Abstract
Interleukin-16 (IL-16), a natural ligand for the CD4 receptor, has been found to modulate T-lymphocyte function and to inhibit human immunodeficiency virus type 1 (HIV-1) replication. Antigen-presenting cells (APC), including macrophages and dendritic cells, are known to express functional surface CD4 molecules, to be susceptible to HIV-1 infection and to play a critical role in different immune processes. Therefore, we evaluated the ability of recombinant IL-16 (rIL-16) to regulate receptor expression and cytokine release in monocyte-derived macrophages (MDM) and monocyte-derived dendritic cells (MDDC). Recombinant IL-16 was found to up-regulate CD25 and CD80 but to down-regulate CD4 and CD86 surface expression in MDM cultures. However, no change could be observed on the level of CD4, CD80 and CD86 expression in IL-16-stimulated MDDC, although a significant up-regulation of CD25 and CD83 was consistently detected. Furthermore, the level of gene expression of the chemokine receptors CCR5 and CXCR4 was significantly reduced in rIL-16-treated MDM and costimulation with IL-2 did not modify the activity of the recombinant cytokine. The effects on chemokine receptor gene expression were less evident in MDDC and only a transient down-regulation of weak intensity could be detected following stimulation with rIL-16. Analysis of supernatants from rIL-16-stimulatedcultures revealed a different profile of released cytokines/chemokines among the two cell populations studied. These findings establish an important role for IL-16 in modulating the activity of APC and may have relevance regarding the protection of reservoir cells against HIV-1 infection.
INTRODUCTION
Interleukin-16 (IL-16) is a pleiotropic cytokine secreted mainly by CD8 T cells, and has chemoattractant activity on CD4-positive lymphocytes, monocytes and eosinophils.1 Following binding to the CD4 receptor on lymphocytes, IL-16 induces an increase in intracellular Ca2+ and inositol triphosphate levels as well as phosphorylation of the CD4 molecule.2 IL-16 has been reported to enhance IL-2 receptor expression but to inhibit the lymphoproliferative responses induced by allogeneic mixed lymphocyte reactions or by anti-CD3 antibodies.3–5 In long-term lymphocyte cultures, this chemoattractant cytokine was found to induce the release of granulocyte–macrophage colony-stimulating factor (GM- CSF) and to synergize with IL-2 in the expansion of CD4 T cells.6 In addition, recombinant IL-16 (rIL-16), corresponding to the C-terminal 130 amino acids of the natural molecule, has been reported recently to inhibit human immunodeficiency virus type-1 (HIV-1) replication in peripheral blood mononuclear cells (PBMC) from infected subjects.7
Among the antigen-presenting cells (APC), macrophages and dendritic cells are known to express the CD4 receptor and to be susceptible to infection with HIV-1. These cells also express the major HIV co-receptors CCR5 and CXCR4 and are believed to act as reservoirs for virus dissemination.8–11 Although the modulation of T-cell activity by IL-16 has been well studied, no or very limited knowledge is currently available on the effects of IL-16 on dendritic cells or on macrophages, respectively. Recently, rIL-16 was reported to activate the stress-activated protein kinase signalling in human macrophages.12 In an attempt to understand the role of IL-16 in regulating the activity of APC, we have analysed the changes induced on the expression of a battery of receptors that mediate accessory cell function (CD80, CD86), that are cell-type specific (CD14, CD1a and CD83) or that are required for HIV-1 entry (CD4, CCR5 and CXCR4). Moreover, the ability of rIL-16 to induce the release of representative inflammatory and HIV-enhancing cytokines, anti-inflammatory cytokines and HIV-suppressive β-chemokines has been examined. Our results demonstrate different effects of the recombinant cytokine on the two cell populations studied, with a marked and selective down-regulation of CD4 and CCR5 expression in macrophages. The presented findings are discussed in the context of a potential role of IL-16 in regulating the immune and inflammatory functions of APC and the control of HIV replication in reservoir cells.
MATERIALS AND METHODS
Reagents used
Murine anti-human monoclonal antibodies [CD4– phycoerythrin (PE), CD95–PE, CD1a–PE, CD3–PE, CD83–PE, CD25–PE, CD14–fluorescein isothiocyanate (FITC), CD80–FITCE, CD86–PE and their matched isotypes) used for cytofluorimetric detection were purchased from Immunotech (Marseille, France), except for CD86–PE which was obtained from Pharmingen (San Diego, CA). Human recombinant tumour necrosis factor-α (rTNF-α), rIL-4 and rIL-2 were purchased from R&D Systems (Abingdon, UK). Human rGM-CSF was provided by Sandoz Pharma (Basel, Switzerland). Recombinant rat IL-5, produced in Escherichia coli, was kindly provided by Dr J. Khalife (Institut Pasteur de Lille, France) and was used as a control of an irrelevant recombinant protein.
Preparation of rIL-16
Recombinant IL-16 was produced and purified as described previously.13 The histidine-tagged 130-amino acid protein produced in E. coli was rendered endotoxin-free (<0·125 endotoxin unit/10 μg protein) by passages over polymyxin-B columns (Pierce, Montluçon, France). Using sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and Coomassie blue staining, rIL-16 was found to migrate as a single band of 20 000 molecular weight (MW) and to be >95% pure. Only 2–10% of rIL-16 corresponded to the active homotetrameric form, whereas the rest of the protein was found as inactive monomers or homodimers.13 The concentration of rIL-16 presented throughout the study was 10 μg/ml of total protein (corresponding to 0·5 μg/ml of homotetrameric form). This concentration was the highest to induce measurable effects and to be free of endotoxin contaminants. Lower concentrations of 3 and 1 μg/ml were evaluated in four separate experiments and were found to produce weaker and dose-dependent effects.
