Abstract
Two cytokines, interferon-γ (IFN-γ) and interleukin-4 (IL-4), which play critical roles in the regulation of serum IgE level by directing the interplay of T helper (Th)1 and Th2 cells, were chosen as targets for gene therapy. Anti-allergic activity was evaluated by determining the serum IgE level, and the functional status of each helper T cell was monitored by the serum concentrations of IgG1 and IgG2a. Experimental animals (BALB/c mice) were divided into four groups: the control group; the ovalbumin (OVA) group; the IFN-γ group; and the IL-4 group. The control group was injected with saline and the OVA group with OVA–alum. The IFN-γ and IL-4 groups were treated with OVA–alum plus the cDNAs of mouse IFN-γ and IL-4 in an expression vector. These treatments were applied intramuscularly on a monthly basis for 4 months. OVA–alum treatment significantly increased the serum IgE and IgG1 concentrations, but did not affect IgG2a. Concomitant treatments with the cDNA of IFN-γ or IL-4 returned the serum IgE almost to the control level and significantly suppressed the OVA-induced increase of IgG1. IFN-γ cDNA increased the serum IgG2a but IL-4 cDNA had no affect. These results suggest that IFN-γ inhibited the OVA-induced IgE production by suppressing the Th2 pathway and by enhancing the Th1 pathway. Administration of IL-4 cDNA suppressed the OVA-induced enhancement of IgE production by inhibiting the Th2 pathway rather than by potentiating it
INTRODUCTION
Individuals prone to immediate hypersensitivity reactions are called atopic and often have higher levels of IgE in blood and more IgE receptors per mast cell. The overproduction of IgE by the immune response is generally believed to be responsible for the pathogenesis of atopic symptoms, such as asthma, eczema, hay fever and urticaria.1–4
Most of the immune response occurring through CD4+ T helper (Th) lymphocytes can be subdivided into Th1 and Th2 responses. The Th1 response predominantly results in cytotoxic T cells and stimulates IgG2a production. In contrast, a Th2 response produces IgE and eosinophilic infiltration, and stimulates the production of IgG1.5–8 These subtypes also produce unique sets of cytokines, that is, Th1 cells generate interferon-γ (IFN-γ) and interleukin (IL)-2, and Th2 cells produce IL-4, IL-5 and IL-13. A number of factors, including cytokines, might contribute to the T-cell differentiation. A cytokine environment dominated by IL-4 and IL-13 stimulates Th2-cell development, but that dominated by IFN-γ and IL-12 stimulates Th1-cell development.
In accordance with the mechanisms discussed above, we examined the effects of IFN-γ and IL-4 cDNAs on a murine model of atopic allergy using ovalbumin (OVA) as the antigen. The cDNAs of IFN-γ and IL-4 were administered for 4 months on a monthly basis and the levels of total serum IgE, IgG1 and IgG2a were measured to understand how they affect the allergic responses and to investigate the possibility of gene therapy for treatment of allergic diseases.
MATERIALS AND METHODS
Animals
Male BALB/c mice were obtained from the Korean Institute of Chemical Technology Animal Centre (Taejeon, Korea) and maintained on 12-hr light/dark cycles. They had free access to food and water. The mice were 6 weeks old at the start of the experiment and were divided into four groups (n = 28).
Mouse IFN-γ and IL-4 constructs
The complete open reading frame of mouse IFN-γ or IL-4 cDNA was subcloned into a eukaryotic expression vector, pZIPneoSV.7 This vector possesses two long-term repeats and is suitable for chromosomal insertion.
Reagents
The antigen was prepared by conjugating OVA with aluminum hydroxide gel, which was produced from 10% potassium alum. All the antibodies (antimouse IgE, purified mouse IgE, biotinylated antimouse IgE, antimouse IgG1, purified mouse IgG1, biotinylated antimouse IgG1, antimouse Ig2a, purified mouse IgG2a and biotinylated antimouse IgG2a) were purchased from Pharmingen (San Diego, CA). Avidin-peroxidase and the 3,3′,5,5′-tetramethylbenzidine (TMB) liquid substrate system were purchased from Sigma (St. Louis, MO).
Immunization and injection
On day 0, the mice belonging to the IFN-γ and IL-4 groups were given 40 μg IFN-γ or IL-4 cDNA by intramuscular injection.9 Subsequent administrations were similarly conducted every 4 weeks, for 3 months, with 40 μg DNA plus 10 μg/ml OVA–alum (OVA absorbed onto 100 μg/ml Al(OH)3 adjuvant). The control group received saline. Four weeks later (at the second injection for the IFN-γ and IL-4 groups), the OVA group received 10 μg/ml OVA–alum. On day 14 after the third and fourth injection, all mice were bled for collection of sera. The samples were placed for 3 hr at room temperature following which the supernatants were removed and stored at −20° until used.
