Abstract
Pectic polysaccharide fraction (BR-2) containing pharmacologically active pectic polysaccharide, bupleuran 2IIc, which was prepared from a medicinal herb, the roots of Bupleurum falcatum L., was administered orally to C3H/HeJ mice for 7 consecutive days. Proliferative responses of spleen cells were enhanced in the presence of the purified pectic polysaccharide, bupleuran 2IIc, but another B-cell mitogen, lipopolysaccharide (LPS) did not give a similar effect. In vitro studies using spleen cells showed that bupleuran 2IIc also stimulated lymphocytes, depleted of adherent cells or T cells. Bupleuran 2IIc treatment increased subpopulation of CD25+ and surface immunoglobulin M-positive (sIgM+) lymphocytes. Non-specific immunoglobulin secretion of spleen cells treated with bupleuran 2IIc was increased according to the culture time, and coexistence of interleukin-6 (IL-6) enhanced the secretion more than that of bupleuran 2IIc alone. These results suggest that bupleuran 2IIc proliferates B cells in the absence of macrophages, and the resulting activated B cells are then induced into antibody-forming cells in the presence of IL-6. Among the structural region of bupleuran 2IIc, ramified region (PG-1), which consists of rhamnogalacturonan core rich in neutral sugar chain, showed the potent mitogenic activity suggesting it to be an active site. Mitogenic activity of bupleuran 2IIc was reduced in the presence of antipolysaccharide antibody (antibupleuran 2IIc/PG-1-IgG), which recognizes the ramified region of bupleuran 2IIc as the antigenic epitope. Mitogenic activity of bupleuran 2IIc was also reduced by the addition of β-d-GlcpA-(1→6)-β-d-Galp-(1→6)-d-Galp or β-d-GlcpA-(1→6)-d-Galp, which are a part of the epitopes of antibupleuran 2IIc/PG-1-IgG. These results suggest that the epitopes in bupleuran 2IIc act as active sites of the polysaccharide during mitogenic activity
INTRODUCTION
Recent observations have accumulated the facts that pectic polysaccharides including pectin from medicinal herbs express various in vitro and in vivo pharmacological activities in addition to the application for drug delivery.1 Therefore, it is possible to consider pectic polysaccharide, not only as one of the active ingredients of medicinal herbs, but also as medicines.
The roots of Bupleurum falcatum L. (Japanese name = Saiko) have been used in Chinese and Japanese herbal medicines for the treatment of chronic hepatitis, inflammatory diseases and ulcer of digestive organs. The pharmacologically active polysaccharides, bupleuran 2IIb and 2IIc,1–4 which have anticomplementary,2 macrophage Fc receptor up-regulating3 and antiulcer activities, were isolated from the hot-water extract of the roots of B. falcatum, and characterized as pectic polysaccharides. Therefore, these pectic polysaccharides are considered to be an important constituent of the immunopharmacological activity of B. falcatum L.
Bupleuran 2IIb and 2IIc consist of a galacturonan region, a ‘ramified’ region composed of a rhamnogalacturonan core having neutral sugar side chains, and a rhamnogalacturonan II-like region;7 the ‘ramified’ region has been considered to be an important part of the expression of these activities.2–4 Therefore, a polyclonal antibody (antibupleuran 2IIc/PG-1-IgG) against the ‘ramified’ region (PG-1) of bupleuran 2IIc was prepared and applied for analysis of absorption and tissue distribution of the polysaccharide after oral administration to mice.8 The results suggested that a part of bupleuran 2IIc was absorbed in the body from the digestive organs, and then mobilized into liver.8 The polysaccharide was also detected in Peyer’s patches of the mucosa-associated lymphoid tissue (MALT) on intestine.8 Because most herbal medicines are taken orally, it is assumed that some active ingredients in the medicines also transport to lymphoid tissue to interact with lymphocytes.
The major antigenic epitope against antibupleuran 2IIc/PG-1-IgG was characterized to be 6-linked galactosyl chains containing terminal glucuronic acid (GlcA) or 4-O-methyl-glucuronic acid (4-O-Me-GlcA), which were substituted to (1→3)-β-d-galactosyl chains in the ‘ramified’ region of bupleuran 2IIc.9
In the present paper, we studied the effect of pectin (bupleuran 2IIc) from Bupleurum falcatum L. on murine lymphocytes in vivo and in vitro. It is important to clarify which carbohydrate chains in pectic polysaccharides are responsible for expression of the pharmacological activities in order to study further mechanism of actions. Therefore, structural requirement of the polysaccharides for the expression of activity was also studied by using the antipolysaccharide antibody.
MATERIALS AND METHODS
Mice
Specific-pathogen-free C3H/HeJ female mice were purchased from SLC (Shizuoka, Japan) and used at 6–8 weeks of age.
Reagents
RPMI-1640 medium and Hanks’ balanced salt solution (HBSS) were obtained from Gibco (Grand Island, NY). Fetal bovine serum (FBS) was obtained from Cell Culture Laboratories (Cleveland, OH). Penicillin, streptomycin, and amphotericin B were from Flow Laboratories (Irvine, UK). Alamar Blue™ was obtained from Alamar Bio-Sciences Inc. (Sacramento, CA), and lipopolysaccharide (LPS) from Escherichia coli (O127:B8) and HEPES were from Sigma (St. Louis, MO).