Culture condition
PBMC were isolated from heparinized blood samples by Ficoll–Hypaque density gradient centrifugation (Pharmacia, Uppsala, Sweden). Monocytes were obtained by adherence as described previously.14 They were allowed to differentiate into macrophages after 5–7 days culture in RPMI containing 10% human AB serum. Monocyte-derived dendritic cells (MDDC) were obtained by culturing monocytes for 7 days in medium supplemented with 1000 U/ml of rGM-CSF, 10 ng/ml of rIL-4 and 200 U/ml of rTNF-α. After differentiation in six-well plates (Falcon, Le Pont de Claix, France), 3×106 monocyte-derived macrophages (MDM) or MDDC were cultured with or without IL-16 (10 μg/ml), IL-2 (100 U/ml) or a combination of the two cytokines. In MDDC cultures, stimulation with IL-16 was performed in the presence of exogenous cytokines that were used to drive the differentiation of monocytes into mature dendritic cells. At different time-points following stimulation, cells were washed in cold phosphate-buffered saline (PBS), removed by gentle scrapping and counted using trypan blue dye. Neither the number of total cells recovered nor the percentage of viable cells (>70%) were different between non-stimulated and IL-16-stimulated cultures from all donors tested.
Levels of secreted cytokines
The levels of TNF-α, IL-6, IL-10, IL-12, IL-1 receptor antagonist (IL-1RA), macrophage inflammatory protein-1α (MIP-1α) and RANTES (regulating upon activation normal T expressed and secreted) in culture supernatants, were determined by using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Abingdon, UK) and following the manufacturer’s instructions. From three preliminary experiments, peak cytokine release was observed after a 24- but not after 6- or 48-hr stimulation period. Therefore, in all subsequent experiments the levels of secreted cytokines were evaluated following 24-hr stimulation with rIL-16.
Flow cytometric analysis
Cells (2×105 cells) were incubated with 1 μg of mouse antibodies for 30 min at 4°, in PBS containing 0·5% bovine serum albumin. Cells were then washed twice with PBS, resuspended and fixed with 1% paraformaldehyde. The percentage of positive cells and mean fluorescence intensity (MFI) were analysed by an Epics Coulter cytofluorimeter (Coultronics France SA, Margency, France). Dead cells were excluded from the analysis by propidium iodide staining and live cells were gated on the basis of their forward scatter and side scatter characteristics.
Semi-quantitative reverse transcription–polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from MDM or MDDC cultures using RNAzol (Bioprobes Systems, Montreuil, France) and following the manufacturer’s protocol. To remove traces of DNA contamination, the RNA samples (5 μg) were treated with 2·5 U of RQ1 Rnase-free Dnase (Promega Corporation, Norwalk, CT) in the presence of 2× rTth reverse transcriptase buffer (Perkin-Elmer Corporation, Foster City, USA), 2 mm MgCl2 and 20 U of RNAsin (Promega). The RT-PCR reaction was carried out in a reaction mixture of 50 μl per tube containing serial 1:5 dilutions of RNA samples (100, 20 or 4 ng), 1× rTth reverse transcriptase buffer, 2·5 mm MnCl2, 300 μm of each dNTP (Pharmacia Biotech, Uppsala, Sweden), 0·4 μm primer pairs (Genset, Paris, France) and 5 U of rTth DNA polymerase (Perkin-Elmer). The sense and antisense primer pairs used to specifically amplify mRNA were, for CCR5: 5′GCT CTC TCC CAG GAA TCA TCT TTA C-3′ and 5′-TTG GTC CAA CCT GTT AGA GCT ACT G-3′;15 for CXCR4: 5′-TGA CTC CAT GAA GGA ACC CTG-3′ and 5′-CTT GGC CTC TGA CTG TTG GTG-3′;16 for CD4: 5′-AAG ACC CTC TCC GTG TCT-3′ and 5′-GTC AGC TTT TCA ACT GTA AAG GCG-3′; for β-actin: 5′-GGG TCA GAA GGA TTC CTA GG-3′ and 5′-GGT CTC AAA CAT GAT CTG GG-3′. The RT reaction started at 55° for 2 min with an additional 30 min at 60°. The different PCR amplification conditions were: CCR5, 30 cycles (94° for 45 seconds, 56° for 45 seconds, 72° for 45 seconds); CXCR4, 45 cycles (94° for 30 seconds, 54° for 30 seconds, 72° for 30 seconds); CD4, 25 cycles (94° for 15 seconds, 56° for 15 seconds, 72° for 15 seconds); β-actin, 20 cycles (94° for 15 seconds, 55° for 15 seconds, 72° for 15 seconds). The PCR reactions were terminated by an incubation at 72° for 7 min. PCR products were separated on a 1·8% agarose gel visualized and photographed under UV light after ethidium bromide staining. Quantification of the PCR products was obtained by densitometric analysis (Image Master 1D prime; Pharmacia Biotech). The mRNA levels of the receptors were normalized to the corresponding β-actin levels, by calculating the ratio of the receptor RNA band volume over that of β-actin, in the linear phase of the amplification. Results are expressed as the percentage of maximum mRNA expression relative to the condition giving the highest gene expression, arbitrarily considered as 100%.
Statistical analysis
Statistical comparisons were made using the Student’s t-test for paired data. Probability values below 0·05 were considered significant.
RESULTS
Phenotypic characterization of MDM and MDDC
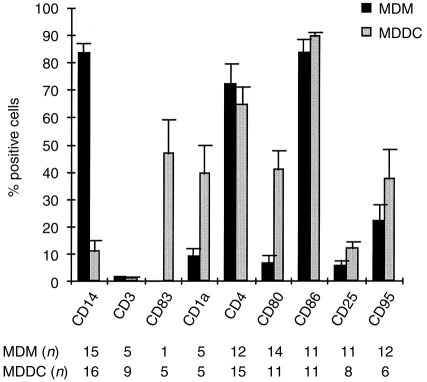
The level of T-cell contamination in MDM and MDDC cultures was found to be below 2%, as revealed by the absence of CD3-positive cells (Fig. 1). MDM exhibited a high-level expression of the monocyte/macrophage marker CD14 (mean percentage positive cells±SEM: 83·6±3·6; MFI±SEM: 97·8±19·8) and over 70% of cells were CD4- or CD86-positive, in contrast to the low expression level of CD1a, CD25, CD80 and CD95 (Fig. 1). On the other hand, MDDC lacked the expression of CD14 and resembled mature dendritic cells, as judged by the expression of the differentiation marker CD83. The CD86-, CD4-, CD80-, CD1a- and CD95-positive cells in MDDC cultures ranged between 35% and 90%, whereas CD25 expression was relatively low (mean percentage positive cells±SEM: 10·7±2·7%). The detected differences in the expression of surface markers clearly indicated that, under our culture conditions, monocytes could be easily differentiated into macrophages or mature dendritic cells. The latter population could also be verified on the basis of morphological criteria, as we observed typical adherent aggregates of dendritic cells with fine membrane projections.
