth. Despite the absence of viable organisms in blood and tissues by culture techniqes, B. henselae DNA was detected by PCR in mouse tissues from 6 hr up to 7 days after i.p. infection (Table 1). Thus, it appears that B. henselae is incapable of maintaining viability for extended periods in BALB/c mice although organ distribution is evidenced by PCR analysis.
Despite failure to maintain viability in vivo, induction of specific humoral immunity following administration of live or killed B. henselae was observed. B. henselae-specific systemic humoral IgG responses were observed by day 21 after administration of live B. henselae via the s.c., i.v. oral and i.n. routes or killed B. henselae s.c. Thus, B. henselae is capable of inducing systemic immunity following a variety of infection routes including mucosal delivery. The development of systemic antibody following mucosal delivery of B. henselae suggests that the organisms may reach the gut-associated lymphoid tissue, where inductive immune responses are induced. Furthermore, the presence of B. henselae-specific faecal IgA following both systemic and mucosal challenge may be indicative of a mucosal component of B. henselae infection.
Although the development of specific immunity following exposure to B. henselae indicates successful immune induction, determination of protective immunity remains elusive. A cellular component of immunity occurs during human CSD,14 and experimentation supports a role for cellular responses in feline protective immunity against B. henselae (our unpublished observation). However, until now the only animal infection model for B. henselae reported to date is the domestic cat,15,18 a model for which immunological reagents are limiting. Thus, analysis of immunity in a murine model provides a model to access the strength of induced immunity. The development of DTH in animals receiving live B. henselae s.c. indicates induction of CD4+ T-cell activity against B. henselae antigen.19 However, mucosal delivery of live or parenteral delivery of killed B. henselae fail to induce statistically significant DTH reactions. In addition, although elevated levels of IFN-γ were observed following in vitro stimulation of naïve as well as all immunized groups, elevated levels of IL-4 along with of IFN-γ induction were detected only following parenteral exposure to live B. henselae. Thus, prior parenteral exposure to live organisms facilitates both T helper 1 (Th1)- and Th2-type immune parameters while only Th1-type cytokine responses are detected following live mucosal or killed parenteral immunizations. The analysis of DTH and cytokine profiles provide evidence of cellular immune induction following B. henselae exposure both parenterally and mucosally. However, it suggests that live parenteral exposure is superior in inducing both aspects of Th1 and Th2 immune responses.
Aside from the analysis of cellular and humoral immunity, detection of bacterial DNA suggests details regarding the fate of B. henselae following parenteral infection. The presence of B. henselae in lymph and liver tissue by PCR 6 hr following i.p. injection suggests the uptake of this organism by professional phagocytes, which then migrate to these organs. In fact, rapid uptake by murine phagocytic cells could explain the absence of B. henselae-induced bacteraemia and viable counts in tissues for extended periods post challenge. The induction of IFN-γ supports a role for phagocyte activity as well as enhanced class I- and class II-mediated immunity, which includes both cellular and humoral components.20 With this in mind, B. henselae may be highly susceptible to immune effectors such as INF-γ and CD4+ T-cell activity. In concordance with this supposition, B. henselae-induced bacteraemia during human infection is typically found only in immunocompromised patients.1,12 Thus, the reduction in CD4+ T-cell activity and IFN-γ may play a role in the development of bacteraemia in these patients. Therefore, although human and murine immune responses to B. henselae may include T-cell activity, IFN-γ and subsequent macrophage-killing activity, the immuno-status of the host may dictate disease progression. By defining immune parameters in a murine model, perhaps more details of B. henselae immunopathogenesis will be unveiled.
