Abstract
There is increasing evidence that spontaneous anticytokine autoantibodies are associated with chronic infections and autoimmune diseases. We report the sporadic occurrence in autoimmune diseases of such autoantibodies to granulocyte–macrophage colony-stimulating factor (GM-CSF), a cytokine involved in inflammation and the regulation of proliferation, differentiation and function of granulocytic and monocytic cell lineages. In 41 of 425 patients tested, we found low to moderate levels of autoantibodies binding to GM-CSF in serum or plasma. These were most prevalent in patients with myasthenia gravis (MG). However, neutralizing autoantibodies against GM-CSF were very rare, being found in only three patients. Two had autoimmune MG, one with thymoma (Patient A) and the other (Patient B) with ‘seronegative’ MG, i.e. without the antiacetylcholine receptor autoantibodies characteristic of most MG patients, and a third (Patient D) had multiple sclerosis. Only very limited amounts of Patient A and Patient D serum/plasma were available for analysis and therefore further studies were carried out on the more plentiful samples from Patient B. The anti-GM-CSF autoantibodies of Patient B were predominantly polyclonal immunoglobulin G and strongly neutralized recombinant human (rh) GM-CSF derived from different expression systems. They had similar immunological and immunochemical characteristics to anti-GM-CSF antibodies that developed in immunocompetent colorectal carcinoma patients following (rh)GM-CSF therapy. In serial samples from Patient B, the anti-GM-CSF autoantibodies were undetectable from diagnosis at age 8 years until at least age 13, but then developed spontaneously during (temporary) withdrawal of immunosuppressive treatment. Their neutralizing activity has persisted since their first detection at age 15 years 1 month, and was at its highest level recently at age 17 years 7 months. There was no obvious association with other autoimmune phenomena, nor were any haematological deficiencies overtly manifested, suggesting that any loss of GM-CSF function may have been compensated for by other cytokines.
INTRODUCTION
‘Spontaneous’ autoantibodies (i.e. occurring without overt antigenic stimulation) can be directed against a spectrum of self-antigens, including serum proteins, carbohydrates and DNA. They are uncommon in healthy individuals and, if present at all, tend to be low-affinity immunoglobulin M (IgM) autoantibodies.1 However, especially in autoantibody-mediated disorders, their frequency, affinity and titre may be greatly increased.2–4 The distinct subsets of myasthenia gravis (MG) patients offer a particularly striking example. The muscle weakness is clearly mediated by autoantibodies to the nicotinic acetylcholine receptor (AChR) at the motor endplate in most cases, including about 30% of those patients with onset before age 40 (early onset, EOMG), 40% with later onset (LOMG) and 10% with a thymoma. However, in another 10–15% of cases with typical generalized MG, they are not detectable using standard radioimmunoassays (with labelled human AChR).2 Nevertheless, these so-called ‘seronegative’ cases apparently have autoantibodies to other endplate antigens that can reproduce their electrophysiological defects when passively transferred to mice.6 In contrast, seropositive MG patients with thymoma always have antibodies to striated muscle antigens, and we have recently found that about 90% of them have antibodies to interferon-α (IFN-α) and about 60% to interleukin-12 (IL-12), both of which neutralize the respective cytokine activities in bioassays in most cases, often to high titre.7 All of these autoantibodies also occur in about 30% of LOMG patients without thymoma (who are clinically indistinguishable from the remaining 70%), but much more rarely in EOMG.
Sporadic autoantibodies to IFN-α8 and a number of other cytokines, including IFN-γ,9 IL-2,10 IL-6,12 IL-8, IL-10,15 and tumour necrosis factor-α (TNF-α),16 have been detected in patients with various autoimmune, malignant, or infectious disorders. These, and autoantibodies to IL-1α and granulocyte–macrophage colony-stimulating factor (GM-CSF), have also been sporadically found in apparently healthy individuals.17–23 While many of them can neutralize their respective cytokine in culture, their in vivo effects and clinical significance remain enigmatic.
Besides MG, thymomas are occasionally associated with other disease states, that apparently also have an autoimmune basis, including red cell aplasia, and agranulocytosis. We therefore tested sera from patients with MG with or without thymoma, or with other autoimmune diseases, or with chronic haematological (malignant or non-malignant) diseases, or with colorectal carcinoma, for the presence of anticytokine antibodies, especially anti-GM-CSF antibodies, that could potentially affect cellular proliferation and immune responses. We now describe characterization of the anti-GM-CSF autoantibodies found in autoimmune patients, including one strongly neutralizing anti-GM-CSF serum/plasma observed in one case of ‘seronegative’ MG.