Figure 1.
Expression of surface receptor markers on MDM and MDDC. Following a 7-day differentiation period of monocytes in vitro, MDM and MDDC were stained with monoclonal antibodies to different CD antigens or with isotype controls and were subjected to flow cytometric analysis. Barograms represent the mean percentage positive cells with SE bars. n reflects the number of different donors tested.
Effect of IL-16 on surface receptors expression
Stimulation of MDM or MDDC for 5–72 hr with rIL-16 resulted in selective modulation of receptor expression that peaked at the 24 hr time-point (Table 1). No significant change in the level of expression of CD14, CD1a and CD95 could be observed in either cell population (data not shown). However, IL-16-stimulated MDM presented a two- to threefold increase in the mean percentage of cells expressing either CD25 or CD80. This was accompanied with a significant decrease in CD4 (Fig. 2) and, to a lesser extent, in CD86 expression (Table 1). In contrast, stimulation of MDDC with IL-16 resulted only in a significant up-regulation of CD25 and CD83 surface expression without any measurable effect on CD4 (Table 1 and Fig. 2). The down-regulation or the lack of modulation of CD4 expression by IL-16 in MDM or in MDDC, respectively, was also evident at the 48 and 72 hr time-points (data not shown). In addition, the specificity of the IL-16 effect was verified in two separate experiments by using 10 μg/ml of rat IL-5 to stimulate MDM and MDDC. This control recombinant protein did not induce any detectable effect on surface receptor expression or on the parameter of cytokine release described below.
Table 1.
Changes in surface receptor expression in MDM and MDDC following 24-hr stimulation with IL-16 (10 μg/ml)
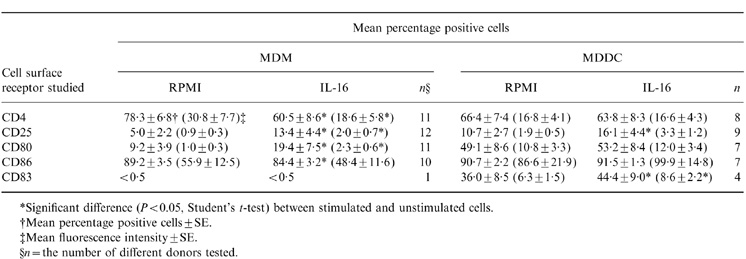
*Significant difference (P < 0.05, Student’s t-test) between stimulated and unstimulated cells.
†Mean percentage positive cells ± SE.
‡Mean fluorescence intensity ± SE.
§ n = the number of different donors tested.
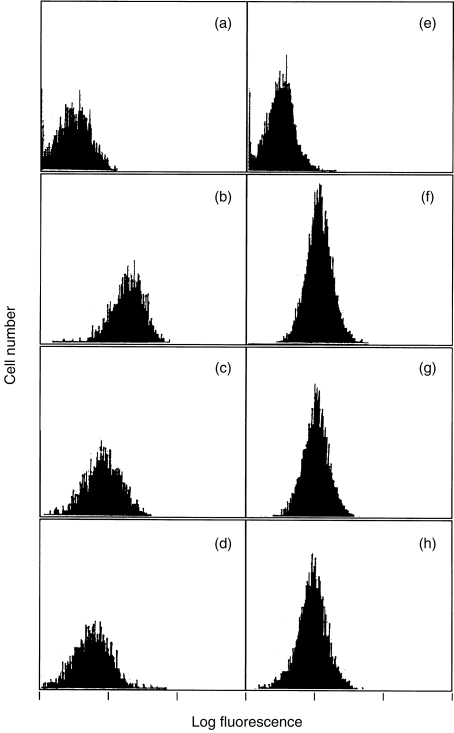
Figure 2.
Effect of rIL-16 on CD4 expression in monocyte-derived macrophages (a–d) and dendritic cells (e–h). Following a 24-hr incubation in the absence (a, b, e and f) or presence of 1 μg/ml (c and g) and 10 emsp14;μg/ml (d and h) of rIL-16, cells were stained either with murine anti-CD4–PE (b–d and f–h) or with isotype-matched control (a and e). Histograms shown are data on cells from one representative donor.
Effect of IL-16 on CD4 gene expression
The stable down-regulation of CD4 in IL-16-treated MDM prompted us to examine whether this effect could be observed at the mRNA level. Total RNA was extracted from unstimulated or stimulated MDM, at 2 or 24 hr post-stimulation, and CD4 gene expression was quantified by RT-PCR. Results shown in Fig. 3, using cells prepared from five different donors, demonstrate significant down-regulation of CD4 mRNA expression, with a mean percentage inhibition of 49% or 55% following, respectively, a 2- or 24-hr treatment with 10 μg/ml of rIL-16. No such effect could be observed in MDDC cultures from three separate donors (data not shown).
Figure 3.