Preparation of bupleuran 2IIc and subfractions
The roots of B. falcatum L. were purchased from Uchida Wakanyaku Co. Ltd. (Tokyo, Japan). A voucher specimen was deposited at the herbarium of Oriental Medicine Research Center of the Kitasato Institute. Bupleurans 2IIb and 2IIc were purified from the pectic polysaccharide fraction (BR-2) of the roots of B. falcatum by anion-exchange chromatography on diethylaminoethyl (DEAE)–Sepharose CL-6B (Pharmacia Fine Chemicals, Uppsala, Sweden) as described previously.5‘Ramified’ region (PG-1; rhamnogalacturonan core possessing side chains rich in neutral sugars) and PG-3 (oligogalacturonide fraction) were prepared from bupleuran 2IIc by endo-(1→4)-α-d-polygalacturonase digestion, as reported previously.5β-d-GlcpA-(1→6)-β-d-Galp-(1→6)-d-Galp and β-d-GlcpA-(1→6)-d-Galp from Acacia gum were prepared as reported by Tsumuraya et al.10β-d-Galp-(1→6)-β-d-Galp-(1→6)-d-Galp was isolated from the partial acid hydrolysate of larch wood arabinogalactan.10
Media
RPMI-1640 medium was supplemented with penicillin (100 U/ml), streptomycin (10 μg/ml) amphotericin B (0·25 μg/ml) and 12·5 mm HEPES. Furthermore, this medium was routinely supplemented with 5% heat-inactivated fetal calf serum (RPMI-1640–FCS). In all experiments, 5 × 10−5 m 2-mercaptoethanol (2-ME) was added to the medium.
Antibodies
The polyclonal antibody (antibupleuran 2IIc/PG-1-immunoglobulin G (IgG)) against the ‘ramified’ region (PG-1) of bupleuran 2IIc was generated and purified as described previously.8 Antibupleuran 2IIc/PG-1-IgG was purified by protein G-Sepharose and bupleuran 2IIc/PG-1 immobilized EAH–Sepharose.8 Anti-bupleuran 2IIc/PG-1-F(ab′)2 was prepared from antibupleuran 2IIc/PG-1-IgG by pepsin digestion.
Oral administration of sample
BR-2 (containing bupleuran 2IIb and bupleuran 2IIc, 250 mg/kg) was dissolved in distilled water, and aliquots (0·2 ml) of the solution were administered orally into mice. As the controls, the mice received distilled water alone instead of the sample solution.
Cell preparation and cell culture
Mice were killed by cervical amputation and their spleens were removed using aseptic techniques. The spleens were passed through a sterilized stainless sieve (150 mesh) to obtain a single-cell suspension. Erythrocytes in the cell suspension were destroyed by 0·75%NH4Cl in 0·017 m Tris–HCl (pH 7·6). Finally, the cells were suspended in RPMI-1640–FCS and cell number was adjusted with the medium to 5 × 106 cells/ml. Peyer’s patches were carefully dissected out using fine scissors from wall of the small intestine, and placed in ice cold RPMI-1640 medium in a flat-bottomed Petri dish (6 cm diameter). The Peyer’s patch cells were dispersed by tapping gently with rubber rod on a 150-gauge sterile stainless sieve. The cell suspensions were passed through 200-gauge sterile stainless sieve and washed twice with HBSS supplemented with 5% FCS (HBSS–FCS). Then the resulting cells were resuspended in RPMI-1640–FCS, and cell number was adjusted, with the medium, to 5 × 106 cells/ml. The cell viability was greater than 95% as determined by trypan blue exclusion, and more than 95% of cells were shown to be lymphocytes and less than 1% were macrophages, with characteristic staining.
Purification of B cells
Spleen cells were incubated in RPMI-1640 medium on plastic plates at 37° for 1 hr, and the non-adherent cells were recovered. The cells (5 × 107) were treated with an anti-Thy-1.2 monoclonal antibody (10 μg; Cedarlane, Canada) at 4° for 30 min followed by incubation for 60 min at 37° with Low-Tox-M rabbit complement (Cedarlane, Canada) at a dilution of 1/10 for 1 hr at 37°. The cells were washed three times and resuspended in the culture medium. Before and after the cell washing, in order to remove cell clumps due to dead cells, the anti-Thy-1.2 antibody- and complement-treated cells were passed through 30 μm nylon mesh. After treatment of spleen cells with anti-Thy-1.2 antibody and complement, CD45R/B220-positive B cells were detected more than 90% by flow cytometry using fluoresceinated antibody. The cell viability was greater than 95%, as determined by trypan blue exclusion.
Flow cytometry
The surface phenotype of cells were identified by using monoclonal antibodies (mAbs). Antibodies against CD45R/B220 (clone RA3-6B2), CD3 (145-2C11), CD25 (interleukin (IL)-2Rα chain, 7D4), IgM, BrdU and CD90 (Thy-1.2) (30-H12) were purchased from PharMingen (San Diego, CA). Fluorescence staining was performed at 4° in 300 μl HBSS–FCS, and all cells were analysed by flow cytometry on EPICS ELITE with logarithmic amplifier (Coulter Corp., Hialeah, FL). Lymphoid cells of spleen and Peyer’s patch cells were gated by the forward- and side-scatter gating method for the analysis of the lymphocyte population. Typically, 10 000 cells were analysed.
