Abstract
The majority of pathogens enter the body through mucosal surfaces and it is now evident that mucosal immunity can provide effective disease protection. However, the induction of mucosal immunity will require efficient targeting of mucosal vaccines to appropriate mucosa-associated lymphoid tissue. An animal model, based upon the surgical preparation of sterile intestinal ‘loops’ (blind-ended segments of intestine), was developed to evaluate mucosal and systemic immune responses to enteric vaccines in ruminants. The effectiveness of end-to-end intestinal anastomoses was evaluated and fetal surgery did not disrupt normal intestinal function in lambs up to 6–7 months after birth. The immunological competence of Peyer’s patches (PP) within the intestinal ‘loops’ was evaluated with a human adenovirus 5 vector expressing the gD gene of bovine herpesvirus-1. This vaccine vector induced both mucosal and systemic immune responses when injected into intestinal ‘loops’ of 5–6-week-old lambs. Antibodies to the gD protein were detected in the lumen of intestinal ‘loops’ and serum and PP lymphocytes proliferated in response to gD protein. The immune competence of ileal and jejunal PP was compared and these analyses confirmed that jejunal PP are an efficient site for the induction of mucosal immune responses. This was confirmed by the presence of gD-specific antibody-secreting cells in jejunal but not ileal PP. Systemic but not mucosal immune responses were detected when the vaccine vector was delivered to the ileal PP. In conclusion, this model provided an effective means to evaluate the immunogenicity of potential oral vaccines and to assess the immunological competence of ileal and jejunal Peyer’s patches.
INTRODUCTION
Mucosal delivery of vaccines induces mucosal immunity more efficiently than parenteral immunization (reviewed in refs 1 and 2) and this mucosal immunity is an important correlate of disease protection.3 However, most vaccines licensed for use in humans and animals are injected intramuscularly or subcutaneously and fail to generate mucosal immunity. Thus, there is a pressing need to develop vaccines and appropriate vaccine delivery systems that can efficiently induce mucosal immunity.
Immune protection at mucosal surfaces is achieved by the activation of effector cells in the mucosa-associated lymphoid tissue. Peyer’s patches (PP) are considered the major inductive site for mucosal immune responses in the small intestine (reviewed in ref. 4). However, in the small intestine of sheep and many other species there are two distinct types of PP that differ markedly in their ontogeny, cell composition and physiology (reviewed in ref. 5). The sheep ileal PP is a major source of cells for the total B-cell pool and appears to play a role in the antigen-independent diversification of the immunoglobulin repertoire.8 In contrast, the B- and T-cell composition9 and the life history of the jejunal PP10 suggest that this is the major site for the induction of mucosal immunity. However, the capacity of the jejunal and ileal PP to respond to antigen has not been clearly examined in sheep.11
To assess the antigen responsiveness of the ileal and jejunal PP we developed a surgical model that facilitated antigen delivery to individual ileal or jejunal PP. We confirmed that the gut-associated lymphoid tissue (GALT) present in intestinal ‘loops’ was functional and then assessed the mucosal and systemic immune responses induced by an adenovirus vaccine vector. In particular, the immune responsiveness of the ileal and jejunal PP were compared.