PATIENTS AND METHODS
Patients
Patients with neurological disease were first diagnosed and subsequently treated at the National Hospital for Nervous Diseases, London, UK or the John Radcliffe Hospital, Oxford, UK. The diagnosis of MG and Lambert–Eaton myasthenic syndrome (LEMS) was based on standard clinical, electromyographic and serological evidence. The multiple sclerosis (MS) patients were all positive for oligoclonal bands in their spinal fluid. The patient groups included in the study are listed in Table 1. Serum or plasma from patients, mostly at diagnosis, was stored at −20°/−70° and archived until tested.
Table 1.
Frequency of binding and neutralizing autoantibodies to rhGM-CSF in various patient groups and healthy controls
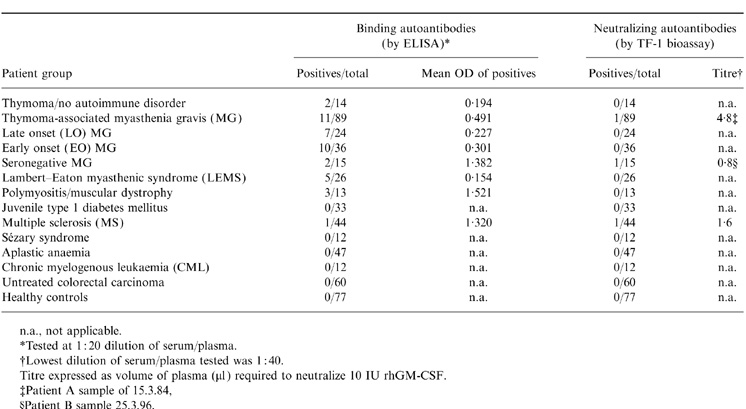
n.a., not applicable.
*Tested at 1:20 dilution of serum/plasma.
†Lowest dilution of serum/plasma tested was 1:40.
Titre expressed as volume of plasma (μ1) required to neutralize 10IU rhGM-CSF.
‡Patient A sample of 15.3.84
§Patient B sample 25.3.96.
Below are the case histories, where available, of the individual patients cited in the text. Sera of three patients (A,B and D) were found to have high levels of neutralizing anti-GM-CSF autoantibodies while that of a fourth patient (C) had only high binding anti-GM-CSF antibodies with no neutralizing activity.
Patient A
A female (date of birth 06.06.11) was first recorded as having MG on 15.02.84. A thymoma was removed on 03.11.83 before onset of MG. Her anti-AChR antibodies were 18·0 nmol on 27.02.84. She was treated with steroids and azathioprine from 15.03.84.
Patient B
A female (date of birth 17.08.80) developed fatiguable weakness of the extraocular, facial, bulbar and (to a lesser extent) limb muscles at age 2·5 years. The diagnosis of MG was established at age 8 years by single fibre electromyography. Since then, despite persistent MG, she has never had detectable anti-AChR autoantibodies. As is typical with seronegative MG cases, her strength improved objectively after plasma exchange, both at diagnosis (October 1988) and 7 years later (January 1996). She was therefore maintained on azathioprine/prednisolone on alternate days from November 1988 to June 1994; also from March 1996 (age 15½ years), since when the dosage of prednisolone has been decreased. To reduce effects on her growth, she was given oxandrolone between June and December 1992 for the 5 months spanning her 12th birthday, and again 1 year later. She has never received intravenous immunoglobulin treatment. Blood cell counts were normal at diagnosis and in March 1996.
Patient C
A female (date of birth 13.04.33) with a history of limb girdle muscular dystrophy from the age of about 20 years. In 1975 she developed type 1 diabetes and hypertension, in 1990 suffered a myocardial infarct, and in 1994 suffered heart failure (part dystrophic, partly ischaemic). She was first seen by neurologists in 1995, and spinal muscular dystrophy and Duchenne muscular dystrophy were excluded. She was found to be negative for anti-AChR autoantibodies. Her haemoglobin and blood cell counts were normal when tested in November 1994 and February 1995. She died in February 1998 aged 64 years.
Patient D
The case history of Patient D, diagnosed with MS, who has neutralizing-anti-GM-CSF, was not available.
Binding ELISA for the detection of anti-GM-CSF and anticytokine autoantibodies
The wells of micro-enzyme-linked immunosorbent assay (ELISA) plates were coated with recombinant human (rh) GM-CSF at 5 μg/ml in 100 μl phosphate-buffered saline (PBS), pH 7·0, overnight at 4°. Wells were then blocked with 5% milk protein in PBS for at least 30 min at room temperature. The sera/plasmas were diluted in 5% milk/PBS and were added to wells at 100 μl per well. Following overnight incubation at 4°, the samples were removed and wells were washed five times with 5% milk/PBS. Horseradish peroxidase (HRP)-conjugated antihuman IgG (Sigma Aldrich Co., Poole, Dorset, UK), diluted to 1:1000 in 5% milk/PBS, was added to all wells (100 μl well) and plates were incubated for 1·5 hr at room temperature. Wells were then washed five times with PBS containing 0·5% Tween-20 prior to addition of tetramethylbenzidine (TMB) substrate (100 μl/well). The colour reaction was terminated after 30 min incubation at room temperature by the addition of 2 m H2SO4 (100 μl/well) and absorbance was measured at 450 nm. Samples with absorbance 2 × SD greater than the mean of normal control values were deemed positive and further quantified by ELISA and neutralization assays. The presence/absence of other anticytokine autoantibodies was determined using the same method, but coating with the cytokine required. Samples with absorbance 2 × SD greater than the mean of normal control absorbance values were deemed positive.