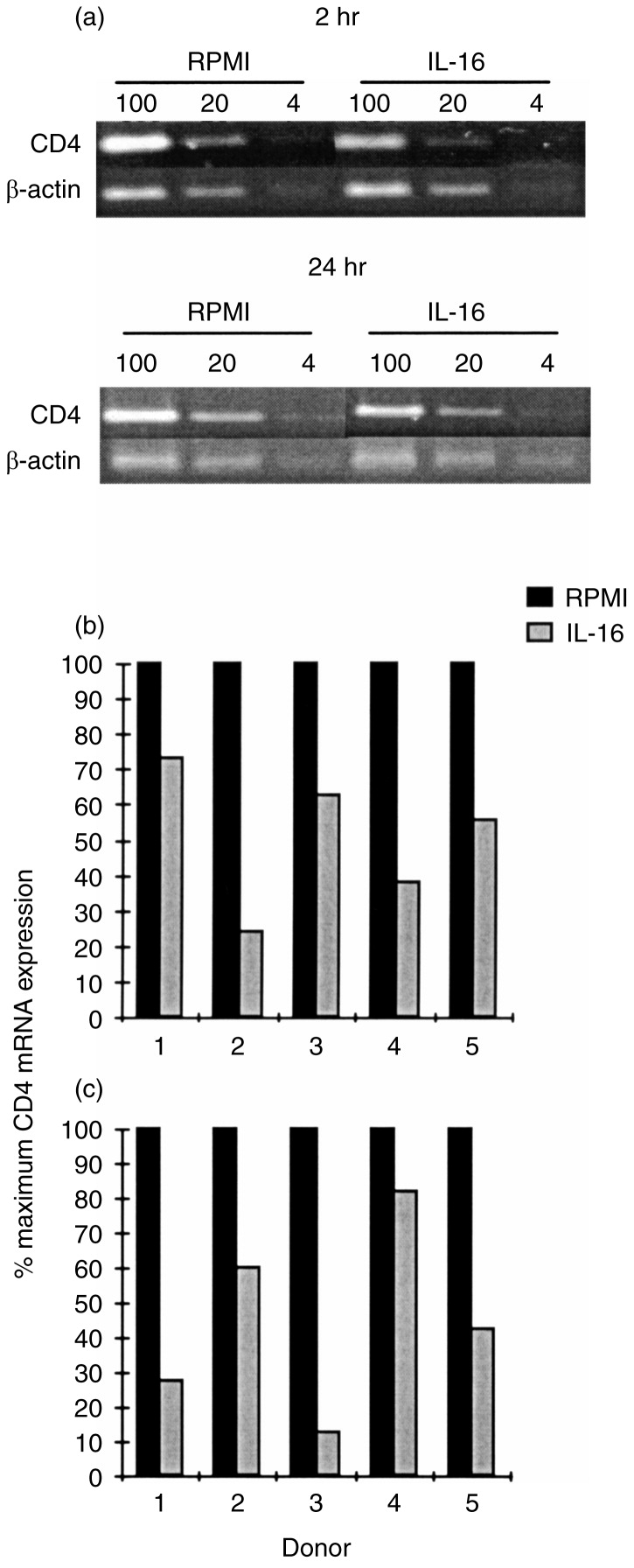
Regulation by IL-16 of CD4 mRNA expression in MDM. Following 2 and 24-hr culture of MDM in the absence or presence of IL-16, total RNA was extracted and subjected to RT-PCR using specific primers for CD4 and β-actin. (a) Representative results of RT-PCR analysis on RNA samples (100, 20 and 4 ng) from MDM of one donor. (b) Relative CD4 mRNA expression in MDM from five different donors following 2-hr and (c) 24-hr stimulation with IL-16.
Effects of cytokines on CCR5 and CXCR4 gene expression
We evaluated the effects of IL-16 or/and IL-2 on chemokine receptor gene expression in MDM and MDDC. A 2-hr stimulation of MDM with IL-2, IL-16 or a combination of the two cytokines significantly inhibited CCR5 mRNA accumulation by 42%, 56% and 67%, respectively (Fig. 4). Similar results were observed when the stimulation period was extended to 24 hr, although the inhibitory effect of IL-16 alone, which was noted on cells from four out of five donors tested, did not attain statistical significance (Fig. 4). The regulation of CCR5 gene expression in MDDC was minimal and a significant inhibition (22%) was only detected following a 2-hr stimulation with rIL-16 (data not shown).
Figure 4.
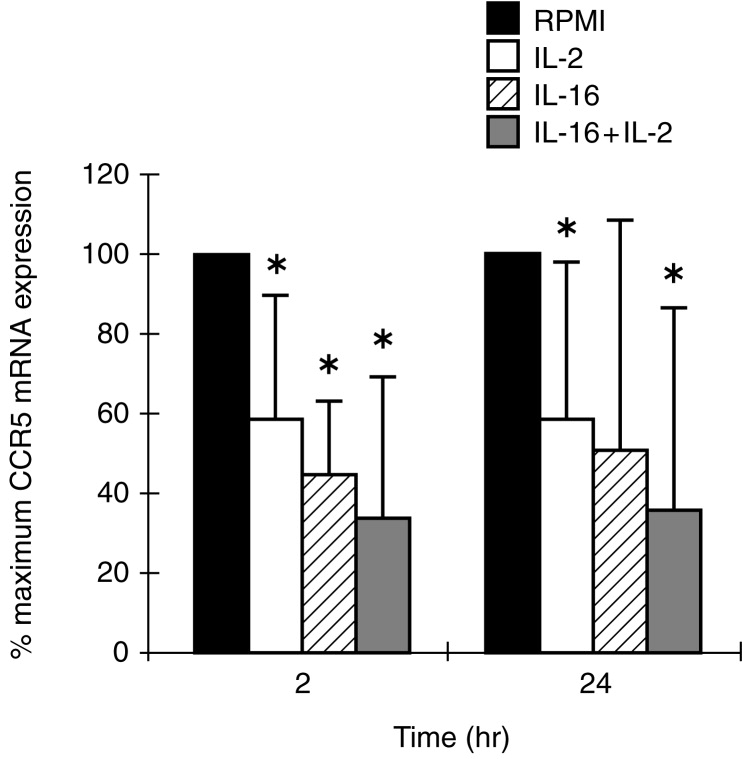
Regulation of CCR5 mRNA expression in MDM following 2 and 24-hr stimulation with IL-2, IL-16 or a combination of the two cytokines. Total RNA, extracted from non-treated (RPMI) and cytokine-treated MDM, was subjected to RT-PCR analysis using specific primers for CCR5 and for β-actin. Barograms represent the means with SE bars of percentage maximum CCR5 mRNA expression from five different donors. *P < 0·05 versus non-treated controls.
Stimulation for 2 hr of MDM (Fig. 5) or MDDC (Fig. 6) with IL-2 had no modulatory effect on the baseline level of CXCR4 gene expression. In contrast, stimulation of either cell population with rIL-16, in the absence or presence of IL-2, resulted in a significant inhibition of CXCR4 mRNA accumulation, and this was more dramatic in MDM than in MDDC (Figs 5 and 6). These effects were transient as no significant modulation of CXCR4 expression could be detected after 24-hr stimulation with the two cytokines, used separately or in combination (data not shown).
Figure 5.