MATERIALS AND METHODS
Animals and surgery
Suffolk sheep were obtained from the Department of Animal and Poultry Science, University of Saskatchewan. Animals were cared for and used humanely, and the experimental protocol was approved by the University of Saskatchewan Committee on Animal Care. Ewes were bred following oestrous synchronization with medoxyprogesterone acetate (Veramix; Upjohn Company, Orangeville, ON, Canada) and injection with pregnant mare serum gonadotrophin (Equinex; Ayerst, Winnipeg, MB, Canada). Pregnancy was confirmed by two successive ultrasound examinations at days 45 and 105 of gestation. Fetal surgery was performed between days 120 and 130 of gestation following previous protocols with the following modifications. After premedication with acepromazine (MTC Pharmaceuticals, Cambridge, ON, Canada), anaesthesia was induced with intravenous thiopental (Abbot Laboratories, St Laurent, PQ, Canada) prior to endotracheal intubation. Anaesthesia was maintained with 2–3% halothane (MTC Pharmaceuticals) in 100% oxygen during intermittent positive pressure ventilation with an Ohio V5A ventilator (Ohio Medical Products, Madison, WI). To prepare an intestinal ‘loop’ (blind-ended segment of intestine) containing an ileal PP, a segment of intestine with a clearly defined vascular arcade was isolated 8–10 cm cranial to the ileo–caecal junction. Each end of the intestinal segment was transected proximal or distal to a haemostat before suturing with a Parker–Kerr oversaw. This created a 5–6-cm long blind-ended intestinal segment (‘loop’) with an intact blood supply. The continuity of the intestinal tract was re-established by doing either an end-to-end or a side-to-side anastomosis using 5-0 Maxon (Sherwood-Davis and Geck, Markham, ON, Canada). The side-to-side anastomoses were performed as described by Partipilo14. For the end-to-end anastomosis, ends of intestine were trimmed at a 45° angle and anastomosis was completed with several interrupted sutures. To prepare a jejunal PP ‘loop’, a discrete PP was identified on the serosal surface of the mid-jejunum and the PP was included in a ‘loop’ using the procedure described for the ileal PP ‘loop.’ Intestinal surgery was performed on a single fetus when twins were present. Butorphanol (Ayerst) was given for analgesia postoperatively.
Adenovirus vaccine vector and immunization protocol
The vaccine vector used was a replication-competent human adenovirus 5 with the gene encoding the glycoprotein D (gD) of bovine herpesvirus-1 inserted in the E3 region (HAd5-gD/E3).15 Preliminary experiments were performed with five lambs to confirm that a single subcutaneous (s.c.) injection of 2 × 109 plaque forming units (PFU) of HAd5-gD/E3 vector induced gD-specific serum antibodies and gD-specific lymphocyte proliferative responses. All subsequent experiments were performed using 2 × 109 PFU HAd5-gD/E3 vector for immunization. Lambs were immunized at 5 weeks of age by injecting the vector either s.c. or into the lumen of intestinal ‘loops’, following a midline laparotomy. Serum was collected weekly for 3–4 weeks before the lambs were humanely killed and tissues were collected.
Cell isolation
PP tissue was collected from intestinal ‘loops’ or adjacent small intestine in lambs that had ‘loops’ lacking a PP. Intestinal ‘loops’ containing jejunal PP were present in s.c. immunized lambs. The PP cells were isolated as described previously.16 With this isolation method, the cell population isolated from the ileal PP is ≈97% surface immunoglobulin M-positive (sIgM+) B cells and less than 1% T cells.9 In contrast, cells isolated from the jejunal PP consist of 60–75% B cells and 15–25% T cells. The T cells are over 90% CD4+ T cells.9 Splenocytes were isolated by mincing tissue in phosphate-buffered saline (PBS) containing 0·1% disodium ethylenediaminetetraacetic acid (EDTA; BDH Inc., Edmonton, AB, Canada). The cell suspension was pelleted, erythrocytes were lysed with distilled water, and the remaining cells were filtered through a 20-μm nylon mesh (Small Parts Inc., Miami Lakes, FL). The number of viable cells was determined by trypan blue exclusion and cell counts were performed with a haemocytometer.
gD-specific lymphocyte proliferative responses
Lymphocyte proliferative responses to gD protein17 were assayed in 96-well U-bottom culture plates (Nunc, Roskilde, Denmark) with 2 × 105 viable splenocytes or PP cells/well. Cells were cultured in AIM-V (Gibco BRL, Canada) supplemented with 2% fetal bovine serum (FBS) (Gibco BRL, Burlington, ON, Canada) and 2 × 10−5 m 2-mercaptoethanol (Sigma, St. Louis, MO). Triplicate cultures were stimulated for 72 hr with either 1·0 μg/ml affinity-purified gD protein or medium alone. Cells were cultured in a final volume of 200 μl medium and pulsed with 0·4 μCi/ml [3H]thymidine (Amersham Canada Ltd, Oakville, ON, Canada) during the final 6 hr of culture. Incorporation of [3H]thymidine was determined using standard scintillation counting methods. Proliferative responses were calculated as a stimulation index (counts per minute with gD protein stimulation/counts per minute with medium alone) and expressed as the mean±SD of triplicate cultures.
