Abstract
The importance of T cells in Chlamydia pneumoniae infection in mice was assessed by comparing wild-type BALB/c mice with nude mice and mice depleted in vivo of either CD4+ or CD8+ T cells. Whereas wild-type mice cleared the primary infection in 3 weeks, nude mice were only able to restrict the infection and could not clear it during the observation period of 56 days. Nude mice exhibited a greater number of macrophages in their lungs and the pulmonary cells secreted a higher level of tumour necrosis factor-α (TNF-α) than wild-type mice. Depletion of CD4+ cells did not change the overall infection kinetics of the primary infection. However, depletion of CD8+ cells resulted in a slightly impaired clearance of the bacteria in the late stages of primary infection. To assess the role of the two T-cell subsets in the acquired immunity that develops during primary infection in wild-type BALB/c mice, in vivo depletions were performed during reinfection. Prior to reinfection, immunocompetent wild-type mice were infected and natural immunity was allowed to form. During reinfection, depletion of CD4+ cells did not have any effect on infection kinetics, whereas depletion of CD8+ cells abolished the protection, reverting the infection kinetics and bacterial load to the same levels found in wild-type mice during primary infection. These results show that T cells are necessary for clearing C. pneumoniae infection in mice. Furthermore, whereas neither of the two main T-cell subsets, separately, were essential for clearance of primary infection, the induced protective immunity was strongly CD8 dependent.
INTRODUCTION
Chlamydia pneumoniae infection is common among the adult population with a seroprevalence of over 50% in many industrialized countries.1 The symptoms of an acute C. pneumoniae infection are often mild, but the accumulating evidence of the association between C. pneumoniae and coronary heart disease,2 a leading cause of mortality in developed countries, has enhanced its significance as a human pathogen. Immune mechanisms that occur during infection with C. pneumoniae are believed to escalate inflammation in the arteries, thus increasing the risk of myocardial infarction.3
As intracellular bacteria, chlamydiae pose an extra challenge for the defence mechanisms of the host. In addition to the neutralizing activity of antibodies, cell-mediated immune responses are decisive, at least in mice.4 In C. trachomatis infection models, both CD4+ and CD8+ cells have been shown to confer protection, although the former are considered of major importance.5–8 However, in C. psittaci infection, CD8+ (Lyt-2+) rather than CD4+ (L3T4+) cells have been reported to confer protection in mice.9
Very little is known of the immunobiology of C. pneumoniae infection and because the overall DNA homology between C. pneumoniae and C. trachomatis or C. psittaci is less than 5 or 10%, respectively,10 the parameters of infection identified with the latter two cannot be directly extrapolated to C. pneumoniae. Thus, even though these pathogens belong to the same genus and share many biological features, a different set of epitopes is probably presented to the immune system. However, a recently developed C. pneumoniae mouse model seems promising for studying the infection in more detail: it resembles human infection in several aspects including respiratory route of challenge, self-restricted infection and mild symptoms. Using this model we have shown that such a mild C. pneumoniae infection in BALB/c mice induces protective immunity to reinfection, manifested as a reduced number of cultivable bacteria, more severe lymphoid reaction and a stronger T helper 1 (Th1)-type local immune response in the lungs.13
In this study our first aim was to clarify the importance of T cells in protection against C. pneumoniae infection in mice. For this purpose we infected thymusless nude mice and compared their infection kinetics with those of immunocompetent mice. In the second part of the study we used an in vivo depletion technique of different T cells to determine more precisely the role of CD4+ and CD8+ T cells in primary infection or in acquired immunity (reinfection). Our results show that, consistent with C. trachomatis and C. psittaci infections, T cells are essential for clearing C. pneumoniae infection in mice. However, in contrast to C. trachomatis infection models, the protective role of CD8+ cells was dominant over that of CD4+ cells and this was accentuated, particularly in acquired immunity.