Measurement of the proliferation of spleen or Peyer’s patch cells (Alamar Blue™ reduction assay)
Cell growth was measured by means of a fluorometric assay, Alamar Blue™ reduction assay.11 Two hundred μl of spleen or Peyer’s patch cells suspension was dispensed into a 96-well plate, and were cultured for 3 days in a humidified atmosphere of 5% CO2–95% air. At 5 hr prior to culture termination, 20 μl of Alamar Blue solution was added to each well, and the cells were then continuously cultured. The fluorescence intensity was measured by Fluoroskan II (Labosystems) at an excitation wavelength 544 nm and emission wavelength 590 nm. The delta soft II (Ver 4.13 FL, BioMetallics, Inc., Princeton, NJ) was used for data management.
Analysis of cell population of bupleuran 2IIc-induced proliferation of spleen cells by flow cytometric analysis12
Spleen cells (1 × 106 cells/ml) from C3H/HeJ mice were cultured with bupleuran 2IIc (50 μg/ml) for 3 days. The proliferated cells incorporated BrdU for last 1 day of the cultivation. Cells were stained with fluoroscein isothiocyanate (FITC)-labelled anti-BrdU, phycoerythrin (PE)-labelled antimouse CD45R/B220 or PE-labelled antimouse Thy-1.2.
Determination of antibody from spleen B cells
IgM or IgG secretion in the culture supernatants of spleen cells were measured by enzyme-linked immunosorbent assay (ELISA). A solution (1 μg/well) of mixture of purified goat antimouse immunoglobulin (IgA, IgM and IgG; ICN Pharmaceuticals Inc. (formerly Cappel), Costa Mesa, CA) in 50 μl of phosphate-buffered saline (PBS) was added to microtitre plates for ELISA overnight at 4°. Unbound antibodies were removed by washing with PBS containing 0·05% Tween-20 (PBS–Tween) four times. The plate was further incubated with 0·8% gelatin in PBS at room temperature for 2 hr (blocking). Conditioned medium of spleen cells were added to the first antibody-coated well at 100 μl/well, and the plates were incubated at room temperature for 1 hr. After the plate was washed four times with PBS–Tween, 100 μl of alkaline-phosphatase-labelled antimouse IgM or antimouse IgG in PBS–Tween containing 0·5% bovine serum albumin (BSA) was added to the wells and the plate was incubated for 4 hr at room temperature. After washing the wells with PBS–Tween containing 0·5% BSA five times, each well was incubated with 150 μl of chromogenic substrate solution (1 mg of p-nitrophenylphosphate disodium salt in 1 ml of diethanolamine buffer, pH 9·8), and subsequently the absorbance at 405 nm was measured using a microplate reader (Bio-Rad, Model 250, Nippon Bio-Rad, Tokyo, Japan).
The effect of antibupleuran 2IIc/PG-1–IgG on mitogenic activity
Spleen cells (1 × 106 cells/ml) from C3H/HeJ mice were cultured with bupleuran 2IIc (50 μg/ml) which preincubated with antibupleuran 2IIc/PG-1-IgG (10 μg/well), antibupleuran 2IIc/PG-1-F(ab′)2 or non-immune-rabbit-F(ab′)2 for 1 hr. After incubation for 3 days, cell growth was measured by means of Alamar Blue™ reduction assay.11
The effect of oligosaccharides on mitogenic activity
Purified B cells (5 × 105 cells/ml) from C3H/HeJ mice were cultured with bupleuran 2IIc (50 μg/ml) after preincubation with β-d-GlcpA-(1→6)-β-d-Galp-(1→6)-d-Galp, β-d-GlcpA-(1→6)-d-Galp or β-d-Galp-(1→6)-β-d-Galp-(1→6)-d-Galp (50 μg/ml each) for 1 hr. After incubation for 3 days, cells proliferation were measured by means of Alamar Blue™ reduction assay.11
Statistics
All results were expressed as mean±SD. The differences between the control and the treatment in these experiment were tested for statistical significance by Student’s t-test. A value of P < 0·01 was considered to indicate statistical significance.
RESULTS
Effect of orally administrated pectic polysaccharide fraction from B. falcatum on proliferation of spleen cells
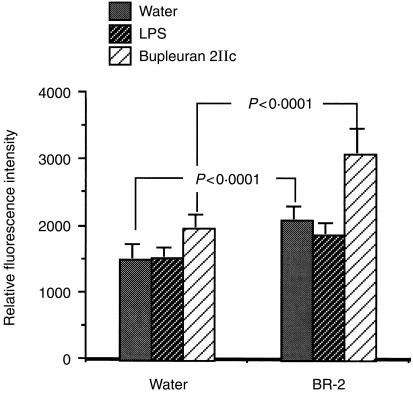
BR-2 (pectic polysaccharide fraction mainly containing bupleuran 2IIb and 2IIc; 250 mg/kg) was administrated orally into C3H/HeJ mice, which are known to have low sensitivity to LPS, for 7 consecutive days, and proliferative response of spleen cells to LPS or bupleuran 2IIc was investigated. As shown in Fig. 1, oral administration of BR-2 enhanced proliferatiion of spleen cells without secondary stimulation. This proliferative response of spleen cells by BR-2 administration was more enhanced after secondary stimulation of bupleuran 2IIc, but LPS did not stimulate this response. These results suggest that the oral administration of BR-2 enhances the proliferative responses of spleen cells.
Figure 1.
Effect of BR-2 on mitogenic activity of spleen cells. C3H/HeJ female mice were orally given BR-2 (250 mg/kg) for 7 days. Resulting spleen cells (5 × 105 cells/200 μl/96-well plate) isolated from C3H/HeJ mice were cultured with various concentrations of water, LPS or bupleuran 2IIc on spleen cells for 3 days and the proliferative responses of cells were assessed by Alamar Blue™ reduction assay.