A standard sandwich ELISA format with rhGM-CSF as antigen and isotype-specific antibody conjugates as secondary reagents was used for the demonstration of immunoglobulin class and subclass and light-chain type of GM-CSF binding antibodies.
Immunoblotting of GM-CSF autoantibodies
Recombinant human GM-CSF (Escherichia coli-derived) was electrophoresed under non-reducing conditions on a 12·5% polyacrylamide gel (≈2 μg of protein-loaded polyacrylamide per track). The separated proteins were transferred to nitrocellulose membranes which were blocked in a solution of 5% (w/v) milk powder in PBS for 30 min on a rotary shaker. The blots were then incubated with antisera (serum or plasma) at approximately 1:200 dilution in 5% milk/PBS or with sheep polyclonal antibody to rhGM-CSF as positive control, also at 1:200 dilution in 5% milk/PBS. After overnight incubation at room temperature on a rotary shaker, the blots were washed five times in 5% milk/PBS and then further incubated with HRP-conjugated antihuman or antiovine (as appropriate) antibody (Sigma Aldrich Co.). The blots were finally washed five times with 0·5% Tween-20/PBS and immunoreactive protein bands were visualized using enhanced chemiluminescence reagents (Amersham International, Bucks, UK).
Detection of neutralizing GM-CSF antibodies
The biological activity of GM-CSF was determined using the human TF-1 erythroleukaemic cell line which proliferates in response to GM-CSF. Briefly, dilution series of various rhGM-CSF preparations, and of the WHO International Standard for rhGM-CSF (88/646), were prepared in 100 μl volumes in 96-well microtitre plates. TF-1 cells, from exponentially growing cultures, were added in RPMI-1640 medium containing 5% fetal calf serum (FCS) at 105 cells/ml, 0·1 ml/well. The plates were incubated for 48 hr at 37°, pulsed for 4 hr with [3H]thymidine, harvested and the radioactivity incorporated into DNA was estimated by scintillation counting.27 For neutralization assays, a twofold dilution series of patients’ sera/plasmas in microtitre plate wells were preincubated with a fixed amount of rhGM-CSF for 1 hr at 37°.
Activity of GM-CSF preparations was determined as International Units (IU) by direct calibration with the WHO International Standard for rhGM-CSF (88/646). The volumes of serum/plasma required to neutralize completely the activity of 1·0 IU of GM-CSF were derived using serum 50% effective dose (ED50) responses obtained from fitting common asymptotes and slope for all test samples, separately for each GM-CSF preparation.28
RESULTS
Occurrence of anti-GM-CSF autoantibodies
Sera/plasmas from patients with various autoimmune and other diseases were screened for the presence of anti-GM-CSF antibodies by ELISA against a Chinese hamster ovary (CHO) cell-derived, glycosylated, rhGM-CSF or E. coli-derived, non-glycosylated rhGM-CSF. None were detected in sera from 77 healthy controls (Table 1). By contrast, a percentage (≈10% overall) of certain groups of autoimmune disease patients showed significant levels of binding autoantibodies (Table 1). They were most frequent in the three major subgroups of AChR antibody-positive MG patients (Table 1), but were also found sporadically in seronegative MG patients, and some other groups of patients, e.g. one among 44 MS patients (Table 1). However, they were not detected in patients with type 1 diabetes, except in one case (Patient C) who developed type 1 diabetes 20 years after limb-girdle muscular dystrophy, nor in aplastic anaemia, chronic myelogenous leukaemia (CML) or Sézary syndrome or in untreated, immunocompetent, colorectal carcinoma patients.
We have previously found and reported the high prevalence of autoantibodies to IFN-α and IL-12 in MG patients, particularly those with thymoma.7 However, apart from the present anti-GM-CSF autoantibodies (as above), autoantibodies to a range of other cytokines, including IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-18, IFN-γ, TNF-α, TNF-β and transforming growth factor-β (TGF-β), were only detected sporadically at very low, borderline, levels as measured by ELISA (data not shown).