gD-specific antibody-secreting cells
The gD-specific antibody-secreting cells (ASC) were detected using a modified ELISPOT assay.18 Briefly, 96-well nitrocellulose filtration plates (Millipore, Molsheim, France) were coated overnight with purified, truncated gD protein.17 Unbound protein was removed and 1 × 106 or 0·5 × 106 cells were added to triplicate wells in a final volume of 200 μl AIM-V supplemented with 2% FBS. After overnight incubation at 37°, the cells were removed and the plates were incubated with biotinylated rabbit anti-sheep immunoglobulin (H+L) (Kirkegaard & Perry Lab., Gaithersburg, MD). The plates were washed and incubated with alkaline phosphatase-conjugated streptavidin (Jackson ImmunoResearch, Lab., Inc., Westgrove, PA) and then developed with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT) insoluble alkaline phosphatase substrate (Sigma). The frequency of gD-specific ASC per 1 × 106 cells was calculated by subtracting the number of ASC detected in uncoated wells from the number of ASC detected in wells coated with truncated gD protein. Three replicate cultures were counted with an inverted light microscope and data presented are mean values for individual lambs.
Enzyme-linked immunosorbent assay
The gD-specific antibody titres were determined by enzyme-linked immunosorbent assay (ELISA). Microtitre plates (Immulon) (Dynex Tech., Inc., Chantilly, VA) were coated overnight with 1·0 μg/ml truncated gD protein.17 Fourfold dilutions of test sera or intestinal ‘loop’ washes were then added to duplicate wells. Total gD-specific immunoglobulin was detected with alkaline phosphatase-conjugated rabbit anti-sheep immunoglobulin (H+L) (Kirkegaard and Perry Lab.) and the plates were developed with p-nitrophenyl phosphate (PNPP) substrate (Sigma). Absorbance was read on a Bio-Rad model 3550 microplate reader (Bio-Rad Laboaratories, Hercules, CA).
Histology
Sections of intestinal tissues were fixed in 10% phosphate-buffered formalin for histology. Fixed sections were stained with haematoxylin and eosin (H&E), and photographed under light microscopy.
Statistical analyses
Statistical analysis was performed using GraphPad Prism 2.01 software (Graphpad Software, Inc., San Diego, CA). A one-way analysis of variance (anova), followed by Tukey’s comparison of the means was used for comparison of multiple groups. Where applicable, differences between two groups were done using a two-tailed t-test. The level of significance was set at P < 0·05.
RESULTS
Fetal surgery
A consistent surgical technique for the preparation of intestinal ‘loops’ was established. A comparison of the surgical success of side-to-side and end-to-end anastomoses is presented in Table 1. There was no significant difference in the level of fetal death and lamb mortality during parturition for the two surgical approaches. The overall success rate of the two surgical techniques, as determined by the number of viable lambs born with intestinal ‘loops’, was also similar for both techniques (70% for side-to-side and 74·1% for end-to-end anastomosis). However, the time required to perform an end-to-end was significantly less (P < 0·05). The average time to perform an end-to-end anastomosis was 104·2±11·6 min (mean±SD; n = 12) and the average time to complete a side-to-side anastomosis was 136·5±16·3 min (mean±SD; n = 10). Finally, within the same flock there was a similar level of lamb mortality during parturition for age-matched, non-surgical (9·5% mortality; n = 50) and surgical ewes (12·3% n = 57).
Table 1.