Proliferative responses of spleen and Peyer’s patch cells in in vitro stimulation with bupleuran 2IIc
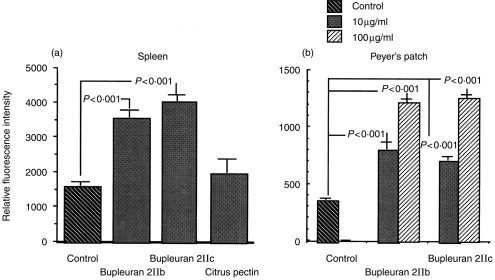
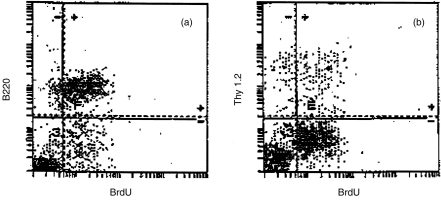
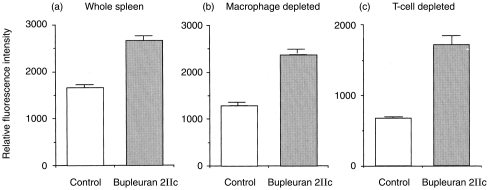
In order to clarify the ability of bupleuran 2IIc to stimulate the proliferation of lymphocytes, mitogenic activity of bupleuran 2IIb and bupleuran 2IIc on spleen and Peyer’s patch cells were investigated in vitro. Both bupleuran 2IIb and bupleuran 2IIc induced the proliferation of whole spleen cells and Peyer’s patch cells, but citrus pectin did not show the mitogenic activity on spleen cells (Fig. 2). Because bupleuran 2IIc was more effective than bupleuran 2IIb on mitogenic activity, we used bupleuran 2IIc in the following experiment. When the cell population of spleen cells responding to the stimulation by bupleuran 2IIc was investigated by flow cytometric analysis, the population of CD45R/B220 (B cell) and BrdU double-positive cells were increased after 3 days cultivation with bupleuran 2Ic; however, no increased population was observed in Thy-1.2 (T cell) and BrdU double-positive cells (Fig. 3). When the adherent cells were depleted from spleen cells, the proliferative response of the resulting spleen cells by bupleuran 2IIc was not changed in comparison with that of whole spleen cells (Fig. 4a,b). This result indicates that bupleuran 2IIc exerts mitogenic activity on spleen cells without adherent cells. When T cells were depleted by treatment with anti-Thy-1.2 antibody and complement in the spleen cells, the proliferative response by bupleuran 2IIc was also not changed (Fig. 4a,c). These results suggest that bupleuran 2IIc was characterized to be a B-cell mitogen, and adherent cells and T cells were not involved in this stimulation.
Figure 2.
Mitogenic activity of bupleuran 2IIb and 2IIc on spleen cells (a) and Peyer’s patch cells (b). Cells (5 × 105 cells/200 μl/96-well plate) isolated from C3H/HeJ mice were cultured with different concentrations (10 μg/ml, 100 μg/ml) of polysaccharides for 3 days. The proliferative responses of cells were assessed by Alamar Blue™ reduction assay.
Figure 3.
Flow cytometric analysis of bupleuran 2IIc-induced proliferation of spleen cells. Spleen cells (1 × 106 cells/ml) from C3H/HeJ mice were cultured with bupleuran 2IIc (50 μg/ml) for 3 days. The proliferated cells were incorporated BrdU for last 1 day of the cultivation. Cells were stained with FITC-labelled anti-BrdU, PE-labelled antimouse B220 and PE-labelled antimouse Thy-1.2. (a) PE-labelled antimouse B220; (b) PE-labelled antimouse Thy-1.2.
Figure 4.
Effects of macrophage and T cells on bupleuran 2IIc induced proliferation of spleen cells. Spleen cells (1 × 106 cells/ml) from C3H/HeJ mice were cultured with and without bupleuran 2IIc (50 μg/ml) for 3 days. The proliferative responses of cells were assessed by Alamar Blue™ reduction assay. (a) whole spleen cells, (b) macrophage-depleted cells, (c) T-cell-depleted cells.
