Abstract
The influence of complement receptor type 1 (CR1; CD35) and decay-accelerating factor (DAF; CD55), both down-regulators of complement activation, on the complement receptor type 2- (CR2) mediated alternative pathway (AP) activation of complement on normal B cells, was assessed. The data indicate that, while neither DAF nor CR1 hinder the function of the AP convertase formed on CR2, CR1 plays a significant role in the remodelling of C3b fragments, generated by the convertase and deposited at secondary acceptor sites on the B-cell surface, such that they become suitable ligands for CR2. The significance of this finding is briefly discussed.
The capacity of complement receptor type 2 (CR2; CD21), on human B cells and cell lines of B-cell origin, to activate complement via the alternative pathway (AP) has been clearly established. Current evidence indicates that the AP convertase is formed at the ligand-binding site of CR2 and consists of hydrolysed C3 (iC3), factor B and Properdin.3 In our original study – using polyclonal anti-C3c and anti-C3d reagants to detect deposition of C3 fragments following in vitro AP activation – we obtained indirect evidence that C3b fragments, generated by this convertase, may become covalently deposited at secondary acceptor sites on the cell surface.1
A comparative study of normal B cells and the Epstein–Barr virus-positive (EBV-positive) Burkitt’s lymphoma cell-line, Raji, revealed that, while the efficiency of C3 fragment deposition did not differ significantly between the two cell types, there was a marked difference in the type of fragment observed on the cell surfaces.4 Thus, the predominant fragment associated with normal B cells was found to be C3dg, whereas Raji cells primarily bore C3b/iC3b fragments. As Raji cells do not express CR1 (CD35), which is the only known cofactor for factor I-mediated cleavage of iC3b to C3c and C3dg,5 it was concluded that the difference observed in the type of fragment deposited may be accounted for by the fact that CR1, on normal B cells, participates in the remodelling of C3b fragments deposited at secondary acceptor sites on these cells.
The main purposes of the present study were (i) to re-examine the question of secondary attachment of C3, by determining the precise stoichiometry of C3 fragment deposition with a monoclonal anti-C3d Ab, and (ii) to examine the influence of CR1 on the degree and nature of C3 fragment deposition, following in vitroAP activation at the B-cell surface. In addition, we deemed it appropriate to investigate whether the complement regulatory protein, decay-accelerating factor (DAF; CD55), had any modulatory influence on the activity of the CR2-generated AP convertase, given that both B cells and Raji cells express this glycosyl-phosphatidylinositol-linked (GPI-linked) protein.4
The studies were performed with Raji cells and normal B cells, in peripheral blood leukocyte (PBL) preparations from normal healthy donors. In the first phase of the study, the cells were submitted to in vitro AP activation in 25% AB-positive pooled normal human serum (NHS) containing 20 mm EGTA/4·4 mm MgCl2, or 10 mm EDTA as negative control. The cells were then probed with a fluorescein isothiocyanate- (FITC) conjugated murine anti-huC3d mAb, of known specific fluorescent activity, and the number of mAb bound per cell was determined by flow cytometry, using Quantum 25 Standard FITC beads to calibrate the Fl1 signal.6 CR2 expression was measured concurrently on untreated cells using the FITC-conjugated immunoglobulin G2a (IgG2a) anti-CR2 mAb, HB135. In the second phase, some of the cells were pretreated with F(ab′)2 fragments of the function blocking anti-CR1 mAb, 3D9 (donated by Dr John O’Shea, Frederick Cancer Research and Development Center, Frederick, MD)7 and then, following AP activation, the deposited C3 fragments were detected using FITC-conjugated IgG preparations of rabbit–anti-huC3c and anti-huC3d Ab, reactive with C3, C3b, iC3b, and C3c, or C3dg, respectively. Finally, the influence of DAF was assessed by pretreating PBL with phosphatidylinositol-specific (PI-specific) phospholipase C (PLC), to cleave the DAF’s GPI anchor, prior to measurement of AP-dependent C3 fragment deposition, with rabbit–anti-huC3d, and parallel determination of residual DAF expression using FITC-labelled BRIC216 mAb. The data were derived by analysis of 10 000 ungated Raji cells or PBL, or 3000 live-gated lymphocytes, using R-phycoerythrin (R-PE)-conjugated mouse–anti-huCD19 mAb to identify the normal B-cell subpopulation.