MATERIALS AND METHODS
Mice
Inbred female BALB/c mice were obtained from the Laboratory Animal Centre, University of Helsinki (Helsinki, Finland), and the thymusless nu/nu mice on a BALB/c background were obtained from Bomholtgård Breeding and Research Centre Ltd (Ry, Denmark). The mice were given food and water ad libitum and they were housed in ventilated containers (Scantainer, Scanbur A/S, Køge, Denmark). This study was approved by the Institutional Animal Care and Use Committee that acts under the provincial board.
Chlamydia
C. pneumoniae isotype Kajaani 614was obtained from P. Saikku (National Public Health Institute, Oulu, Finland). It was propagated and purified as described in reference 13. For in vitro assays the organism was inactivated with formalin and protein concentration was determined by the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL) (1 μg corresponds to ≈106 inclusion-forming units, IFUs).
Experimental infection
The mice were challenged with intranasal inoculation of 106–107 IFU of C. pneumoniae, in 40 μl of sucrose–phosphate–glutamate (SPG), under light carbon dioxide anaesthesia.11 During reinfection, the same dose was given using the same procedure, 36–55 days after the primary challenge.
Depletion of lymphocytes
The 6–8-week-old mice were depleted of a specific lymphocyte subset by 0·5 mg/dose of anti-CD8 (clone YTS 169, a kind gift from Dr R. L. Coffman, DNAX Research Institute, Palo Alto, CA) or anti-CD4 (clone GK 1·5, DNAX Research Institute) monoclonal antibodies (mAbs) that had been produced in serum-free media (by DiaBor Ltd, Oulu, Finland). The antibodies were injected intraperitoneally (i.p.) 1 day before challenge and every 3 to 4 days thereafter. In depletion studies during reinfection, the primary infection was given to immunocompetent mice and the first dose of specific antibodies was given 1 day before rechallenge and every 3 to 4 days thereafter. The success of depletion was monitored by flow cytometric analysis, either in lungs or blood. In wild-type mice the range of CD8+ and CD4+ cells, as a proportion of all lymphocytes, was 4·1–17% and 22–39%, respectively. In CD8-depleted mice the range of CD8+ cells as a proportion of all lymphocytes was 0–1·6% and in CD4-depleted mice the range of CD4+ cells as a proportion of all lymphocytes was 3·0–3·2%. Thus, the mean depletion efficiency was 86% and 89% with YTS 169 and GK 1·5 antibodies, respectively.
Culture of C. pneumoniae from the lungs
At predetermined days after challenge, between two and 10 mice were killed using carbon dioxide and the lungs were dissected and mechanically homogenized. In some sets of experiments the bronchoalveolar lavage (BAL) fluid was obtained before dissecting the lungs. In these experiments the culture results of lung supernatants and BAL fluids were combined. The lung supernatants and the BAL fluids were cultured in several dilutions on Vero cell monolayers using centrifugation and cycloheximide.13 After 48–72 hr of incubation, the cells were fixed with methanol (Riedel-de Haen, Sleeze, Germany) and stained with fluorescein isothiocyanate (FITC)-conjugated Chlamydia-specific antibodies (Kallestad, Chaska, MN). Intracellular inclusions were counted using UV microscopy. Results are expressed as the mean of logarithmic values of IFUs per lung. After the dilution factors were taken into account, one inclusion seen by microscopy corresponded to a logarithmic value of 1·3 IFU/lung (=detection limit). If no inclusions were detected, an arbitrary value of one-half of log10 was used for calculating means and for statistical analysis.
Isolation of pulmonary mononuclear cells
Pulmonary cells were isolated from mechanically homogenized pooled lungs after red-cell lysis, as described in reference 15. Mononuclear cells were counted under light microscopy and the cells were suspended into complete growth media containing: RPMI-1640 (Sigma, St Louis, MD), 10% fetal calf serum (FCS), 10 mm HEPES (Sigma), 0·3 mg/ml l-glutamine (Gibco BRL, Life Technologies Ltd, Paisley, Strathclyde, UK), 10 U/ml penicillin (Sigma), 10 μg/ml streptomycin (Sigma) and 50 μm 2-mercaptoethanol (Sigma).