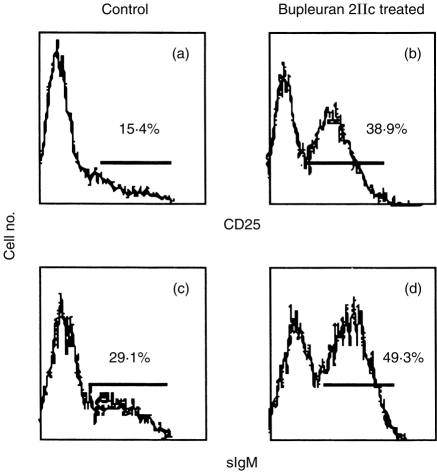
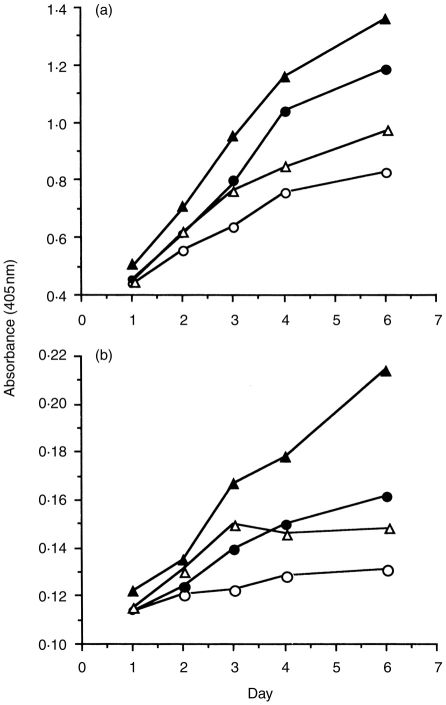
Because CD25 and sIgM are known as cell-surface markers of differentiation of B cells, spleen cells treated with bupleuran 2IIc (25 μg/ml) for 3 days were analysed to measure the extent of lymphocyte differentiation by the flow cytometry. The population of CD25+ and sIgM+ cells were increased by the treatment with bupleuran 2IIc (Fig. 5). IgM and IgG secretions by bupleuran 2IIc were increased with cultivating time as shown in Fig. 6. In the presence of IL-6, which is known to affect the differentiation of B cells at the late stage (from activated B cells to antibody-forming cell) in the B-cell lineage, the increased secretions by treatment with bupleuran 2IIc were enhanced. IL-6 production from B cells were also increased by treatment with bupleuran 2IIc (data not shown). These results suggest that bupleuran 2IIc induces maturation of B cells at least to activated B cells, and bupleuran 2IIc also induces immunoglobulin and IL-6 productions.
Figure 5.
Flow cytometric analysis of bupleuran 2IIc-induced differentiation of B cells. Spleen cells (1 × 106 cells/ml) from C3H/HeJ mice were cultured with bupleuran 2IIc (50 μg/ml) for 3 days. Cells were stained with PE-labelled antimouse B220, FITC-labelled antimouse CD25 (a, b) and FITC-labelled antimouse sIgM (c, d). (a) and (c) control, (b) and (d) bupleuran 2IIc-treated.
Figure 6.
Effect of bupleuran 2IIc on antibody production. Spleen cells (1 × 106 cells/ml) from C3H/HeJ mice were cultured with bupleuran 2IIc (• 50 μg/ml), IL-6 (▵ 10 unit), bupleuran 2IIc+IL-6 (▴) or water (○) for 3 days. IgM and IgG in the culture supernatants were measured by ELISA. (a) IgM, (b) IgG.
Elucidation of the active site of bupleuran 2IIc on mitogenic activity by endo-α-(1→4)-polygalacturonase digestion
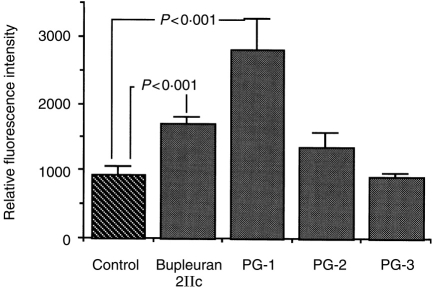
Endo-α-(1→4)-polygalacturonase digestion of bupleuran 2IIc gives a ‘ramified’ region (PG-1), which is composed of rhamnogalacturonan core having side chains rich in neutral sugars, rhamnogalacturonan II-like region (PG-2) and oligogalacturonide fraction (PG-3) by gel filtration of Bio-gel P-30. When these carbohydrate fragments obtained from the digest were tested for mitogenic activity, only PG-1 showed the potent activity (Fig. 7). This result suggests that the ‘ramified’ region (PG-1) is the active site for the mitogenic activity of bupleuran 2IIc.
Figure 7.
Mitogenic activity of the digestion products of bupleuran 2IIc by endo-β-(1→4)-polygalacturonase treatment on spleen cells. Cells (5 × 105 cells/200 μl/96-well plate) isolated from C3H/HeJ mice were cultured with various concentrations of polysaccharides for 3 days. The proliferative responses of cells were assessed by Alamar Blue™ reduction assay.
The effect of antipolysaccharide antibody on mitogenic activity of bupleuran 2IIc
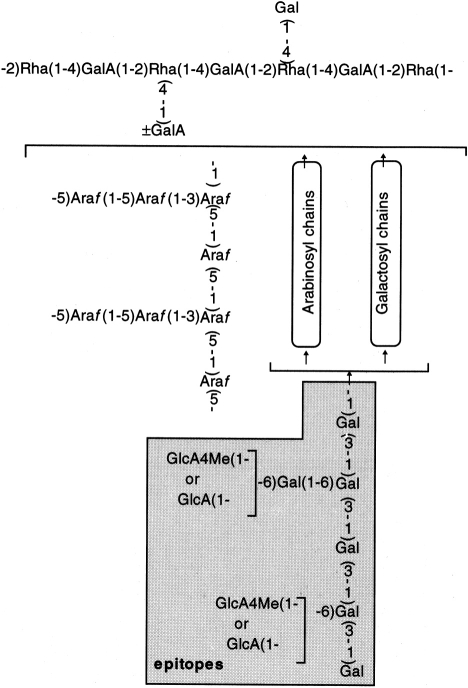
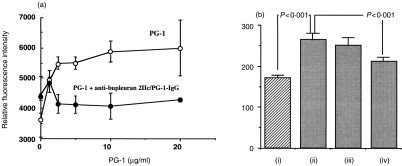
Polyclonal antibody (antibupleuran 2IIc/PG-1-IgG) against the purified ramified region (PG-1) of bupleuran 2IIc was prepared from rabbit, and the major antigenic epitopes were characterized to be 6-linked galactosyl chains containing terminal GlcA or 4-O-Me-GlcA, which were substituted to (1→3)-β-d-galactosyl chains in the ‘ramified’ region (PG-1) of bupleuran 2IIc (Fig. 8).9 When PG-1 was preincubated with the antibody, mitogenic activity of PG-1 was inhibited as shown in Fig. 9(a). F(ab′)2 of this antibody also inhibits the mitogenic activity of bupleuran 2IIc significantly, but F(ab′)2 from non-immune-rabbit IgG had no effect (Fig. 9b). These results suggest that the antigenic epitope against antibupleuran 2IIc/PG-1-IgG recognizes a part of the active sites on the mitogenic activity of bupleuran 2IIc.