Table Comparison of surgical success with end-to-end and side-to-side anastomoses
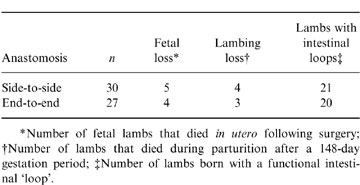
*Number of fetal lambs that died in utero following surgery; †Number of lambs that died during parturition after a 148-day gestation period; ‡Number of lambs born with a functional intestinal ‘loop’.
Intestinal ‘loop’ lymphoid tissue and gut function
Intestinal ‘loops’ were easily identified 6 weeks and 6–7 months after birth. End-to-end anastomosis resulted in minor constriction of the intestine at 6 weeks of age but this constriction was not more pronounced at 6–7 months of age. Furthermore, intestinal function was not compromised since the lambs with intestinal ‘loops’ were similar in weight to non-surgical twins, passed normal faeces and did not display abdominal discomfort.
Reynolds and Morris11 reported an accelerated involution of ileal PP isolated in intestinal ‘loops’. Thus, it was necessary to establish that the PP within intestinal ‘loops’ were functional. HAd5-gD/E3 vector was injected into ileal and jejunal ‘loops’ of 5-week-old lambs. Three weeks later the PP were collected from ‘loops’ and the histology was compared with PP collected from adjacent small intestine. Lymphoid follicles were present in the ileal ‘loop’ (Fig. 1b) but the size of these follicles and the development of the mucosal villi was reduced when compared to normal intestine (Fig. 1a). However, in the absence of HAd3-gD/E3 vaccine vector there was extensive follicular involution in ileal ‘loops’ of 9-week-old lambs (data not shown). In contrast, lymphoid follicles in PP of immunized jejunal ‘loops’ (Fig. 1d) and adjacent jejunum (Fig. 1c) were similar in size and cellularity. Lymphoid follicles in the immunized jejunal ‘loops’ displayed a pronounced corona that included the dome region and the underlying follicle (Fig. 1d). This corona was suggestive of marked antigen activation.
Figure 1.
Comparison of PP tissue in ileal and jejunal ‘loops’ and the adjacent intestine of an 8-week-old lamb. HAd5-gD/E3 vector was injected into the intestinal ‘loops’ 3 weeks prior to tissue collection. Lymphoid follicles in normal ileal PP (a) were larger and more cellular than lymphoid follicles present in an immunized ileal ‘loop’ (b). Lymphoid follicles in normal jejunal PP (c) and the PP contained in a jejunal ‘loop’ (d) were similar in size and cellularity. However, there was a marked corona of cells in the dome region of follicles present in the immunized jejunal ‘loop’.
Mucosal immune responses to gD following immunization with the HAd5-gD/E3 vector
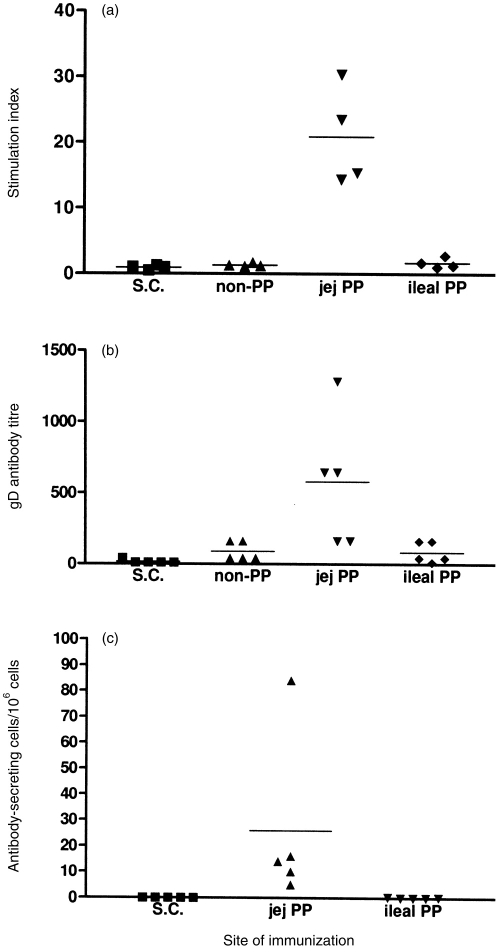
Approximately 45 million cells (range 29·2 × 106–63·6 × 106 cells) could be isolated from the PP tissue of an ileal or jejunal ‘loop.’ This cell number was adequate to perform assays for the detection of gD-specific T- and B-lymphocyte responses. The gD-specific proliferative responses were detected only with PP cells isolated from jejunal ‘loops’ (Fig. 2a). No gD-specific proliferative responses were detected with cells isolated from PP of ileal ‘loops’, PP isolated from intestine adjacent to jejunal ‘loops’ that did not contain a PP, or jejunal PP in ‘loops’ of lambs injected s.c. with the HAd5-gD/E3 vector. Thus, delivery of the vector directly to a jejunal PP appeared necessary for the induction of a mucosal immune response.