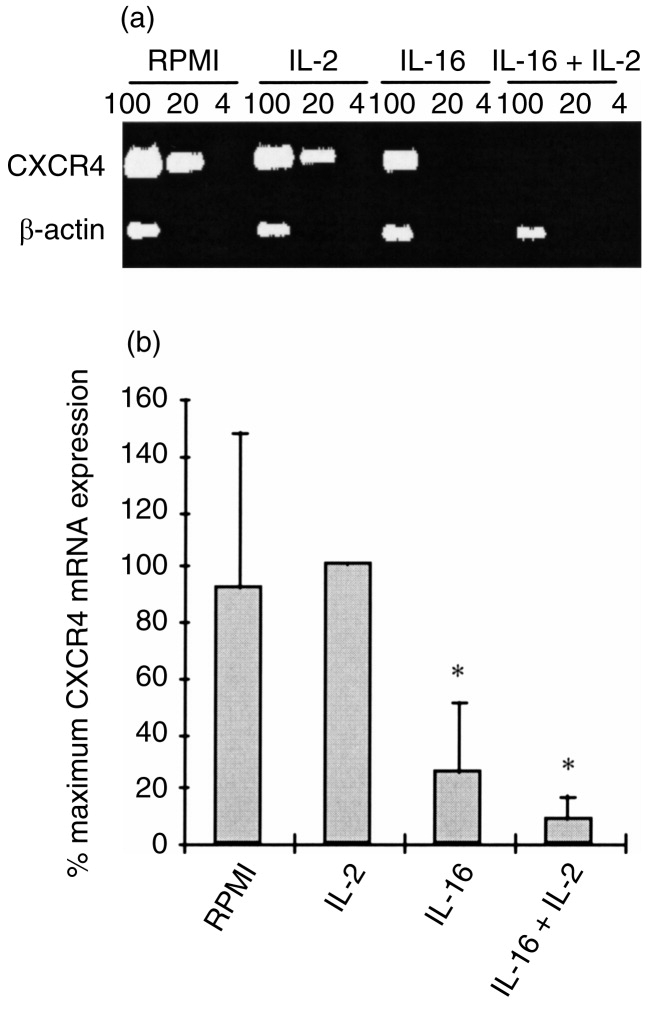
Regulation of CXCR4 mRNA expression in MDM following 2 hr stimulation with IL-2, IL-16 or a combination of the two cytokines. (a) Representative results of RT-PCR analysis on RNA samples (100, 20 and 4 ng) from MDM of a single donor using specific primers for CXCR4 and for β-actin. (b) Relative CXCR4 mRNA expression in non-treated and cytokine-treated MDM represented as means with SE bars of percentage maximum gene expression from five different donors. P < 0·05 versus non-treated controls.
Figure 6.
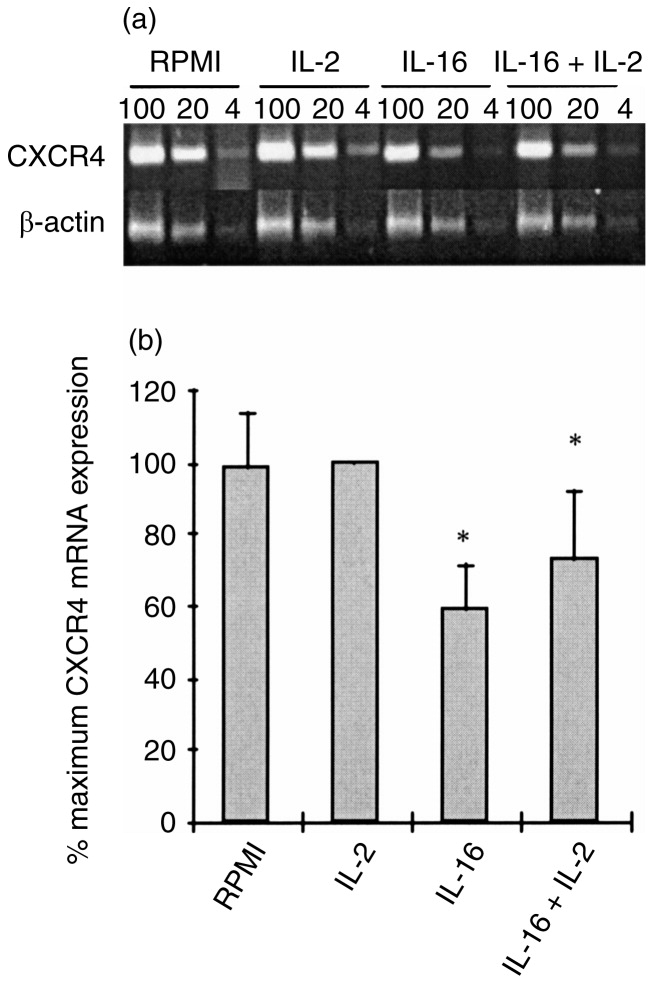
Regulation of CXCR4 mRNA expression in MDDC following 2 hr stimulation with IL-2, IL-16 or a combination of the two cytokines. (a) Representative results of RT-PCR analysis on RNA samples (100, 20 and 4 ng) from MDDC of a single donor using specific primers for CXCR4 and for β-actin. (b) Relative CXCR4 mRNA expression in non-treated and cytokine-treated MDDC represented as means with SE bars of percentage maximum mRNA expression from five different donors. P < 0·05 versus non-treated controls.