REFERENCES
- 1.Anderson BE, Neuman MA. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:269. doi: 10.1128/cmr.10.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loutit JS. Bartonella infections. Curr Clin Top Infect Dis. 1997;17:269. [PubMed] [Google Scholar]
- 3.Koehler JE. Bartonella infections. Adv Pediatr Infect Dis. 1996;11:1. [PubMed] [Google Scholar]
- 4.Liston TE, Koehler JE. Granulomatous hepatitis and necrotizing splenitis due to Bartonella henselae in a patient with cancer: case report and review of hepatosplenic manifestations of Bartonella infection. Clin Infect Dis. 1996;22:951. doi: 10.1093/clinids/22.6.951. [DOI] [PubMed] [Google Scholar]
- 5.Wong MTMJ, Dolan CP, Lattuada RL, et al. Neuroretinitis, aseptic meningitis, and lymphadenitis associated with Bartonella (Rochalimaea) henselae infection in immunocompetent patients and patients infected with human immunodeficiency virus type 1. Clin Infect Dis. 1995;21:352. doi: 10.1093/clinids/21.2.352. [DOI] [PubMed] [Google Scholar]
- 6.Koehler JE, Glassen CA, Tappero JW. Rochalimea henselae infection: a new zoonosis with the domestic cat as reservoir. JAMA. 1994;271:531. doi: 10.1001/jama.271.7.531. [DOI] [PubMed] [Google Scholar]
- 7.Dolan MJ, Wong MT, Regnery RL. Syndrome of Rochalimea henselae adenitis suggesting cat scratch disease. Ann Intern Med. 1993;118:331. doi: 10.7326/0003-4819-118-5-199303010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Koehler JE, Quinn FD, Gerger TG, Leboit PE, Tappero JW. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med. 1992;327:1625. doi: 10.1056/NEJM199212033272303. [DOI] [PubMed] [Google Scholar]
- 9.Slater LN, Welch DF, Min KW. Rochalimaea henselae causes bacillary angiomatosis and peliosis hepatitis. Arch Intern Med. 1992;152:602. [PubMed] [Google Scholar]
- 10.Jackson LA, Perkins BA, Wenger JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. 1993;83:1707. doi: 10.2105/ajph.83.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margileth AM. Cat scratch disease and nontuberculous mycobacterial disease: diagnostic usefulness of PPD-battery, PPD-T and cat scratch skin test antigens. Ann Allergy. 1992;68:149. [PubMed] [Google Scholar]
- 12.Regnery RL, Olson JG, Perkins BA, Bibb W. Serological response to Rochalimaea henselae antigen in suspected cat-scratch disease. Lancet. 1992;339:1443. doi: 10.1016/0140-6736(92)92032-b. [DOI] [PubMed] [Google Scholar]
- 13.Mcgill SL, Regnery RL, Karem KL. Characterization of human immunoglobulin (Ig) isotype and IgG subclass response to Bartonella henselae infection. Infect Immun. 1998;66:5915. doi: 10.1128/iai.66.12.5915-5920.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moriarity RA, Margileth AM. Cat scratch disease. Infect Dis North Am. 1987;1:575. [PubMed] [Google Scholar]
- 15.Regnery R, Rooney J, Johnson A, et al. Experimentally induced Bartonella henselae infections followed by challenge exposure and antimicrobial therapy in cats. Am J Vet Res. 1996;57:1714. [PubMed] [Google Scholar]
- 16.Minnick MR, Barbian KD. Identification of Bartonella using PCR; genus- and species-specific primer sets. J Microbiol Methods. 1998;31:51. [Google Scholar]
- 17.Karem KL, Chatfield S, Kuklin N, Rouse BT. Differential induction of carrier antigen-specific immunity by Salmonella typhimurium live-vaccine strains after single mucosal or intravenous immunization of BALB/c mice. Infect Immun. 1995;63:4557. doi: 10.1128/iai.63.12.4557-4563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene CE, Mcdermott M, Jameson PH, Atkins CL, Marks AM. Bartonella henselae infection in cats: evaluation during primary infection, treatment, and rechallenge infection. J Clin Microbiol. 1996;34:1682. doi: 10.1128/jcm.34.7.1682-1685.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cher DJ, Mossman TR. Two types of murine helper T cell clones. II. Delayed-type hypersensitivity is mediated by Th-1 clones. J Immunol. 1987;138:3688. [PubMed] [Google Scholar]
- 20.Abbas AK, Lichtman AH, Pober JS. Cellular and Molecular Immunology. 3. Saunders, Philadelphia: 1997. [Google Scholar]
- 21.Regnery RL, Anderson BE, Clarridge JE, Rodrigeuz-Barradas MC, Jones DC, Carr JH. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J Clin Microbiol. 1992;30:265. doi: 10.1128/jcm.30.2.265-274.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]