Flow cytometric analysis
Freshly isolated pulmonary mononuclear cells (0·4 × 106 for each test) were stained with 5 μl of each antibody: phycoerythrin (PE)-conjugated rat IgG2b (for controlling non-specific binding) (Caltag, South San Francisco, CA), anti-CD4 (YTS 191·1, Caltag), FITC-conjugated anti-CD8 (α-chain specific, CT-CD8a, Caltag) and anti-Mac-1 (CD11b) (M1/70·15, Caltag). After a 30-min incubation, the cells were washed with phosphate-buffered saline (PBS) and fixed with 1% paraformaldehyde (Sigma). Unstained cells were used for adjustment of the fluorescence-activated cell sorter (FACScan; Becton-Dickinson, San Jose, CA); gating of lymphocytes was performed by size. Data were typically collected from 10 000 gated events.
Lymphoproliferation assay
The proliferative response of isolated mononuclear cells to 1 μg/ml formalin-inactivated C. pneumoniae was measured by incorporation of 1 μCi/well [3H]thymidine (Amersham, Aylesbury, Bucks, UK) over the last 16–20 hr of a 2-day culture period at +37° in an atmosphere of 5% CO2, as described previously.15 Control wells received medium alone (background control) or 5 μg/ml concanavalin A (Con A) (positive control). The proliferation index was calculated as follows:
 |
Cytokine EIA
Freshly isolated pulmonary mononuclear cells were plated in 24-well plates (Grainer, Frickenhausen, Germany) at 2 × 106 cells per well. Formalin-inactivated C. pneumoniae was added at 1 μg/ml and the final volume was adjusted to 1 ml with complete growth media. Control wells received medium alone (background control) or 5 μg/ml Con A (positive control). The cells were incubated at +37° in an atmosphere of 5% CO2 for 72 hr, after which the supernatants were collected, frozen and later analysed for interferon-γ (IFN-γ), interleukin-10 (IL-10) and tumour necrosis factor-α (TNF-α) using enzyme-linked immunosorbent assay (ELISA).
Detection of IFN-γ and IL-10 was performed as described in reference 15 and detection of TNF-α was performed using a commercial mouse TNF-α DuoSet (Genzyme, Cambridge, MA), according to the manufacturer’s instructions. The sensitivity of the cytokine ELISAs were, in our hands, typically 0·5 ng/ml for IFN-γ, 0·2 ng/ml for IL-10 and 0·2 ng/ml for TNF-α. The results are shown as C. pneumoniae-induced cytokine production subtracted from the background production.
Statistical analysis
Statistical significances were evaluated by the non-parametric Mann–Whitney U-test.
RESULTS
C. pneumoniae infection kinetics in nude mice
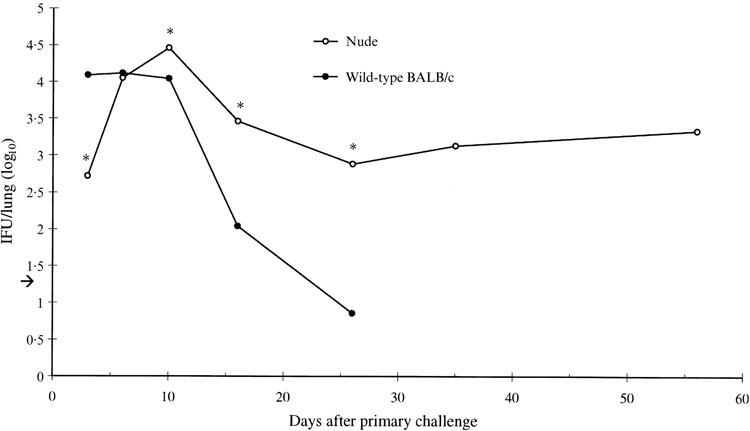
While wild-type BALB/c mice cleared the C. pneumoniae infection in ≈3 weeks (Fig. 1; also see reference 13), intranasal challenge of athymic, T-cell deficient, nude mice resulted in a significantly prolonged infection in the lungs, with no sign of elimination of the bacteria during the observation period of 56 days (Fig. 1). The nude mice did, however, control the infection by limiting the numbers of IFU to a ‘steady-state’ level of 103–104 IFU/lung starting ≈2 weeks after challenge.