Effect of IL-16 on cytokines production
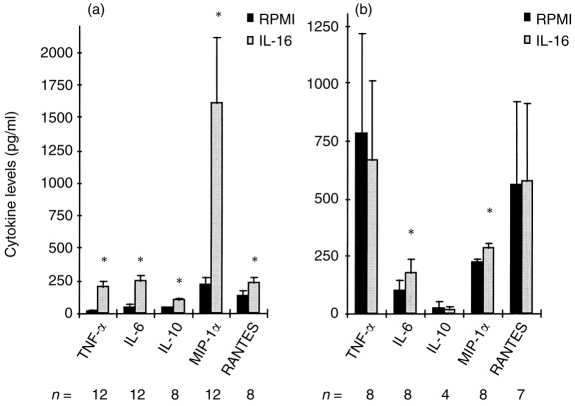
MDM and MDDC were incubated for 24 hr with rIL-16 and supernatants were then collected for titration of different cytokines and chemokines. Among the interleukins tested, IL-12 was always below the detection limit (<5 pg/ml) in either non-stimulated or IL-16-stimulated supernatants of MDM and MDDC cultures from seven separate donors. However, results shown in Fig. 7a demonstrate a significant increase in MIP-1α release in IL-16-stimulated MDM. This was accompanied by a significant up-regulation, although of smaller magnitude, in RANTES, TNF-α, IL-6 and IL-10 secretion. Moreover, MDM displayed a high spontaneous release of IL-1RA (mean±SEM: 57 315±6735 pg/ml) that was significantly up-regulated following stimulation with rIL-16 (mean±SEM: 72 747±8824 pg/ml). On the other hand, stimulation of MDDC with rIL-16 did not induce a significant increase in the secreted levels of RANTES, IL-10, TNF-α (Fig. 7b) or IL-1RA (data not shown). However, the levels of IL-6 and MIP-1α were found to be significantly elevated in IL-16-stimulated MDDC compared with unstimulated cultures (Fig. 7b). Finally, it is worth pointing out that the high spontaneous TNF-α levels detected in MDDC cultures were mainly attributed to the exogenous recombinant cytokine added to induce cellular differentiation.
Figure 7.
Profile of cytokines induced in cultures of MDM (a) and MDDC (b) following 24-hr stimulation with IL-16. Barograms represent the means with SE bars of the levels of cytokines in supernatants of unstimulated (RPMI) and IL-16-stimulated cultures from 4–12 different donors (n =). *P < 0·05 versus controls.
DISCUSSION
Macrophages and dendritic cells are integral components of the immune system capable of regulating immune responses via antigen presentation, cytokine secretion and the delivery of costimulatory signals for T-cell activation.17,18 The present study demonstrates that rIL-16 can interact with either macrophages or with dendritic cells, and can induce the modulation of certain parameters relevant to the functional activities of the two cell types. The need for relatively high concentrations (10 μg/ml) of rIL-16 to achieve measurable effects in vitro is attributed to the low content (2–10%) of the active homotetrameric form in the bacterially derived recombinant cytokine.13
Certain cytokines, such as interferon-γ (IFN-γ) and IL-10, have been reported to regulate the expression of costimulatory molecules on APC.19–21 Our findings demonstrate, for the first time, the capacity of the chemoattractant cytokine IL-16 to modulate CD80 and CD86 expression in MDM but not in MDDC. Indeed, differential regulation between both types of cells has already been reported for CD80, which was found to be up-regulated in macrophages but to be down-regulated in Langerhans’ cells following stimulation with IL-10 and IFN-γ.19,21 Furthermore, the signals transduced following CD4 binding on MDM by rIL-16 appear to enhance CD80 but to suppress CD86 expression. Other cytokines, such as IL-10, IL-4 and TNF-α, have also been shown to exert differential effects on CD80 and CD86 expression in monocytes.21 It has been suggested that these two costimulatory molecules influence T-helper (Th) cell differentiation into Th1 or Th2 cell subsets.22 In this regard, CD86 has been implicated in the development of IL-4-producing cells23 while CD80 appears more potent in inducing IFN-γ secretion by T cells.24 Thus, it will be of interest to determine whether IL-16 could affect, either directly or indirectly via macrophages, the differentiation of Th cell populations.
The ability of rIL-16 to enhance CD25 expression has been previously observed on T cells.3 Our results extend this finding to show a similar effect on both MDM and MDDC. Although the role of CD25 is not yet clearly established in dendritic cells, up-regulation of this receptor as well as of CD83 has been associated with dendritic cells maturation.25 Thus, based on our findings demonstrating the ability of rIL-16 to up-regulate CD25 and CD83 expression in MDDC, it is tempting to suggest that this cytokine might be an enhancing factor for dendritic cell maturation. Moreover, it will be of interest to examine, in future studies, whether rIL-16 could induce the expression of the β and γ chains of the IL-2 receptor, which have been reported to be lacking in dendritic cells.26 In this regard, an up-regulation by IL-16 of the IL-2 receptor β chain has already been observed in T lymphocytes.6
The down-regulation of CD4 expression by rIL-16 in MDM but neither in MDDC nor in T lymphocytes, could imply different signal transduction pathways mediated by the same receptor in different cell populations. Differential modulation of the expression of one defined receptor by the same stimulus in different cell types has been reported previously. For instance, CD4 cross-linking by anti-CD4 monoclonal antibodies or by HIV envelope glycoprotein gp120 was found to up-regulate CD95 ligand expression in monocytes but not in T lymphocytes.27 Furthermore, this selective effect of rIL-16 in MDM could not simply be explained on the basis of higher receptor expression on macrophages, as T cells are known to express much higher levels of CD4 than MDM. Although the mechanism of down-regulation of CD4 in T cells, by other ligands, was found to involve the dissociation of CD4 with the protein kinase p56lck,28 this mechanism cannot explain the observed IL-16 effect in MDM, which are known to lack p56lck kinase activity.28 In addition, the finding that CD4 down-regulation was stable and detectable at the mRNA level, argues against internalization of the receptor as a major mechanism of the IL-16-induced effect.
Macrophages and dendritic cells have been shown to express the chemokine receptors CCR5 and CXCR4, which were identified as co-receptors for macrophage-tropic and T-cell tropic HIV-1 strains, respectively.8,9 Regulation of the expression of chemokine receptors has been observed with different cytokines and with HIV antigens.29,30 The ability of IL-16 to induce rapid and sustained down-regulation of CCR5 mRNA expression in MDM is similar to the effect observed with agents known to increase the cellular level of cyclic adenosine monophosphate.31 Whether the IL-16-induced down-regulation of CCR5 gene expression in MDM, and to a lesser extent in MDDC, is mediated via the adenosine monophosphate pathway, would need to be addressed in future studies.