Figure 8.
Proposed structure of the antigenic epitopes in the ramified region of bupleuran 2IIc for antibupleuran 2IIc/PG-1-IgG.
Figure 9.
The effect of antibupleuran 2IIc/PG-1-IgG on mitogenic activity. Spleen cells (1 × 106 cells/ml) from C3H/HeJ mice were cultured with (a) several concentrations of PG-1which was preincubated with and without antibupleuran 2IIc/PG-1-IgG (10 μg/well) (b) (i) control (ii) bupleuran 2IIc (50 μg/ml) (iii) bupleuran 2IIc (50 μg/ml) + non-immune-rabbit-F(ab′)2 (10 μg/well) (iv) bupleuran 2IIc (50 μg/ml)+antibupleuran 2IIc/PG-1-F(ab′)2 (10 μg/well). After incubation for 3 days, cell growth was measured by means of Alamar Blue™ reduction assay.
The effect of oligosaccharides on mitogenic activity of bupleuran 2IIc
Oligosaccharides, consisting of galactosyl and glucuronosyl residues contained in bupleuran 2IIc, were assayed for their ability to compete for mitogenic activity of bupleuran 2IIc. As shown in Table 1, mitogenic activity of bupleuran 2IIc was reduced significantly by the preincubation of β-d-GlcpA-(1→6)-d-Galp, which is one of the epitopes of antibupleuran 2IIc/PG-1-IgG. Although β-d-GlcpA-(1→6)-β-d-Galp-(1→6)-d-Galp was also reduced in activity, β-d-Galp-(1→6)-β-d-Galp-(1→6)-d-Galp had no effect. These results suggest that β-d-GlcpA-(1→6)-d-Galp and β-d-GlcpA-(1→6)-β-d-Galp-(1→6)-d-Galp in bupleuran 2IIc may be able to interact with the cells through carbohydrate receptor-like molecules on the cell surface in order to express their activity.
Table 1.
The effect of several kinds of oligosaccharides on mitogenic activity by bupleuran 2IIc
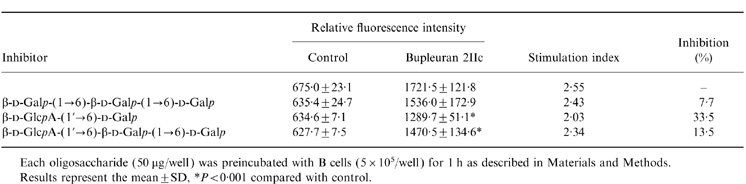
Each oligosaccharide (50μg/well) was preincubated with B cells (5 × 105/well) for 1 h as described in Materials and Methods.
Results represent the mean ±SD, *P < 0·001 compared with control.
DISCUSSION
Pectic polysaccharides, bupleuran 2IIb and 2IIc having anticomplementary activity, Fc receptor up-regulating activity on macrophages and antiulcer activity were isolated from an important Sino-Japanese medicinal herb, the roots of Bupleurum falcatum.2–4 Present results indicated that bupleurans 2IIb and 2IIc also have mitogenic activity on murine spleen and Peyer’s patch cells.
Mitogenic activity has been observed in a few pectic polysaccharides and acidic polysaccharide fractions from plant extracts.1 For example, the pectic polysaccharide, GR-2IIc from roots of Glycyrrhiza uralensis Fisch. et DC showed not only complement activating activity but also mitogenic activity against spleen B lymphocyte.15 However, detailed mechanisms of B-cell proliferation by pectic polysaccharides have not been reported. In this study, the responder cells to bupleuran 2IIc were characterized to be B cells, and the responsiveness was not changed after depletion of macrophages and T cells. These results suggest that bupleuran 2IIc is classified to the category of T-cell independent (TI) antigens. TI antigens were divided into two categories, TI-1 and TI-2. Generally, TI-2 antigens stimulate B cells by cross-linking of surface IgM (sIgM), and cytokines such as IL-1 and/or IL-6 are required in several steps in the B-cell responses, including differentiation into immunoglobulin-secreting cells, the regulation of secreted immunoglobulin level, and isotype switching. In this study, enhanced immunoglobulin secretion by bupleuran 2IIc needed the presence of IL-6; therefore it is classified as TI-2 antigen. Another B-cell mitogen, F-5-2, which is an acidic pectic polysaccharide fraction from one of the Kampo (Japanese herbal) medicines, Juzen-taiho-to, required the adherent cells for proliferation of spleen cells.18 F-5-2 did not induce differentiation into immunoglobulin-secreting cells but induced IgM secretion in the presence of IL-6. These biological differences might be caused by the different carbohydrate sequences of the pectic polysaccharides. However, detailed essential carbohydrate chains to exert the mitogenic activity have not been well known. Therefore, it is necessary to clarify which carbohydrate chains in pectic polysaccharides are responsible for expression of mitogenic activity, in order to study further mechanism of actions.