Figure 1.
Chlamydia pneumoniae was cultured from the supernatants of homogenized lung samples of wild-type BALB/c mice and athymic nude mice after primary challenge with 106–107 inclusion-forming units (IFUs) of C. pneumoniae given intranasally in a volume of 40 μl. Data represents the mean logarithmic values obtained from individual mice. Fifteen to 33 wild-type mice and 17–30 nude mice were used per time-point. The arrow shows the detection limit of the culture assay: 1·3 IFU/lung. *Statistically significant difference between nude and wild-type mice (P = 0·001, P = 0·001, P < 0·01, P < 0·001) as determined by the Mann–Whitney U-test.
Additional challenge was given to some of the nude mice 35 days after primary challenge, and the mice were followed-up further, up to 37 days. There was no significant increase or decrease in the number of IFUs, which continued to remain at the same ‘steady-state’ level as seen during primary infection. Of all 151-infected nude mice, challenged once or twice, four died during the C. pneumoniae infection experiments (at day 37 after primary challenge), whereas none of the 244-infected wild-type BALB/c mice died.
Effect of in vivo depletion of CD4+ or CD8+ cells on C. pneumoniae infection kinetics
In vivo depletion of CD4+ or CD8+ cells was performed by i.p. injection of 0·5 mg mAbs, 1 day prior to challenge or rechallenge of C. pneumoniae and every 3 or 4 days thereafter. In reinfection experiments, the primary challenge was given to immunocompetent mice.
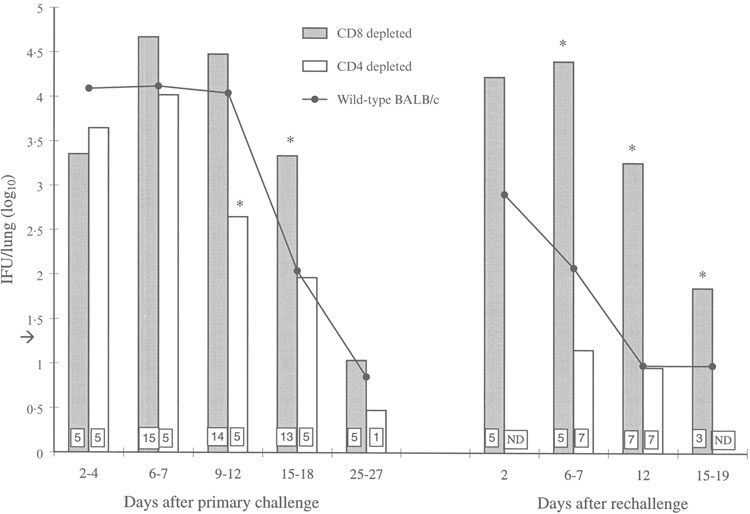
Depletion of CD4+ cells did not impair clearance of the primary infection. In fact, at days 9–12 after infection, the number of bacteria in the lungs of the CD4-depleted mice was decreased compared with the number of bacteria in lungs of wild-type mice at the same time-point (P < 0·01). In contrast, depletion of CD8+ cells resulted in elevated numbers of IFU in the lungs compared with wild-type mice at several time-points; the difference was statistically significant on days 15–18 (P < 0·01) after primary infection. Nevertheless, both the CD4- and the CD8-depleted mice eliminated the primary infection by days 25–27, similar to the wild-type mice (Fig. 2).
Figure 2.
The numbers of cultured Chlamydia pneumoniae inclusion-forming units (IFUs) from the lungs of wild-type and in vivo CD8- or CD4-depleted BALB/c mice during primary infection and reinfection. Data represents the mean logarithmic values obtained from individual mice. The data for wild-type mice are the same as detailed in the legend to Figure 1. Numbers of mice used in the depletion experiments are shown in boxes inside the bars. The detection limit of the culture assay, 1·3 IFU/lung, is shown by an arrow. *Statistically significant difference compared with wild-type mice (P < 0·01, P < 0·01 after primary challenge and P = 0·001, P < 0·001, P < 0·05 after rechallenge). ND, not determined.