Inhibition of CCR5 mRNA accumulation was also observed in MDM stimulated with either IL-2 or with the combination of IL-2 and IL-16. These findings agree with a recent report demonstrating the capacity of IL-2 to down-regulate CCR5 surface expression in macrophages.32 Although, in this latter study, continuous culture of monocytes for 10 days with IL-2 resulted in CD4 down-regulation, our results indicate that stimulation of already differentiated macrophages with the same cytokine had no significant effect on CD4 expression. On the other hand, an effect of IL-2 on CXCR4 mRNA accumulation was lacking in MDM and in MDDC. In contrast, IL-16 signalling via CD4, in both cell populations, resulted in a rapid but reversible down-regulation of CXCR4 gene expression that was not significantly modified by the co-presence of IL-2. However, it would be unlikely that this transient effect on the level of gene expression could induce a considerable down-regulation of CXCR4 surface expression. Nevertheless, our results suggest that signalling through the IL-2 receptor selectively modulates CCR5 expression, whereas signalling via CD4 can modulate, to a variable extent, both CCR5 and CXCR4 gene expression. On the other hand, the implications for some of the selective effects of IL-16 in MDM but not in MDDC need to be considered. For example, through its capacity to induce sustained CD4 and CCR5 down-regulation in MDM, rIL-16 may have a role to play in protecting macrophages, but not dendritic cells, against HIV-1 infection. Similarly, the selective modulation of the expression of costimulatory molecules in MDM suggests a potential regulatory effect of the cytokine on T-cell responses that are dependent on antigen presentation by macrophages. However, it is essential to determine, in future studies, the relevance of these findings to the immune and antiviral responses in vivo.
The release of cytokines by IL-16-stimulated MDM was of relatively low magnitude compared with the previously reported levels induced in macrophages by another CD4 ligand, gp120.33 Moreover, rIL-16 did not appear to trigger significant release of cytokines in MDDC, except for MIP-1α and IL-6. These results confirm earlier reports on the low capacity of mature dendritic cells to secrete cytokines.34 In addition, the profile of IL-16-induced cytokines in APC cultures, including the absence of IL-12 release, is unlikely to be a major factor contributing to the previously suggested inflammatory property of IL-161 or to a potential capacity to drive Th1 responses. Nevertheless, with the limited number of tested cytokines in our study, it is not possible to delineate the spectrum of cytokines induced by IL-16 or to rule out a potential role for other cytokines in mediating some of its biological activities.
It is of interest to point out that CD4 cross-linking with its natural ligand IL-16 appears to result in a different spectrum of activities when compared with other CD4 ligands (gp120 and CD4 monoclonal antibodies). This has already been observed in normal T cells where stimulation with anti-CD4 antibodies or with gp120, in contrast to stimulation with rIL-16, resulted in an increase in phosphatidyl serine synthesis.35 In addition, the level of HIV replication and of activation-induced cell death in infected T lymphocytes was found to decrease following stimulation with IL-165,7,13 but to increase upon CD4 ligation with gp120.36,37 Taken together, these results substantiate the findings that the CD4 molecule can transmit either activating or inhibiting intracellular signals depending on the CD4 ligand used.35 Finally, the protective effect of IL-16 against HIV-1 replication in PBMC7,13 together with our findings on the capacity of this cytokine to down-regulate HIV-1 receptor and co-receptors in MDM, suggest an important role for IL-16 in protecting reservoir cells against HIV-1 infection. However, the efficacy of IL-16 to inhibit virus replication in APC still needs to be established and is currently being investigated in our laboratory.
REFERENCES
- 1.Center DM, Kornfeld H, Cruikshank WW. Interleukin 16 and its function as a CD4 ligand. Immunol Today. 1996;17:476. doi: 10.1016/0167-5699(96)10052-i. [DOI] [PubMed] [Google Scholar]
- 2.Cruikshank WW, Greenstein JL, Theodore AC, Center DM. Lymphocyte chemoattractant factor induces CD4-dependent intracytoplasmic signalling in lymphocytes. J Immunol. 1991;146:2928. [PubMed] [Google Scholar]
- 3.Cruikshank WW, Berman JS, Theodore AC, Bernardo J, Center DM. Lymphokine activation of T4+ T lymphocytes and monocytes. J Immunol. 1987;138:3817. [PubMed] [Google Scholar]
- 4.Theodore AC, Center DM, Nicoll J, Fine G, Kornfeld H, Cruikshank WW. CD4 ligand IL-16 inhibits the mixed lymphocyte reaction. J Immunol. 1996;157:1958. [PubMed] [Google Scholar]
- 5.Cruikshank WW, Lim K, Theodore AC, et al. IL-16 inhibition of CD3-dependent lymphocyte activation and proliferation. J Immunol. 1996;157:5240. [PubMed] [Google Scholar]
- 6.Parada NA, Center DM, Kornfeld H, et al. Synergistic activation of CD4+ T cells by IL-16 and IL-2. J Immunol. 1998;160:2115. [PubMed] [Google Scholar]
- 7.Amiel C, Darcissac E, Truong M-J, et al. Interleukin-16 (IL-16) inhibits human immunodeficiency virus replication in cells from infected subjects and serum IL-16 drops with disease progression. J Infect Dis. 1998;179:83. doi: 10.1086/314550. [DOI] [PubMed] [Google Scholar]
- 8.Zaitseva M, Blauvelt A, Lee S, et al. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nature Med. 1997;12:1369. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]
- 9.Granelli-Piperno A, Moser B, Pope M, et al. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gendelman HE, Orenstein JM, Baca LM, et al. The macrophage in the persistence and pathogenesis of HIV infection. AIDS. 1989;3:475. doi: 10.1097/00002030-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Graziosi C, Soudeyns H, Rizzardi GP, et al. Immunopathogenesis of HIV infection. AIDS Res Hum. Retrovir. 1998;14:S135. [PubMed] [Google Scholar]
- 12.Krautwald S. IL-16 activates the SAPK signaling pathway in CD4+ macrophages. J Immunol. 1998;160:5874. [PubMed] [Google Scholar]