Figure 2.
The gD-specific responses in ileal and jejunal PP following immunization with the HAd5-gD/E3 vector. The vector was injected either subcutaneously (s.c.) or directly into intestinal ‘loops’ that lacked a PP (non-PP) or contained either a jejunal (jej PP) or ileal (ileal PP) PP. (a) gD-specific proliferative responses were present only in jejunal PP isolated from intestinal ‘loops’ injected with the adenovirus vector. (b) The gD-specific antibody titre was measured by an ELISA that measured total antibody. Significantly higher antibody titres were detected in jejunal PP ‘loops’ injected with the adenovirus vector. (c) gD-specific ASC were measured by an ELISPOT. ASC were detected in jejunal but not ileal PP isolated from intestinal ‘loops’ injected with the adenovirus vector. Horizontal bars indicate mean value for each group and values for individual lambs are presented.
The level of gD-specific antibody in intestinal ‘loops’ provided further evidence that targeting antigen to jejunal PP was the most efficient route for the induction of a mucosal immune response (Fig. 2b). The highest antibody titres were detected in ‘loops’ containing a jejunal PP and these titres were significantly higher than the antibody level detected in intestinal ‘loops’ containing ileal PP (P < 0·01) or ‘loops’ containing no PP (P < 0·05). The antibody level in intestinal ‘loops’ containing ileal PP was similar to ‘loops’ that contained no PP. This suggested that the induction of an immune response may not be restricted to GALT. Subcutaneous immunization did not induce detectable antibody levels in intestinal ‘loops’ despite the presence of high serum antibody levels (Fig. 3a). To characterize further the source of antibody in gut ‘loops’ an ELISPOT assay was performed to detect gD-specific ASC in PP associated with intestinal ‘loops’. Numerous ASC were detected in the PP isolated from jejunal ‘loops’ but no ASC were detected in PP isolated from ileal ‘loops’ or jejunal PP isolated from ‘loops’ of lambs immunized s.c. (Fig. 2c). Thus, there was a correlation between the level of antibody in intestinal ‘loops’ and the frequency of ASC in the PP.
Figure 3.
The gD-specific immune responses in 8–9-week-old lambs immunized by injecting HAd5-gD/E3 either subcutaneously (s.c.) or into intestinal ‘loops’ [non-Peyer’s patch ‘loop’ (non-PP), jejunal PP ‘loop’ (jej PP) and ileal PP ‘loop’ (ileal PP)]. Lambs were killed 3–4 weeks after immunization. (a) Antibody titre in serum were determined with an ELISA that detected total gD-specific antibody. (b) Stimulation index in splenocytes was determined by [3H]thymidine incorporation. Horizontal bars indicate mean value for each group and values for individual lambs are presented.