The effect of CD4 depletion on reinfection was assessed on days 6–7 and 12 after rechallenge. The protective immunity seen in the wild-type mice was not impaired by CD4 depletion. By contrast, depletion of CD8+ cells had a profound effect on the kinetics of reinfection. The number of culturable bacteria on each of the assay days from days 6–19 was significantly elevated when compared with reinfection of wild-type mice (P = 0·001, P < 0·001 and P < 0·05) (Fig. 2). Indeed, the pattern and kinetics of reinfection in the CD8-depleted mice were similar to those seen in the wild-type mice during primary infection.
Characterization of lung-derived cells in nude, CD8-depleted and wild-type mice
Pulmonary mononuclear cells isolated during primary infection from nude, CD8-depleted and wild-type mice, were restimulated in vitro with inactivated C. pneumoniae, and their response was evaluated by analysing proliferation and cytokine secretion (TNF-α, IFN-γ and IL-10). Culture media alone or stimulation with a T-cell mitogen, Con A, were used as background and positive controls, respectively. In addition, the proportion of macrophages was determined by flow cytometry.
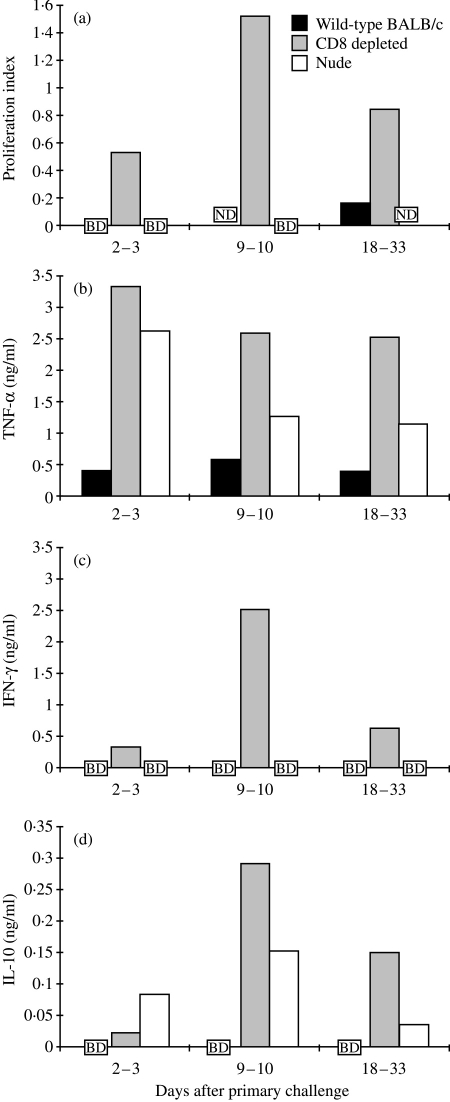
The nude mice exhibited elevated proportions of macrophages (Mac-1+ cells represented a mean of 20%, relative to all cells, on days 6–25 after primary challenge) in the lungs, compared with the wild-type mice (mean 11%, on days 6–25 after primary challenge), and the cells isolated from nude mice secreted higher levels of TNF-α and IL-10 than wild-type mice (P < 0·05 in both cases, calculated from pooled data from all time-points) (Fig. 3b,3d). However, no IFN-γ secretion (Fig. 3c) or proliferation (Fig. 3a) was detected in cells from nude mice, in response to in vitro stimulation. In contrast, in the cells from CD8-depleted mice, the proliferative response was elevated compared with cells from wild-type mice (Fig. 3a), and when stimulated with C. pneumoniae they secreted elevated levels of all three cytokines compared with wild-type mice (TNF-α, P < 0·05; IFN-γ, P < 0·01, IL-10, not statistically significant) (Fig. 3b,3c,3d).
Figure 3.