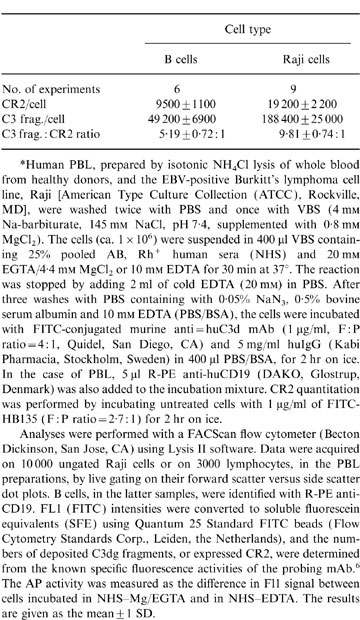
Following in vitro AP activation, an average of 49 200±6900 and 188 400±25 000 anti-C3d mAb molecules were bound to normal B cells and Raji cells, respectively (Table 1). Assuming that the mAb reacts monovalently with its antigen, the figure obtained for C3 fragment deposition on B cells corresponds well with that previously derived (30 000–50 000 per cell)1 using polyclonal anti-C3d as a probe. The ratios of fragment deposition to CR2 expression were 5·2:1, for normal B cells, and 9·8:1 for Raji cells (Table 1). Even assuming that all the CR2 binding sites bear AP convertases, and that the relevant epitope in the C3dg region of the iC3 component of the convertase is fully accessible to the anti-C3d mAb, this finding implies that 80–90% of the detectable C3 fragments are deposited at secondary acceptor sites on these cells; either on the CR2 molecules themselves or on other cell surface glycoproteins. Furthermore, the data indicate that C3 fragment deposition occurs with a significantly greater efficiency (1·9-fold, P < 0·001) on Raji cells than on B cells. A similar trend (1·5-fold difference) was previously observed, using polyclonal anti-C3d as a probe, although the difference, in that case, was not significant; reflecting, perhaps, a lack of precision in measurements with the polyclonal reagent. The question of whether the difference in CR2 performance on Raji cells versus normal B cells reflects greater stability of the AP convertase on Raji cells, or a higher efficiency of secondary deposition of C3b fragments generated by the convertase, remains to be resolved.
Table 1.
C3 fragment deposition on normal B cells, Raji and CR2-transfected K562 cells*
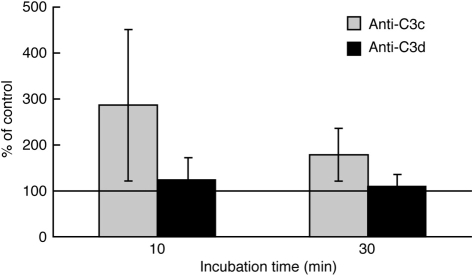
Pre-incubation of PBL with 3D9 resulted in a significant increase in the reactivity of the B cells with FITC anti-C3c, after both 10 and 30 min of AP activation, without significantly altering their reactivity with FITC anti-C3d Ab (Fig. 1). Similar pretreatment of Raji cells did not alter their reactivity with anti-C3c (data not shown). The lack of change in B-cell reactivity with anti-C3d, which recognizes all the membrane-bound C3 fragments (i.e. C3b, iC3b and C3dg) indicates that the efficiency of the AP convertase associated with CR2 is not altered by functional blockade of CR1. Thus it would appear that CR2 effectively protects the convertase against the decay acceleration and co-factor activities of CR1. Furthermore, this finding effectively rules out the possibility that the stability of the AP convertase on Raji cells is enhanced by the absence, from these cells, of CR1. On the other hand, the rise in reactivity with anti-C3c indicates that an increased proportion of unprotected C3 fragments, bound to secondary acceptor sites on the cell surface, remain as C3b or iC3b, confirming our previous assumption that the presence of CR1 on the cell surface is required for the conversion of these fragments to their terminal cleavage product, C3dg.
Figure 1.
 |
1 |
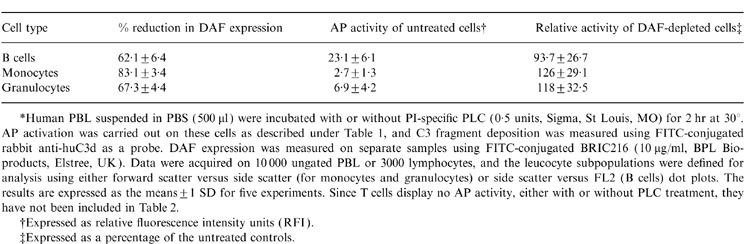
Treatment of PBL with PLC resulted in the removal of 62, 67 and 83% of the membrane bound DAF from B cells, granulocytes and monocytes, respectively (Table 2). On the other hand, the AP-activating capacity of B cells was unaffected by this depletion, while the low-grade activation, seen with the other two cell subpopulations, displayed a marginal, but non-significant, increase. Thus, DAF does not appear to regulate the activity of the CR2-generated AP convertase.