Systemic immune responses to gD following immunization with the HAd5-gD/E3 vector
Serum gD-specific antibody levels were assayed to determine if ileal and jejunal PP played a significant role in the induction of systemic humoral immune responses. Subcutaneous injection of HAd5-gD/E3 vector induced a significantly higher serum antibody titre than immunization in intestinal ‘loops’ (P < 0·05;Fig. 3a). In contrast, there was no significant difference in serum antibody levels following immunization in intestinal ‘loops’ that contained either an ileal PP, jejunal PP, or no PP. However, significant differences were apparent in the capacity of GALT to induce systemic cellular immune responses when splenocyte proliferative responses were assayed. Strong splenocyte proliferative responses to gD protein were observed in lambs immunized s.c. and in lambs immunized in intestinal ‘loops’ that contained jejunal PP (Fig. 3b). Splenocyte proliferative responses (stimulation index >2·5) were not detected when the vector was injected into ‘loops’ that did not contain a PP (Fig. 3b) but three of four lambs displayed a positive proliferative response following immunization in ‘loops’ containing ileal PP. These observations indicated that GALT was necessary for the induction of systemic cellular immune responses following intestinal delivery and that the ileal PP possessed some capacity for the induction of a systemic immune response after intestinal antigen delivery.
DISCUSSION
Intestinal ‘loop’ models have been used previously in a variety of species to study intestinal immunity.19–21 In these previous studies, loops were not prepared in the fetus and contained environmental antigens. Thus, we established a consistent surgical technique in fetal lambs to prepare sterile intestinal ‘loops’ that contained or excluded PP. The GALT in the intestinal ‘loops’ was functional for at least 5–6 weeks after birth. This lamb model offers a unique model with which to study antigen responses in the absence of other environmental antigens. However, the usefulness of this model may be restricted by the premature involution of GALT, especially the ileal PP, in the sterile intestinal ‘loops’.11 Further investigations will be necessary to determine if jejunal PP remain functional longer than the 8–9-week period evaluated in the present investigation. A further limitation of this model is the relatively small number of cells isolated from a single PP, which may restrict the number of assays that can be performed with each animal.
In this model it was possible to compare the capacity of jejunal and ileal PP to respond to antigen. For sheep, it is hypothesized that jejunal PP play a central role in mucosal immunity and ileal PP play a distinct role in the development of the total B-cell pool (reviewed in ref. 5). The present investigation provides direct evidence that the jejunal PP is an efficient site for the induction of both mucosal and systemic immune responses (Figs 2 and 3). In contrast, neither cellular nor humoral mucosal immune responses were detected following antigen stimulation of ileal PP. These observations provide clear evidence that jejunal and ileal PP function differently during the induction of a mucosal immune response. It is interesting to note that immunization in the intestinal ‘loop’ gave good proliferative responses in the spleen but minimal antibody responses in blood. We speculate that immunization in the intestinal ‘loop’ may result in antigen-specific T-cell traffic to other tissues (e.g. spleen). In contrast, B cells may have a more restricted capacity to traffic, primarily to the gut.19 It may also be that immunization in a single ‘loop’, that contains one PP, induces a humoral immune response that is below the level of detection when sampling the large volume of blood.
Several observations suggest that the ileal PP may have some capacity to respond to antigen. First, the involution of lymphoid follicles was delayed following the injection of the HAd5-gD/E3 vector into the ileal ‘loops’ (Fig. 1). Second, gD-specific proliferative responses were detected in the spleen following immunization in ileal ‘loops’ (Fig. 3b). These responses may be explained by leakage of gD protein or the spread of the vaccine vector to the mesenteric lymph node. However, no proliferative response was detected when the vector was injected into ‘loops’ that did not contain a PP. This argues against antigen or vector leakage from the ‘loops’. The absence of a gD-specific proliferative response in assays with cells isolated from the PP of ileal ‘loops’ was not surprising since this cell population contains fewer than 1% T cells.9 Furthermore, the absence of gD-specific antibody and ASC in ileal ‘loops’ may be explained by the observation that B lymphoblasts leave the ileal PP and return primarily to other regions of the gut.22 Thus, the present investigation suggests that antigen may have an impact on lymphocyte development in the ileal PP through the positive selection of emigrant lymphocytes.