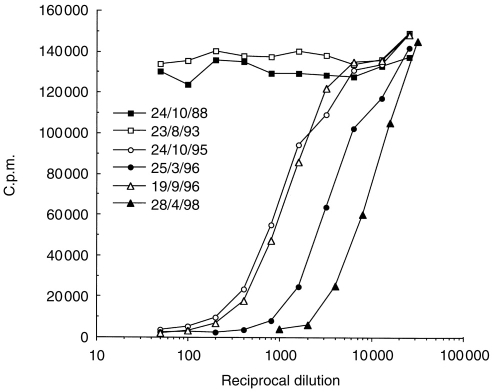
Only three of the sera/plasmas above showed neutralizing autoantibodies to GM-CSF, all of which had previously tested positive for binding antibodies. One was from a MG thymoma-associated patient (Patient A) from whom only one early sample (15.3.84) was available. Another was from a MS patient (Patient D) and was also of limited volume. However, several samples from one seronegative MG patient (Patient B) showed high titres of neutralizing anti-GM-CSF antibodies (Fig. 1). They were first detected at age 15 after temporary withdrawal of immunosuppressive therapy about 1 year previously. They appeared to rise till age 15 years 6 months and then declined when corticosteriods were re-introduced. However, a recent sample at age 17 years 7 months has shown a significantly higher anti-GM-CSF level (Fig. 1), despite continuing immunosuppressive therapy. The volumes of plasma required to neutralize 10 IU of rhGM-CSF were 2·80 μl, 0·80 μl, 2·57 μl and 0·27 μl for ages 15 years 1 month, 15 years 6 months, 16 years and 17 years 7 months, respectively.
Figure 1.
Neutralization dose–response curves of Patient B plasmas against E. coli-derived rhGM-CSF. Dilutions of plasmas taken from Patient B at successive dates were preincubated with a fixed amount (100 pg/ml) of rhGM-CSF. The subsequent proliferation of the TF-1 cell line, measured as [3H]thymidine incorporation (c.p.m.), is plotted against the plasma dilution (reciprocal)
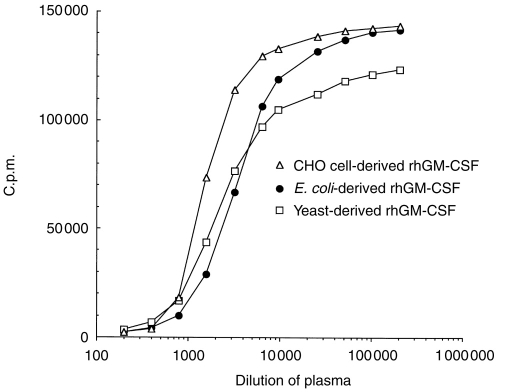
The proliferative responses of TF-1 cells to rhGM-CSF derived from various expression systems, e.g. E. coli, yeast, CHO cells, were neutralized to approximately the same degree (Fig. 2). The volumes of plasma (25.3.96 sample, age 15 years 6 months) required to neutralize 10 IU of rhGM-CSF were calculated to be 0·91 μl, 1·3 μl and 2·0 μl for E. coli, yeast and CHO cell-derived rhGM-CSF, respectively. In this patient, we detected no significant neutralization of the activities of other cytokines, including IL-1α, IL-1β, IL-3, IL-5, IL-12, IL-13, IFN-α, -β, -γ, -ω, granulocyte colony-stimulating factor (G-CSF), stem-cell factor (SCF), oncostatin M, leukaemia inhibitory factor, nerve growth factor, TGF-β1 and TNF-α, or -β (data not shown). By contrast, the plasma from Patient A (which contained neutralizing activity against rhGM-CSF) also neutralized IFN-α, as in many other MG/thymoma patients.7
Figure 2.
Neutralization dose–response curves of Patient B plasma (25.3.96 sample) against rhGM-CSF derived from different expression systems. Plasma was serially diluted dilutions mixed with a fixed amount of rhGM-CSF, and TF-1 assays carried out as in Fig. 1.
Other immunological and immunochemical characteristics of anti-GM-CSF autoantibodies
The immunological/immunochemical properties of the anti-GM-CSF plasma from Patient B were compared with:
A typical strong anti-GM-CSF binding, non-neutralizing, plasma, in this case from a patient with limb-girdle muscular dystrophy (Patient C);
Positive sera from colorectal cancer patients treated with rhGM-CSF;
A sheep polyclonal anti-GM-CSF serum raised against yeast-derived rhGM-CSF.