Induction of (a) proliferation and (b) secretion of tumour necrosis factor-α (TNF-α), (c) interferon-γ (IFN-γ) and (d) interleukin-10 (IL-10) by pulmonary cells, isolated on the indicated days after primary Chlamydia pneumoniae infection from wild-type, CD8-depleted and nude mice, in response to in vitro stimulation with inactivated C. pneumoniae. In the late stage of infection (days 18–33), data from typically two different time-points are combined. In panel (d) the scale is one-tenth that of panels (b) and (c). The proliferation index was calculated as described in the Materials and methods. Cytokine secretion results are expressed as C. pneumoniae-induced cytokine secretion after subtraction of the background secretion. BD, below the detection limit; ND, not determined.
DISCUSSION
Protective immunity against a wide range of intracellular bacteria is mostly dependent on the activation of T-cell-mediated immune defence mechanisms.16 A first conclusion from our data was that the clearance of C. pneumoniae from the lungs of BALB/c mice was T-cell dependent. As we show here, C. pneumoniae infection of athymic nude mice resulted in persistent infection that was not cleared from the lungs, although was controlled at a certain level of culturable bacteria. In addition to the impaired cellular immunity, the nude mice exhibited poor antibody responses without T-cell help (data not shown). Antibodies are not, however, likely to have a major role in the clearance of C. pneumoniae infection because passively transferred convalescent serum of outbred NIH/S mice (i.p., 3 × 100 μl on 9–10-day intervals) did not confer protection in nude or BALB/c mice, as assessed on days 6 and 25 after infection (our unpublished data). The lungs of the nude mice exhibited higher proportions of macrophages and increased secretion of TNF-α by pulmonary mononuclear cells than seen in the wild-type mice, suggesting that these were the mechanisms by which nude mice controlled the infection. The importance of TNF-α has been established in many studies of other intracellular infections, including C. trachomatis mouse pneumonitis19 and genital tract infection caused by C. trachomatis.20
The second aim of this study was identification of the decisive T-cell class in protection against C. pneumoniae. The results from depleted mice showed that during the primary C. pneumoniae infection other defence mechanisms were able to compensate for the lack of CD4+ and CD8+ T cells. While absence of all T cells, as in nude mice, resulted in persistent infection, the overall clearance kinetics of primary C. pneumoniae infection were not dependent on the presence of either CD4+ or CD8+ cells alone. The depleted mice cleared the infection as fast and as completely as the wild-type mice. However, small differences were seen between days 7 and 25 and indicated contrasting effects of CD4+ and CD8+ cells on the bacterial load in the lungs: CD8-depleted mice were less efficient than wild-type mice in controlling the level of IFUs, whereas CD4 depletion appeared to enhance bacterial clearance.
Acquired immunity, studied here as responses to rechallenge, is of particular importance in chlamydial infections in which repeated infections are typically associated with more severe disease.21In vivo depletion is an effective method for studying acquired immunity because natural immunity is allowed to form in immunocompetent mice during primary infection, and different cell types can be depleted specifically during reinfection. Therefore, the second conclusion from the data was most interesting: acquired immunity was strongly CD8 dependent. After rechallenge, the CD8-depleted mice had a worse course of infection, and indeed behaved like the immunocompetent mice during primary infection, both in respect to the number of IFUs cultured from the lungs and the kinetics of the infection. Depletion of CD4+ cells had no effect on reinfection. However, the role of CD4+ cells in the development of CD8+ memory cells remains to be established, e.g. by studies in which CD4+ cells are depleted during primary infection and acquired immunity is evaluated after secondary challenge.