Our observations are consistent with a previous report that oral immunization of ruminants can induce both mucosal and systemic immune responses.23 Collectively, these data indicate that enteric delivery of vaccines has great potential in ruminants. However, a major challenge for oral vaccination of ruminants is to ensure that the immunogen reaches the GALT in appropriate concentrations and conformation. The present investigation also reveals that targeting the appropriate GALT may also be very important in ruminants. Induction of mucosal (Fig. 2) and systemic (Fig. 3) immune responses was most efficient when vaccine was targeted to jejunal rather that ileal PP. With this model, it was also evident that s.c. immunization did not stimulate secretion of antibody into gut loops, despite high serum antibody titres (Fig. 3). This is consistent with observations in mice which indicated that oral immunization was approximately 10-fold more efficient than intramuscular injection for the induction of enteric immunity.2
In conclusion, the intestinal ‘loop’ model offered several advantages: first, consistent delivery of a defined dose of vaccine antigen without potential loss during passage through the rumen or abomasum; second, collection of lymphoid tissues (Peyer’s patches) and intestinal secretions from the site of vaccine delivery; and third, the capacity to target antigens to specific GALT. This model allowed us to evaluate the capacity of a vaccine to induce mucosal and systemic immune responses and to define the capacity of specific types of GALT to respond to the vaccine.
Acknowledgments
We thank Dave Dixon and the Animal Science Department, University of Saskatchewan, for providing pregnant ewes and for their assistance with lambing. We also thank the following people from VIDO: the Animal Care staff for assistance with surgeries and rearing lambs; M. Snider and D. Dent for assisting in the development of immunological assays; and C. Bateman for her technical assistance throughout this project. Financial support for this research was provided by the Alberta Agricultural Research Institute and the Saskatchewan Health Services Utilization and Research Commission. This work was published with permission of the Director of VIDO as journal series # 261.
Glossary
Abbreviations
- ASC
antibody-secreting cell
- GALT
gut-associated lymphoid tissue
- HAd5-gD/E3
replication-competent human adenovirus 5 vector with the gD gene of bovine herpesvirus-1 inserted in the E3 region of the adenovirus genome; PP, Peyer's patch(es)
REFERENCES
- 1.Czerkinsky C, Holmgren J. The mucosal immune system and prospects for anti-infectious and anti-inflammatory vaccines. The Immunologist. 1995;3:97. [Google Scholar]
- 2.Coffin SE, Klinek M, Offit PA. Induction of virus-specific antibody production by lamina propria lymphocytes following intramuscular inoculation using rotavirus. J Infect Diseases. 1995;172:874. doi: 10.1093/infdis/172.3.874. [DOI] [PubMed] [Google Scholar]
- 3.Matson DO, O’ryan ML, Herrera I, Pickering LK, Estes MK. Fecal antibody responses to symptomatic and asymptomatic rotavirus infections. J Infect Diseases. 1993;167:577. doi: 10.1093/infdis/167.3.577. [DOI] [PubMed] [Google Scholar]
- 4.Cebra JJ, Shroff KE. Peyer’s patches as inductive sites for IgA commitment. In: Ogra P L, Mestecky J, Lamm M E, Strober W, McGhee JR, Bienenstock J, editors. Handbook of Mucosal Immunology. London: Academic Press; 1994. p. 151. [Google Scholar]
- 5.Griebel PJ, Hein WR. Expanding the role of Peyer’s patches in B-cell ontogeny. Immunology Today. 1996;17:30. doi: 10.1016/0167-5699(96)80566-4. [DOI] [PubMed] [Google Scholar]