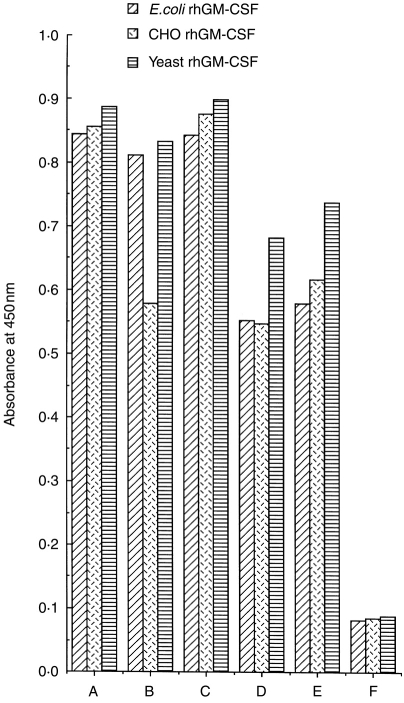
These all showed similar binding to ELISA wells coated with three different rhGM-CSF preparations, including both glycosylated and non-glycosylated forms (Fig. 3). Moreover, when tested against rhGM-CSF, the antibodies from Patients B and C and several rhGM-CSF-treated colorectal carcinoma patients were all found to be mainly IgG, with the IgG1 subclass predominating, though with smaller minor amounts of IgG2, IgG3, IgG4 and IgM also present. The IgG predominance was confirmed by BiaCore analysis (Schering-Plough, Kenilworth, NJ). The presence of both κ and λ light chains, as well as all four IgG subclasses, in the anti-GM-CSF antibodies of Patients B and C indicated a polyclonal origin (data not shown).
Figure 3.
Comparison of binding profiles of anti-GM-CSF antibodies from different patient groups and a sheep immunized with rhGM-CSF for three different rhGM-CSF preparations. ELISA plates were coated at 5 μg/ml of various rhGM-CSF preparations and sera/plasmas were tested at 1:20 dilution. A, sheep antiserum; B, patient B plasma; C, patient C plasma; D and E, sera from two different rhGM-CSF-treated immunocompetent colorectal carcinoma patients; F, medium control
In immunoblotting tests with two different E. coli-derived rhGM-CSF products, a neutralizing plasma sample from Patient B (at age 15 years 6 months) stained positively, yielding the typical banding pattern (Fig. 4) observed with the sheep polyclonal anti-GM-CSF antiserum. Similar results were obtained with non-neutralizing plasma/sera from Patient C and from colorectal carcinoma patients with circulating neutralizing anti-GM-CSF antibodies developed as a consequence of rhGM-CSF therapy (data not shown).
Figure 4.
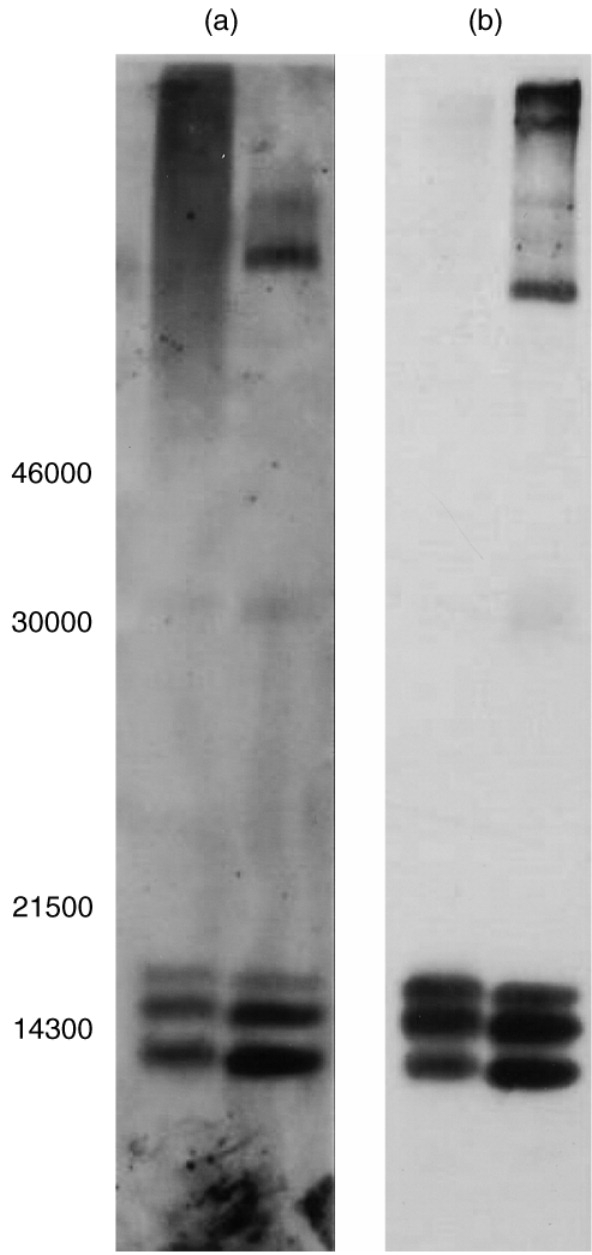
Analysis of sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE)/immunoblotting of a plasma sample (date 25.3.96) from patient B and sheep polyclonal anti-GM-CSF serum. Approximately 2 μg rhGM-CSF from two different batches of E. coli-derived rhGM-CSF per lane were subjected to SDS–PAGE (14% gel) under non-reducing conditions. Proteins were then electrophoretically transferred onto a nitrocellulose membrane and immunoblotted with patient B plasma or sheep polyclonal antiserum as described in the Patients and Methods. Left-hand panel (a), patient B plasma; right-hand panel (b), sheep polyclonal antiserum
DISCUSSION
In a large number of plasmas/sera from various groups of autoimmune disease patients, we have found that binding anti-GM-CSF autoantibodies are present in the circulation of a significant proportion (12·3%) of MG patients with thymomas. These were recently shown to have high frequencies of spontaneous anti-IFN-α and anti-IL-12 autoantibodies at the point of diagnosis and thereafter.7 However, the anti-GM-CSF autoantibodies were mainly found in the LOMG and EOMG patient groups, albeit at relatively low levels. Their sporadic occurrence was also detected in patients with ‘seronegative’ MG, LEMS, polymyositis, muscular dystrophy, MS and in patients with thymoma but no autoimmune disease. They were not detected in juvenile type 1 diabetes, aplastic anaemia, CML or Sézary syndrome, or in untreated colorectal carcinoma patients (before GM-CSF therapy), or in healthy controls.