While the cells isolated from lungs of wild-type mice during primary infection did not respond to in vitro stimulation with inactivated C. pneumoniae (results reported here and in reference 13), depletion of CD8+ cells resulted in an increase of both proliferation and secretion of IFN-γ. As the cells from wild-type mice were, however, responsive to treatment with a T-cell mitogen, Con A, it seems that the presence of CD8+ cells inhibited these functions. This initial CD8+ cell-mediated inhibition may be especially pronounced in BALB/c mice because it has not been demonstrated in outbred NIH/S (our unpublished data) or C57BL/6 mice.22 Furthermore, in the BALB/c mice this inhibition of both proliferation and secretion of IFN-γ, which was detected during primary infection, was undetectable during reinfection.13 An increased secretion of TNF-α was also observed in CD8-depleted mice, although a low level of secretion was detected also in the wild-type mice. Similarly to the thymusless mice, the increased production of TNF-α in CD8-depleted mice may have been a compensatory mechanism involved in controlling the infection.
The protective effect of the CD8+ cells during reinfection may have been mediated by direct cytotoxicity. Cytotoxic cells have an important role in protection against intracellular infection by bacteria that can resist, or even profit from, IFN-γ-mediated bactericidal functions of macrophages.23 The necessity of cytotoxic functions in chlamydial infection is not clear. Development of Chlamydia-specific cytotoxic T cells during C. trachomatis infection has been demonstrated24,25–27 but no studies demonstrating cytotoxic T-lymphocyte activity during C. pneumoniae infection have been published. Also, production of cytokines, alone or combined with cytotoxic activity, may be a relevant part of protection mediated by CD8+ T cells. In addition to CD4+ T cells, CD8+ T cells can be subdivided into type 1 or 2, according to the cytokines they secrete.28 Furthermore, in the C. trachomatis model the protective effect of cytotoxic T lymphocytes has recently been reported to be dependent on IFN-γ production.29
Given the commonness of C. pneumoniae infection in humans and the accumulating evidence of its association with coronary heart disease, a detailed understanding of the relationship between immunological responses and protection is of paramount importance, for example for the design and development of antichlamydial vaccines. In this study, we show that T cells are essential for clearing C. pneumoniae infection. During primary infection, multiple mechanisms (e.g. innate immunity) overlapped and were equally effective in protection, whereas in acquired immunity CD8-mediated functions were clearly dominant.
Acknowledgments
This study was partially supported by the Academy of Finland (grant no. 8400) and contract no. BIO4-CT96-0152 of Biotechnology programme of the Commission of the European Union. We are greatful for the skilful technical assistance of Outi Rautio, Irene Viinikagas, Raili Haikala and Leena Erkkilä.
REFERENCES
- 1.Kuo C-C, Jackson LA, Campbell LA, Grayston JT. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet. 1997;350:430. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 3.Ward ME. The immunobiology and immunopathology of chlamydial infections. APMIS. 1995;103:769. doi: 10.1111/j.1699-0463.1995.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 4.Cotter TW, Byrne GI. Immunity to Chlamydia: comparison of human infections and murine models. Res Immunol. 1996;147:587. doi: 10.1016/s0923-2494(97)85226-1. [DOI] [PubMed] [Google Scholar]
- 5.Magee DM, Williams DM, Smith JG, et al. Role of CD8 T cells in primary Chlamydia infection. Infect Immun. 1995;63:516. doi: 10.1128/iai.63.2.516-521.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su H, Caldwell HD. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams DM, Grubbs BG, Pack E, Kelly K, Rank RG. Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis. Infect Immun. 1997;65:2876. doi: 10.1128/iai.65.7.2876-2882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buzoni-Gatel D, Guilloteau L, Bernard F, Bernard S, Chardès T, Rocca A. Protection against Chlamydia psittaci in mice conferred by Lyt-2+ T cells. Immunology. 1992;77:284. [PMC free article] [PubMed] [Google Scholar]