Table 2.
.The influence of DAF on the activity of the CR2-generated AP convertase*
In conclusion, it would appear that AP activation on normal B cells results in substantial deposition of C3b fragments at secondary acceptor sites on the cell surface, where they become degraded to C3dg fragments, under the influence of CR1 and factor I, and are, thereby, converted to a form capable of binding to unoccupied CR2 receptors. The physiological relevance of this phenomenon may be two-fold. On the one hand, it may provide a mechanism for enhancing the interaction of B cells and other CR2-bearing cells involved in an immune response, such as follicular dendritic cells or activated T cells. Evidence for this mechanism is provided in a recent study, using the murine B lymphoblastoid cell line, A20, or concanavalin A-stimulated murine peritoneal macrophages (which also activate the AP) as antigen-presenting cells, where it has been shown that the T-cell proliferative response to suboptimal antigen doses is significantly enhanced when the presenter cells bear C3 fragments on their surface.8 On the other hand, it could constitute a mechanism for cross-linking CR2, which is a co-stimulatory receptor for B-cell activation by antigen (reviewed in ref. 9), to C3 fragment-bearing B-cell glycoproteins (i.e. secondary acceptor molecules), with either positive or negative signalling activities (e.g. sIgM or Fc′R11b, respectively). The possibility that such interactions might play a role in modulating B-cell responsiveness to external stimuli remains to be investigated.
Glossary
Abbreviations
- AP
alternative pathway (of complement activation)
- C3
complement component 3
- CR1 and CR2
complement receptor types 1 and 2
- DAF
decay-accelerating factor
- Epstein–Barr virus
EBV
- FITC
fluorescein isothiocyanate
- hu
human
- NHS
normal human serum
- PBL
peripheral blood leukocytes
- PI
phosphatidylinositol
- PLC
phospholipase C
References
- 1.Marquart HV, Svehag S-E, Leslie RGQ. CR2 is the primary acceptor site for C3 during alternative pathway activation of complement on human peripheral B lymphocytes. J Immunol. 1994;153:307. [PubMed] [Google Scholar]
- 2.Budzko DB, Lachmann PJ, McConnel I. Activation of the alternative complement pathway by lymphoblastoid cell lines derived from patients with Burkitt’s lymphoma and infectious mononucleosis. Cell Immunol. 1976;22:98. doi: 10.1016/0008-8749(76)90011-3. [DOI] [PubMed] [Google Scholar]
- 3.Schwendinger MG, Spruth M, Schoch J, Dierich MP, Prodinger WM. A novel mechanism of alternative pathway complement activation accounts for the deposition of C3-fragments on CR2-expressing homologous cells. J Immunol. 1997;158:5455. [PubMed] [Google Scholar]
- 4.Marquart HV, Olesen EH, Johnson AA, Damgaard G, Leslie RGQ. A comparative study of normal B cells and the EBV-positive Burkitt’s lymphoma cell line, Raji, as activators of the complement system. Scand J Immunol. 1997;46:246. doi: 10.1046/j.1365-3083.1997.d01-122.x. [DOI] [PubMed] [Google Scholar]
- 5.Ross GD, Lambris JD, Cain JA, Newman SL. Generation of three different fragments of bound C3 with purified factor I or serum. I. Requirements for factor H vs. CR1 cofactor activity. J Immunol. 1982;129:2051. [PubMed] [Google Scholar]
- 6.Christensen J, Leslie RGQ. Quantitative measurement of Fc receptor activity on human peripheral blood monocytes and the monocyte-like cell line, U937, by laser flow cytometry. J Immunol Methods. 1989;132:211. doi: 10.1016/0022-1759(90)90032-q. [DOI] [PubMed] [Google Scholar]
- 7.O’shea JJ, Brown EJ, Seligman BE, Metcalf JA, Frank MM, Gallin JI. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. J Immunol. 1985;134:2580. [PubMed] [Google Scholar]
- 8.Kerekes K, Prechl J, Bajtay Z, Józsi M, Erdei A. A further link between innate and adaptive immunity: C3 deposition on antigen-presenting cells enhances the proliferation of antigen-specific T cells. Int Immunol. 1998;10:1923. doi: 10.1093/intimm/10.12.1923. [DOI] [PubMed] [Google Scholar]
- 9.Carroll MC. The role of complement and complement receptors in induction and regulation of immunity. Annu Rev Immunol. 1998;16:545. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]