Interestingly, while the majority of the spontaneous anti-GM-CSF autoantibodies bound in ELISA without detectable neutralizing activity, neutralizing autoantibodies were seen in two patients with autoimmune MG, one with a thymoma and one with ‘seronegative’ MG, and also in one patient with MS. To our knowledge, this is the first report of such neutralizing anti-GM-CSF autoantibodies in autoimmune patients. In one previously reported patient with acquired cyclic amegakaryocytic thrombocytopenia, the IgG blocked the action of GM-CSF, but appeared to be directed against some cellular component of haemopoietic cells, for example the GM-CSF receptor, rather than against GM-CSF itself.30 A very low prevalence of binding anti-GM-CSF autoantibodies in apparently healthy individuals (four out of 1258) has recently been reported, but the autoantibodies were not tested for neutralizing activity.22
The nature and characteristics of spontaneous anti-GM-CSF autoantibodies from Patients A and B were similar to those of neutralizing anti-GM-CSF antibodies that developed in response to recombinant human GM-CSF therapy in a cohort of immunocompetent colorectal carcinoma patients. They were polyclonal IgG, with the IgG1 subclass predominating. They were able to bind rhGM-CSF derived from various different expression systems, e.g. E. coli, yeast, CHO cells, and in Patient B, they also neutralized their biological activity. Moreover, they showed identical immunoblotting specificities to those of therapy-induced anti-GM-CSF antibodies or anti-GM-CSF antibodies raised in a sheep. The distinct binding and neutralizing activities in certain sera clearly show that GM-CSF has at least two different epitopes, one associated with neutralization and the other merely with binding.
The clinical significance of neutralizing anti-GM-CSF autoantibodies in autoimmune disease patients remains unclear. In Patient B, who had a long clinical history of ‘seronegative’ MG, the anti-GM-CSF autoantibodies were first detected during a temporary cessation of immunosuppressive therapy at age 15 years, and declined after its subsequent re-introduction. Nevertheless, although they have apparently persisted since then, and have recently risen again (age 17 years 7 months), we have seen no haematological deficiencies or other obvious clinical correlates; the patient’s condition has remained largely unchanged. There was no evidence of cyclic amegakaryocytic thrombocytopenia that was previously attributed to an antibody that selectively blocked the action of GM-CSF on megakaryocyte progenitor cells.30 In contrast, another MG patient with thymoma did develop neutropenia that was probably autoimmune, since it remitted on immunosuppressive therapy.31 Ironically, this patient had autoantibodies to IFN-α and IL-12,7 but not to GM-CSF.
That no haematological deficiencies were observed in Patient B is consistent with the normal steady-state haematopoiesis that occurs in mice lacking in the GM-CSF gene.32 Possibly, in these mice there is compensation by other cytokines having similar (overlapping) activity, e.g. G-CSF and IL-3. It is not known whether the neutralizing anti-GM-CSF autoantibodies in Patient B abrogate endogenous GM-CSF activity in vivo, but even if this did occur, the growth-promoting activities of other cytokines, e.g. IL-3, G-CSF, macrophage colony-stimulating factor, SCF, etc., should remain available to support haematopoiesis; so far we have been unable to show the presence of any other anticytokine autoantibodies in Patient B plasma/serum.
Since the plasma from Patient B thus shows the unique capacity to neutralize GM-CSF specifically and strongly, we have prepared a lyophilized preparation [National Institute for Biological Stabdards and Control (NIBSC) catalogue number 97/538] as a reference reagent for monitoring the performance of biological assays to measure neutralization of GM-CSF activity. (The preparation 97/538 is available on request from NIBSC, South Mimms, UK).