- 10.Cox RL, Kuo C-C, Grayston JT, Campbell LA. Deoxyribonucleic acid relatedness of Chlamydia sp. strain TWAR to Chlamydia trachomatis and Chlamydia psitaci. Int J Syst Bacteriol. 1988;38:265. [Google Scholar]
- 11.Kaukoranta-Tolvanen S-SE, Laurila AL, Saikku P, Leinonen M, Liesirova L, Laitinen K. Experimental infection of Chlamydia pneumoniae in mice. Microb Pathog. 1993;15:293. doi: 10.1006/mpat.1993.1079. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z-P, Kuo C-C, Grayston JT. A mouse model of Chlamydia pneumoniae strain TWAR pneumonitis. Infect Immun. 1993;61:2037. doi: 10.1128/iai.61.5.2037-2040.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penttilä JM, Anttila M, Puolakkainen M, et al. Local immune responses to Chlamydia pneumoniae in the lungs of BALB/c mice during primary infection and reinfection. Infect Immun. 1998;66:5113. doi: 10.1128/iai.66.11.5113-5118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekman M-R, Grayston JT, Visakorpi R, Kleemola M, Kuo C-C, Saikku P. An epidemic of infections due to Chlamydia pneumoniae in military conscripts. Clin Infect Dis. 1993;17:420. doi: 10.1093/clinids/17.3.420. [DOI] [PubMed] [Google Scholar]
- 15.Penttilä JM, Pyhälä R, Sarvas M, Rautonen N. Expansion of a novel pulmonary CD3− CD4+ CD8+ cell population in mice during Chlamydia pneumoniae infection. Infect Immun. 1998;66:3290. doi: 10.1128/iai.66.7.3290-3294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann SHE. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 17.Nauciel C, Espinasse-Maes F. Role of gamma interferon and tumour necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992;60:450. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith D, Hänsch H, Bancroft G, Ehlers S. T-cell-independent granuloma formation in response to Mycobacterium avium: role of tumour necrosis factor-α and interferon-γ. Immunology. 1997;92:413. doi: 10.1046/j.1365-2567.1997.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams DM, Magee DM, Bonewald LF, et al. A role in vivo for tumor necrosis factor alpha in host defense against Chlamydia trachomatis. Infect Immun. 1990;58:1572. doi: 10.1128/iai.58.6.1572-1576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darville T, Andrews JRCW, Laffoon K, Shymasani W, Kishen LR, Rank RG. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beatty WL, Byrne GI, Morrison RP. Repeated and persistent infection with Chlamydia and the development of chronic inflammation and disease. Trends Microbiol. 1994;2:94. doi: 10.1016/0966-842x(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 22.Rottenberg ME, Rothfuchs ACG, Gigliotti D, Svanholm C, Bandholte L, Wigzell H. Role of innate and adaptive immunity in the outcome of primary infection with Chlamydia pneumoniae as analyzed in genetically modified mice. J Immunol. 1999;162:2829. [PubMed] [Google Scholar]
- 23.Ottenhoff THM, Mutis T. Role of cytotoxic cells in the protective immunity against and immunopathology of intracellular infections. Eur J Clin Invest. 1995:371. doi: 10.1111/j.1365-2362.1995.tb01716.x. [DOI] [PubMed] [Google Scholar]
- 24.Beatty PR, Stephens RS. CD8+ T lymphocyte-mediated lysis of Chlamydia-infected L cells using an endogenous antigen pathway. J Immunol. 1994;153:4588. [PubMed] [Google Scholar]
- 25.Beatty PR, Rasmussen SJ, Stephens RS. Cross-reactive cytotoxic T-lymphocyte-mediated lysis of Chlamydia trachomatis- and Chlamydia psittaci-infected cells. Infect Immun. 1997;65:951. doi: 10.1128/iai.65.3.951-956.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasmussen SJ, Timms P, Beatty PR, Stephens RS. Cytotoxic-T-lymphocyte-mediated cytolysis of L cells persistently infected with Chlamydia spp. Infect Immun. 1996;64:1944. doi: 10.1128/iai.64.6.1944-1949.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starnbach MN, Bevan MJ, Lampe MF. Protective cytotoxic T lymphocytes are induced during murine infection with Chlamydia trachomatis. J Immunol. 1994;153:5183. [PubMed] [Google Scholar]
- 28.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lampe MF, Wilson CB, Bevan MJ, Starnbach MN. Gamma interferon production by cytotoxic T lymphocytes is required for resolution of Chlamydia trachomatis infection. Infect Immun. 1998;66:5457. doi: 10.1128/iai.66.11.5457-5461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]