- 6.Gerber HA, Morris B, Trevella W. The role of gut associated lymphoid tissue in the generation of immunoglobulin bearing lymphocytes in sheep. Aust J Biol Med Sci. 1986;64:201. doi: 10.1038/icb.1986.22. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds JD, Kennedy L, Peppard J, Pabst R. Ileal Peyer’s patch emigrants are predominantly B cells and travel to all lymphoid tissues in sheep. Eur J Immunol. 1991;21:283. doi: 10.1002/eji.1830210207. [DOI] [PubMed] [Google Scholar]
- 8.Reynaud C-A, Garcia C, Hein WR, Weill J-C. Hypermutation generating the sheep immunoglobuloin repertoire is an antigen-independent process. Cell. 1995;80:115. doi: 10.1016/0092-8674(95)90456-5. [DOI] [PubMed] [Google Scholar]
- 9.Griebel PJ, Ferrari G. CD40 signaling in ileal Peyer’s patch B cells: implications for T-cell dependent antigen selection. Int Immunol. 1995;7:369. doi: 10.1093/intimm/7.3.369. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds JD, Morris B. The evolution and involution of the Peyer’s patches in fetal and postnatal sheep. Eur J Immunol. 1983;13:627. doi: 10.1002/eji.1830130805. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds JD, Morris B. The effect of antigen on the development of Peyer’s patches in sheep. Eur J Immunol. 1984;14:1. doi: 10.1002/eji.1830140102. [DOI] [PubMed] [Google Scholar]
- 12.Smeaton TC, Cole GJ, Simpson-Morgan MW, Morris B. Techniques for the long-term collection of lymph from the unanesthetized fetal lamb in utero. Aust J Exp Biol Med Sci. 1969;47:565. doi: 10.1038/icb.1969.150. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds JD, Morris B. The influence of gut function on lymphoid cell populations in the intestinal mucosa of lambs. Immunology. 1983;49:501. [PMC free article] [PubMed] [Google Scholar]
- 14.Partipilo AV. Surgical Techniques and Principles of Operative Surgery. Philadelphia: Lea & Febiger; 1957. p. 757. [Google Scholar]
- 15.Mittal S, Papp Z, Tikoo SK, et al. Induction of systemic and mucosal immune responses in cotton rats immunized with human adenovirus type 5 recombinants expressing the full and truncated forms of bovine herpesvirus type 1 glycoprotein gD. Virology. 1996;222:299. doi: 10.1006/viro.1996.0427. [DOI] [PubMed] [Google Scholar]
- 16.Griebel PJ. Isolation of lymphoid follicles from Peyer’s patches. In: Lefkovits I, editor. Immunology Methods Manual. Vol. 3. London: Academic Press; 1997. p. 2079. [Google Scholar]
- 17.Van Drunen Little-Van Den Hurk S, Gifford GA, Babiuk LA. Epitope specificity of the protective immune response induced by individual bovine herpesvirus-1 glycoproteins. Vaccine. 1990;8:358. doi: 10.1016/0264-410x(90)90095-4. [DOI] [PubMed] [Google Scholar]
- 18.Czerkinsky CC, Nilsson LA, Nygren H, Ouchterlony O, Tarkowski A. A solid-phased enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 19.Husband AJ. Kinetics of extravasation and redistribution of IgA-specific antibody-containing cells in the intestine. J Immunol. 1982;128:1355. [PubMed] [Google Scholar]
- 20.Husband AJ, Gowans JL. The origin of antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978;148:1146. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mcaleer FT, Silbert LK, Van Kruiningen HJ, Koudelka J, Tobias A. A simplified procedure for studies of inetstinal immunity in rabbits. J Immunol Methods. 1996;194:49. doi: 10.1016/0022-1759(96)00055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pabst R, Reynolds JD. Peyer’s patches export lymphocytes throughout the lymphoid system in sheep. J Immunol. 1987;139:3981. [PubMed] [Google Scholar]
- 23.Bowerstock TL, Hogenesch H, Torregroa SS, et al. Induction of pulmonary immunity in cattle by oral administration of ovalbumin in alginate microspheres. Immunol Lett. 1998;60:37. doi: 10.1016/s0165-2478(97)00131-4. [DOI] [PubMed] [Google Scholar]