Present results showed that the ‘ramified’ region (PG-1) contained the active site for the mitogenic activity of bupleuran 2IIc. Citrus pectin, which predominantly consists of polygalacturonan region but a little of the ‘ramified’ region, did not show the mitogenic activity. Previous studies also demonstrated that mitogenic activity of the pectic polysaccharide from G. uralensis15 was also caused by the ‘ramified’ region. These observations suggest that certain carbohydrate chains in the ‘ramified’ region of the pectins play important roles for expression of mitogenic activities. Previously, we prepared the antibody against the ‘ramified’ region of bupleuran 2IIc (antibupleuran 2IIc/PG-1-IgG), and the antigenic epitopes were characterized to be 6-linked galactosyl chains containing terminal GlcA or 4-O-Me-GlcA, which were substituted to (1→3)-β-d-galactosyl chains (Fig. 8)9.
The present study showed that mitogenic activity of bupleuran 2IIc was inhibited in the presence of antibupleuran 2IIc/PG-1-IgG or β-d-GlcpA-(1→6)-d-Galp, which is a part of the epitopes of antibupleuran 2IIc/PG-1-IgG. This result indicates that the same carbohydrate sequences, which are recognized by the antibody, are involved in the proliferation of splenic B cells. It has been considered that the polysaccharide antigens contain repeating structures of the antigenic epitope which are capable of cross linking with membrane B-cell receptor (BCR) in a multivalent fashion on the surface of a polysaccharide-responsible B cell. Therefore, antibupleuran 2IIc/PG-1-IgG, β-d-GlcpA-(1→6)-d-Galp and β-d-GlcpA-(1→6)-β-d-Galp-(1→6)-d-Galp might inhibit the recognition between the active sites in bupleuran 2IIc and BCR for the proliferation of B cells by bupleuran 2IIc. Because bupleuran 2IIc also has a complement-activating activity,2 its B-cell activation might be mediated, not only by BCR, but also CD19/CD21 as a costimulatory receptor complex having complement-binding ability.21 Details on the interaction between bupleuran 2IIc and B cells including identification of receptor and signal transduction must await further study.
Because most herbal medicines are taken orally, it is assumed that some active substances in the medicines are also transported to lymphoid tissue and interact with lymphocytes. When the pectin fraction, BR-2, containing mainly of bupleuran 2IIb and 2IIc was administrated to the mice orally, bupleuran 2IIc was detected in Peyer’s patches of the mucosa-associated lymphoid tissue (MALT) on intestine using antipolysaccharide antibody.8 Flow cytometric analysis also showed that B-cell population was increased in Peyer’s patch by oral administration of BR-2 to the mice (M. Sakurai et al. unpublished). These results suggest that oral administration of bupleuran 2IIc proliferates B lymphoid cells in Peyer’s patch. MALT are continuously exposed to antigens from foods, normal microbial flora, pathogenic microorganisms and parasites, and then function as the first line of defence against pathogens.22 It is known that specialized epithelial cells, M cells, exist among the ordinary epithelial cells overlying the lymphoid follicles of Peyer’s patch.23 M cells transport soluble antigens, bacteria and viruses from the intestinal lumen and deliver them to the underlying lymphoid tissue; therefore M cells may be involved in the absorption of bupleuran 2IIc from the intestine. Recently, we also demonstrated that bupleuran 2IIc was detected in the liver after oral administration to the mice by ELISA-used antipolysaccharide antibody.8 In the present study, oral administration of the pectins (BR-2) showed the enhancement of proliferative responses of murine spleen cells against bupleuran 2IIc. These results suggest that bupleuran 2IIc modulates, not only the gastric mucosal immune system, but also the systemic immune system. The detailed mechanism by which the orally administered pectins activate the splenic lymphocytes was not clear, it must await further study.
Acknowledgments
We would like to thank Ms Hiromi Noto for technical assistance. A part of this work was supported by funds from Uehara Memorial Foundation, Japan Keirin association, and Tsumura & Co. Ltd, Japan.