In conclusion, we have positively identified the presence of binding and neutralizing anti-GM-CSF autoantibodies in a small number of autoimmune patients. These autoantibodies have proved to be mainly IgG, with similar immunological properties to the anti-GM-CSF antibodies derived from immunocompetent colorectal carcinoma patients receiving GM-CSF therapy. In one patient (Patient B) who could be studied serially over several years, the first appearance and increase in levels of neutralizing anti-GM-CSF autoantibodies was followed. However, we saw no obvious haematological or clinical effects, leaving open the question of their relevance/significance to the patient’s autoimmunity. The cause of the development of autoantibodies also remains enigmatic. In theory, the relatively high incidence of anti-GM-CSF autoantibodies in MG patients suggests that they may develop through molecular mimicry, e.g. between GM-CSF and AChR subunits or other neurological antigens. However, this possibility seems unlikely since anti-GM-CSF autoantibodies were present not only in a MG patient seronegative for AChR autoantibodies, but also in patients with other unrelated autoimmune diseases. Another possibility is that autoimmunizing cell types in these disorders happen not only to produce and but also to immunize against GM-CSF. If so, our findings might hold valuable clues to their identification.
Acknowledgments
We are very grateful to Drs D. Dunger, D. Hilton-Jones and H. Chapel for access to patient sera, and to Sister E. Goodger for help in collecting them. This work was partly supported by the Sir Jules Thorn Charitable Trust. We thank Mrs J. Haynes for expertly typing this manuscript.
REFERENCES
- 1.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7:812. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 2.Newsom Davis J. The Hughlings Jackson Lecture: autoimmunity and the nervous system. J R Soc Med. 1995;88:639. doi: 10.1177/014107689508801110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotzin B. Systemic lupus erythematosus. Cell. 1996;85:303. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 4.Song YH, Li Y, Maclaren NK. The nature of autoantigens targeted in autoimmune endocrine diseases. Immunol Today. 1996;17:232. doi: 10.1016/0167-5699(96)10008-6. [DOI] [PubMed] [Google Scholar]
- 5.De Baets M. Autoimmune diseases against cell surface receptors: myasthenia gravis, a prototype anti-receptor disease. Neth J Med. 1994;45:294. [PubMed] [Google Scholar]
- 6.Mossman S, Vincent A, Newsom-Davis J. Myasthenia gravis without acetylcholine receptor antibody: a distinct disease entity. Lancet. 1986:116. doi: 10.1016/s0140-6736(86)92259-2. [DOI] [PubMed] [Google Scholar]
- 7.Meager A, Willcox N, Vincent A, Newsom-Davis J. Spontaneous neutralising antibodies to interferon-alpha and interleukin-12 in thymoma-associated autoimmune disease. Lancet. 1997;350:1596. doi: 10.1016/s0140-6736(05)64012-3. [DOI] [PubMed] [Google Scholar]
- 8.Prummer O, Seyfarth C, Scherbaum WA, Drees N, Porzsolt F. Interferon-alpha antibodies in autoimmune diseases. J Interferon Res. 1989;9:67. [PubMed] [Google Scholar]
- 9.Caruso A, Bonfanti C, Colombrita D, et al. Natural antibodies to IFN-γ in man and their increase during viral infection. J Immunol. 1990;144:685. [PubMed] [Google Scholar]
- 10.Balsari A, Caruso A. Natural antibodies to IL-2. Biotherapy. 1997;10:25. doi: 10.1007/BF02678214. [DOI] [PubMed] [Google Scholar]
- 11.Hansen MB, Svenson M, Diamant M, Bendtzen K. Anti-interleukin-6 antibodies in normal human serum. Scand J Immunol. 1991;33:777. doi: 10.1111/j.1365-3083.1991.tb02552.x. [DOI] [PubMed] [Google Scholar]
- 12.Takemura H, Suzuki H, Yoshizaki K, et al. Anti-interleukin-6 autoantibodies in rheumatic diseases. Increased frequency in the sera of patients with systemic sclerosis. Arthritis Rheum. 1992;35:940. doi: 10.1002/art.1780350814. [DOI] [PubMed] [Google Scholar]
- 13.Amiral J, Marfaing Koka A, Wolf M, et al. Presence of autoantibodies to interleukin-8 or neutrophil-activating peptide-2 in patients with heparin-associated thrombocytopenia. Blood. 1996;88:410. [PubMed] [Google Scholar]
- 14.Kurdowska A, Miller EJ, Noble JM, et al. Anti-IL-8 autoantibodies in alveolar fluid from patients with the adult respiratory distress syndrome. J Immunol. 1996;157:2699. [PubMed] [Google Scholar]