- 13.Idziorek T, Billaut-Mulot O, Hermann E, et al. Recombinant human IL-16 inhibits HIV-1 replication and protects against activation-induced cell death (AICD) Clin Exp Immunol. 1998;112:84. doi: 10.1046/j.1365-2249.1998.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermann E, Idziorek T, Kusnierz J-P, Mouton Y, Capron A, Bahr GM. Role of nitric oxide in the regulation of lymphocyte apoptosis and HIV-1 replication. Int J Immunopharmacol. 1997;19:387. doi: 10.1016/s0192-0561(97)00060-x. [DOI] [PubMed] [Google Scholar]
- 15.Yi Y, Rana S, Turner JD, Gaddis N, Collman RG. CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:772. doi: 10.1128/jvi.72.1.772-777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fear WR, Kesson AM, Naif H, Lynch GW, Cunningham AL. Differential tropism and chemokine receptor expression of human immunodeficiency virus type 1 in neonatal monocytes, monocyte-derived macrophages, and placental macrophages. J Virol. 1998;72:1334. doi: 10.1128/jvi.72.2.1334-1344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doherty TM. T-cell regulation of macrophage function. Curr Opin Immunol. 1995;7:400. doi: 10.1016/0952-7915(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 18.Hart DNJ. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245. [PubMed] [Google Scholar]
- 19.Ozawa H, Aiba S, Nakagawa, Tagami H. Interferon-gamma and interleukin-10 inhibit antigen presentation by Langerhans cells for T helper type 1 cells by suppressing their CD80 (B7-1) expression. Eur J Immunol. 1996;26:648. doi: 10.1002/eji.1830260321. [DOI] [PubMed] [Google Scholar]
- 20.Buelens C, Willems F, Delvaux A, et al. Interleukin-10 differentially regulated B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur J Immunol. 1995;25:2668. doi: 10.1002/eji.1830250940. [DOI] [PubMed] [Google Scholar]
- 21.Creery WD, Diaz-Mitoma F, Filion L, Kumar A. Differential modulation of B7-1 and B7-2 isoform expression on human monocytes by cytokines which influence the development of T helper cell phenotype. Eur J Immunol. 1996;26:1273. doi: 10.1002/eji.1830260614. [DOI] [PubMed] [Google Scholar]
- 22.Kuchroo VK, Prabhu Das M, Brown JA, et al. B7-1 and B7-2 costimulatory molecules activate preferentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 23.Ranger AM, Prabhu Das M, Kuchroo VK, Glimcher LH. B7-2 (CD86) is essential for the development of IL-4-producing T cells. Int Immunol. 1996;8:1549. doi: 10.1093/intimm/8.10.1549. [DOI] [PubMed] [Google Scholar]
- 24.Fleischer J, Soeth E, Reilling N, et al. Differential expression and function of CD80 (B7-1) and CD86 (B7-2) on human peripheral blood monocytes. Immunology. 1996;89:592. doi: 10.1046/j.1365-2567.1996.d01-785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delgado E, Finkel V, Baggiolini M, Mackay CR, Steinman RM, Granelli-Piperno A. Mature dendritic cells respond to SDF-1, but not to several β-chemokines. Immunobiol. 1997/1998;198:490. doi: 10.1016/s0171-2985(98)80073-9. [DOI] [PubMed] [Google Scholar]
- 26.Kronin V, Vremec D, Shortman K. Does the IL-2 receptor α chain induced on dendritic cells have a biological function. Int Immunol. 1998;10:237. doi: 10.1093/intimm/10.2.237. [DOI] [PubMed] [Google Scholar]
- 27.Oyaizu N, Adachi Y, Hashimoto F, et al. Monocytes express Fas ligand upon CD4 cross-linking and induce CD4+ T cells apoptosis. J Immunol. 1997;158:2456. [PubMed] [Google Scholar]
- 28.Geleziunas R, Morin N, Wainberg MA. Mechanisms of down-modulation of CD4 receptors on the surface of HIV-1 infected cells. CR Acad Sci Paris. 1996;319:653. [PubMed] [Google Scholar]
- 29.Wang J, Roderiquez G, Oravecz T, Norcross MA. Cytokine regulation of human immunodeficiency virus type 1 entry and replication in human monocytes/macrophages through modulation of CCR5 expression. J Virol. 1998;72:7642. doi: 10.1128/jvi.72.9.7642-7647.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueda H, Howard OMZ, Grimm MC, et al. HIV-1 envelope gp41 is a potent inhibitor of chemoattractant receptor expression and function in monocytes. J Clin Invest. 1998;102:804. doi: 10.1172/JCI3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thivierge M, Le Gouill C, Tremblay MJ, Stankova J, Rola-Pleszczynski M. Prostaglandin E2 induces resistance to human immunodeficiency virus-1 infection in monocyte-derived macrophages: downregulation of CCR5 expression by cyclic adenosine monophosphate. Blood. 1998;92:40. [PubMed] [Google Scholar]
- 32.Kutza J, Hayes MP, Clouse KA. Interleukin-2 inhibits HIV-1 replication in human macrophages by modulating expression of CD4 and CC-chemokine receptor-5. AIDS. 1998;12:F59–F64. doi: 10.1097/00002030-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Merrill JE, Koyanagi Y, Chen IS.Y. Interleukin-1 and tumor necrosis factor α can be induced from mononuclear phagocytes by human immunodeficiency virus type 1 binding to the CD4 receptor. J Virol. 1989;63:4404. doi: 10.1128/jvi.63.10.4404-4408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghanekar S, Zheng L, Logar A, et al. Cytokine expression by human peripheral blood dendritic cells stimulated in vitro with HIV-1 and herpes simplex virus. J Immunol. 1996;157:4028. [PubMed] [Google Scholar]
- 35.Dumaurier M-J, Pelassy C, Breittmayer J-P, Aussel C. Regulation of the serine-base exchange enzyme system by CD4: effects of monoclonal antibodies, jacalin, interleukin 16 and the HIV membrane protein gp120. Biochem J. 1998;329:49. doi: 10.1042/bj3290049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Than S, Oyaizu N, Tetali S, Romano J, Kaplan M, Pahwa S. Upregulation of human immunodeficiency virus (HIV) replication by CD4 cross-linking in peripheral blood mononuclear cells of HIV-infected adults. J Virol. 1997;71:6230. doi: 10.1128/jvi.71.8.6230-6232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oyaizu N, Mccloskey TW, Than S, Hu R, Kalyanaraman VS, Pahwa S. Cross-linking of CD4 molecules upregulates Fas antigen expression in lymphocytes by inducing interferon-γ and tumor necrosis factor-α secretion. Blood. 1994;84:2622. [PubMed] [Google Scholar]