Glossary
Abbreviations
- sIgM
surface immunoglobulin M
- Galp
galactopyranose
- GlcAp
glucopyranosyluronic acid
- 4-O-Me-GlcAp
4-O-methyl-glucopyranosyluronic acid
- Rha
rhamnose
- Ara
arabinose
- GalA
galacturonic acid
- LPS
lipopolysaccharide
REFERENCES
- 1.Yamada H. Contribution of pectins on health care. In: Visser J, Voragen A G J, editors. Pectin and Pectinases. Amsterdam: Elsevier Science; 1996. p. 173. [Google Scholar]
- 2.Yamada H, Ra K-S, Kiyohara H, Cyong J-C, Otsuka Y. Structural characterization of an anti-complementary pectic polysaccharide from the roots of Bupleurum falcatum L. Carbohydr Res. 1989;189:209. doi: 10.1016/0008-6215(89)84098-4. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto T, Cyong J-C, Kiyohara H, et al. The pectic polysaccharides from Bupleurum falcatum L. enhances immune-complexes binding to peritoneal macrophages through Fc receptor expression. Int J Immunopharmacol. 1993;15:683. doi: 10.1016/0192-0561(93)90141-k. [DOI] [PubMed] [Google Scholar]
- 4.Yamada H, Sun X-B, Matsumoto T, Ra K-S, Hirano M, Kiyohara H. Purification of anti-ulcer polysaccharides from the roots of Bupleurum falcatum L. Planta Med. 1991;57:555. doi: 10.1055/s-2006-960205. [DOI] [PubMed] [Google Scholar]
- 5.Sun X-B, Matsumoto T, Yamada H. Effects of a polysaccharide fraction from the roots of Bupleurum falcatum L. on experimental gastric ulcer models in rats and mice. J Pharm Pharmacol. 1991;43:699. doi: 10.1111/j.2042-7158.1991.tb03461.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamada H, Hirano M, Kiyohara H. Partial structure of an anti-ulcer pectic polysaccharide from the roots of Bupleurum falcatum L. Carbohydr Res. 1991;219:173. doi: 10.1016/0008-6215(91)89050-p. [DOI] [PubMed] [Google Scholar]
- 7.Hirano M, Kiyohara H, Matsumoto T, Yamada H. Structural studies of endo-polygalacturonase-resistant fragments in anti-ulcer pectin from the roots of Bupleurum falcatum L. Carbohydr Res. 1994;251:145. doi: 10.1016/0008-6215(94)84282-5. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai MH, Matsumoto T, Kiyohara H, Yamada H. Detection and tissue distribution of anti-ulcer polysaccharides from Bupleurum falcatum L. by polyclonal antibody. Planta Med. 1996;62:341. doi: 10.1055/s-2006-957898. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai MH, Kiyohara H, Matsumoto T, Tsumuraya Y, Hashimoto Y, Yamada H. Characterization of antigenic epitopes in anti-ulcer pectic polysaccharides from Bupleurum falcatum L. using several carbohydrases. Carbohydr Res. 1998;311:219. doi: 10.1016/s0008-6215(98)00217-1. [DOI] [PubMed] [Google Scholar]
- 10.Tsumuraya Y, Mochizuki N, Hashimoto Y, Kovac P. Purification of an exo-β-(1→3)-d-galactanase of Irpex lacteus (Polyporus tulipiferae) and its action on arabinogalactan-proteins. J Biol Chem. 1990;265:7207. [PubMed] [Google Scholar]
- 11.Page B, Page M, Noël C. A new fluorometric assay for cytotoxicity measurements in vitro. Int J Oncol. 1993;3:473. [PubMed] [Google Scholar]
- 12.Staquet M-J, Fraissinette AD, Dezutter-Dambuyant C, Schmit D, Thivolet J. A combined method for detection of cell surface marker expression and bromodeoxyuridine (BrdU) uptake by epidermal cells in suspension. J Immunol Methods. 1989;116:287. doi: 10.1016/0022-1759(89)90215-9. [DOI] [PubMed] [Google Scholar]
- 13.O’garra A, Umland S, De France T, Christiansen J. B-cell factors are pleiotropic. Immunol Today. 1988;9:45. doi: 10.1016/0167-5699(88)91259-5. [DOI] [PubMed] [Google Scholar]
- 14.Kishimoto T, Hirano T. A new interleukin with pleiotropic activities. Bioassays. 1988;9:11. doi: 10.1002/bies.950090104. [DOI] [PubMed] [Google Scholar]
- 15.Zhao JF, Kiyohara H, Yamada H, Takemoto N, Kawamura H. Heterogeneity and characterization of mitogenic and anti-complementary pectic polysaccharides from the roots of Glycyrrhiza uralensis Fisch et. DC. Carbohydr Res. 1991;219:149. doi: 10.1016/0008-6215(91)89049-l. [DOI] [PubMed] [Google Scholar]
- 16.Paul WE. The Immune System. an Introduction Fundamental Immunology. 3. New York: Raven Press; 1993. p. 1. [Google Scholar]
- 17.Bonclada S, Garg M. Thymus independent antigens. In: Snow E C, editor. Handbook of B and T Lymphocytes. New York: Academic Press; 1994. p. 343. [Google Scholar]
- 18.Takemoto N, Kiyohara H, Maruyama H, Komatsu Y, Yamada H, Kawamura H. A novel type of B-cell mitogen isolated from Juzen-taiho-to (TJ-48), a Japanese traditional medicine. Int J Immunopharmacol. 1994;16:919. doi: 10.1016/0192-0561(94)00056-5. [DOI] [PubMed] [Google Scholar]
- 19.Andersson B, Blomgren H. Evidence for thymus-independent humoral antibody production in mice against polyvinylpyrrolidone and E. coli lipopolysaccharide. Cell Immunol. 1971;2:411. doi: 10.1016/0008-8749(71)90052-9. [DOI] [PubMed] [Google Scholar]
- 20.Dorris R, Schimpl A, Wecker E. Action of dextran sulfate as direct and general B cell mitogen. Eur J Immunol. 1974;4:230. doi: 10.1002/eji.1830040315. [DOI] [PubMed] [Google Scholar]
- 21.Cambier JC, Pleiman CM, Clark MR. Signal transduction by the B cell antigen receptor and its coreceptors. Annu Rev Immunol. 1994;12:457. doi: 10.1146/annurev.iy.12.040194.002325. [DOI] [PubMed] [Google Scholar]
- 22.Mowat AM. The cellular basis of gastrinal immunity. In: Marsh M N, editor. Immunopathology of the Small Intestine. Dorchester: Dorset Press; 1987. p. 41. [Google Scholar]
- 23.Sneller MC, Strober W. M cells and host defense. J Infect Dis. 1986;154:737. doi: 10.1093/infdis/154.5.737. [DOI] [PubMed] [Google Scholar]