- 15.Menetrier-Caux C, Briere F, Jouvenne P, Peyron E, Banchereau J. Identification of human IgG autoantibodies specific for IL-10. Clin Exp Immunol. 1996;104:173. doi: 10.1046/j.1365-2249.1996.d01-646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sioud M, Dybwad A, Jespersen L, Suleyman S, Natvig JB, Forre O. Characterization of naturally occurring autoantibodies against tumour necrosis factor-alpha (TNF-alpha): in vitro function and precise epitope mapping by phage epitope library. Clin Exp Immunol. 1994;98:520. doi: 10.1111/j.1365-2249.1994.tb05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svenson M, Hansen M, Bendtzen K. Distribution and characterization of autoantibodies to interleukin-1α in normal human sera. Scand J Immunol. 1990;32:695. doi: 10.1111/j.1365-3083.1990.tb03212.x. [DOI] [PubMed] [Google Scholar]
- 18.Hansen M, Svenson M, Diamant M, Bendtzen K. High affinity IgG autoantibodies to IL-6 in sera of normal individuals are competitive inhibitors of IL-6 in vitro. Cytokine. 1993;5:72. doi: 10.1016/1043-4666(93)90026-2. [DOI] [PubMed] [Google Scholar]
- 19.Tiberio L, Caruso A, Pozzi A, et al. The detection and biological activity of human antibodies to IL-2 in normal donors. Scand J Immunol. 1993;38:472. doi: 10.1111/j.1365-3083.1993.tb02590.x. [DOI] [PubMed] [Google Scholar]
- 20.Sylvester I, Yoshimura T, Sticherling M, et al. Neutrophil attractant protein-1-immunoglobulin G immune complexes and free anti-NAP-1 antibody in normal human serum. J Clin Invest. 1992;90:471. doi: 10.1172/JCI115883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Meide PH, Schellekens H. Anti-cytokine autoantibodies: epiphenomenon or critical modulators of cytokine action. Biotherapy. 1997;10:39. doi: 10.1007/BF02678216. [DOI] [PubMed] [Google Scholar]
- 22.Svenson M, Hansen MG, Ross C, et al. Antibody to granulocyte-macrophage colony-stimulating factor is a dominant anti-cytokine activity in human IgG preparations. Blood. 1998;91:2054. [PubMed] [Google Scholar]
- 23.Bendtzen K, Hansen MB, Ross C, Svenson M. High avidity autoantibodies to cytokines. Immunol Today. 1998;19:209. doi: 10.1016/s0167-5699(98)01252-3. [DOI] [PubMed] [Google Scholar]
- 24.Ammus SS, Yunis AA. Acquired pure red cell aplasia. Am J Hematol. 1987;24:311. doi: 10.1002/ajh.2830240312. [DOI] [PubMed] [Google Scholar]
- 25.Yip D, Rasko JE, Lee C, Kronenberg H, O’neill B. Thymoma and agranulocytosis: two case reports and literature review. Br J Haematol. 1996;95:52. doi: 10.1046/j.1365-2141.1996.d01-1880.x. [DOI] [PubMed] [Google Scholar]
- 26.Kitamura T, Tange T, Terasawa T, et al. Establishment and characterisation of a unique human cell line that proliferates despondently on GM-CSF, IL-3, or erythropoietin. J Cellul Physiol. 1989;140:323. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- 27.Wadhwa M, Bird C, Page LA, Mire-Sluis AR, Thorpe R. Quantitative biological assays for individual cytokines. In: Balkwill F R, editor. Cytokines a Practical Approach. 2. Oxford: IRL Press; 1995. p. 357. [Google Scholar]
- 28.Wadhwa M, Bird C, Fagerberg J, et al. Production of neutralizing granulocyte-macrophage colony-stimulating factor (GM-CSF) antibodies in carcinoma patients following GM-CSF combination therapy. Clin Exp Immunol. 1996;104:351. doi: 10.1046/j.1365-2249.1996.11704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ragnhammar P, Friesen H-J, Frödin J-E, et al. Induction of anti-recombinant human granulocyte-macrophage colony-stimulating factor (Escherichia coli-derived) antibodies and clinical effects in non-immunocompromised patients. Blood. 1994;84:4078. [PubMed] [Google Scholar]
- 30.Hoffman R, Briddell RA, van Besien K, et al. Acquired cyclic amegakaryocytic thrombocytopenia associated with an immunoglobulin blocking the action of granulocyte-macrophage colony-stimulating factor. N Engl J Med. 1989;321:97. doi: 10.1056/NEJM198907133210207. [DOI] [PubMed] [Google Scholar]
- 31.Mathieson PW, O’neill JH, Durrant STS, Henderson SJ, Green PJ, Newsom-Davis J. Antibody-mediated pure neutrophil aplasia, recurrent myasthenia gravis and previous thymoma: case report and literature review. Quart J Med New Series. 1990;74:57. [PubMed] [Google Scholar]
- 32.Stanley E, Lieschke G, Grail D, Metcalf D, Hodgson G. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of haematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci, USA. 1994;91:5592. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]