Measurement of IgE, IgG1 and IgG2a concentrations
Sandwich enzyme-linked immunosorbent assay (ELISA) was performed, in 96-well disposable microtitre plates, to determine the levels of IgE, IgG1 and IgG2a. Phosphate-buffered saline (PBS), pH 7·0, containing 0·5% Tween-20 was used for dilution of samples and for washing. For blocking non-specific binding sites, the same buffer containing 1% bovine serum albumin (BSA) (blocking buffer) was used. Antimouse IgE was used to coat the plates. After the samples and the purified mouse IgE were applied, the plates were washed, and biotin antimouse IgE was added. Avidin-peroxidase diluted to 1 in 10 000 was applied for 30 min. The plates were washed and TMB as a substrate was used to develop the reaction for 20 min. This reaction was stopped with 1 m H3PO4 and the plates were read at 405 nm using an ELISA reader. The same procedure was performed for evaluation of IgG1 and IgG2a. The concentrations of each were determined using the calibration standard curve.
RESULTS
Effect of IFN-γ and IL-4 cDNA on OVA-induced serum IgE
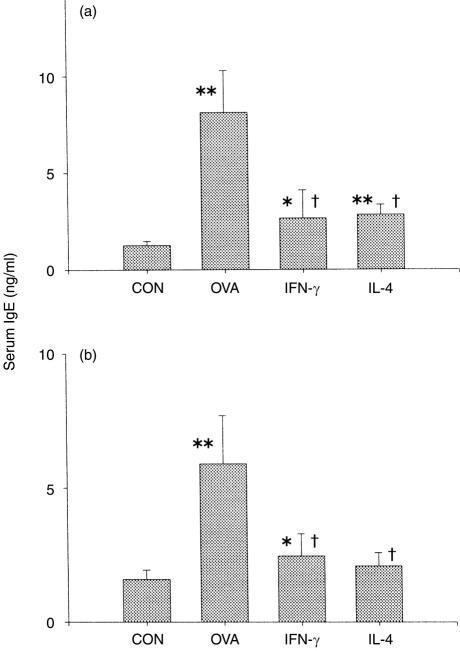
The expression of mouse IFN-γ or IL-4 cDNA subcloned into a eukaryotic expression vector, pZIPneoSV, was confirmed at the protein level (from LM fibroblasts transfected with those constructs) and at the mRNA level (from cell-vaccinated mice).7 After the third injection, the serum IgE level of each group was measured. As previously reported, the serum IgE in the OVA group was significantly elevated compared with the control group (Fig. 1a). The concentration of serum IgE from OVA group was compared with that of standard IgE, which had been routinely used for passive cutaneous anaphylaxis (PCA) in the author’s laboratory, and the PCA titre of OVA-specific IgE was calculated to be ≈25. Because the cytokines produced by Th2 cells are known to play an important role in the enhancement of the allergic response, we examined the effect, on IgE production, of injection of IFN-γ and IL-4 cDNAs. The serum IgE level was significantly decreased by IFN-γ cDNA injection, and unexpectedly also by IL-4 cDNA injection. The same pattern was observed after the fourth injection (Fig. 1b).
Figure 1.
Effect of interferon-γ (IFN-γ) and interleukin-4 (IL-4) cDNAs on serum IgE levels of ovalbumin (OVA)-treated mice. Mice from the IFN-γ and IL-4 groups were injected intramuscularly with 40 μg IFN-γ or IL-4 DNA 4 weeks earlier than the OVA group. Subsequent administrations were every 4 weeks for 3 months with 40 μg DNA plus 10 μg/ml OVA–alum (OVA absorbed onto 100 μg/ml Al(OH)3 adjuvant). The control group (CON) received saline and the OVA group received 10 μg/ml OVA–alum. On day 14 after the third and fourth injections, all mice were bled to collect sera. (a) Blood samples were collected on the 14th day after the third injection. (b) Blood samples were collected on the 14th day after the fourth injection. *P < 0·05 compared with the control group; **P < 0·01 compared with the control group. †P < 0·01 compared with the OVA group
Effect of OVA and cytokines on serum IgG1 and IgG2a levels
The serum levels of IgG1 and IgG2a in each group were measured from the sera obtained after the fourth injection to determine the functional status of both Th1 and Th2 cells following treatment with IFN-γ or IL-4 cDNA.
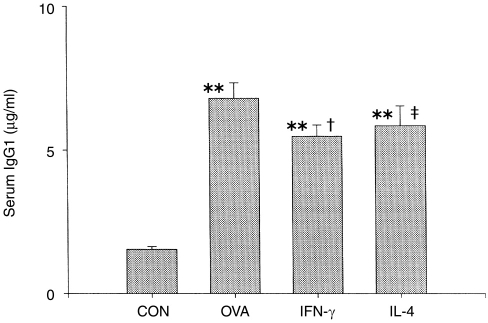
As reported previously, IgG1, along with isotype switching of immunoglobulins, is capable of sensitizing the anaphylactic reaction by stimulating the Th2-cell immune response.16 The IgG1 level of the OVA group was elevated by almost fourfold when compared with the control group (Fig. 2). Concomitant treatment of the animals with OVA–alum and IFN-γ cDNA or IL-4 cDNA significantly decreased the levels of IgG1 compared with the OVA group (P < 0·01 for IFN-γ and P < 0·05 for IL-4).
Figure 2.
Effect of interferon-γ (IFN-γ) and interleukin-4 (IL-4) cDNAs on the serum IgG1 level from ovalbumin (OVA)-treated mice. Animal treatments were performed as described in the legend to Figure 1. Blood samples were collected on the 14th day after the fourth injection. **P < 0·01 compared with the control (CON) group; †P < 0·05 compared with the OVA group; ‡P < 0·01 compared with the OVA group.
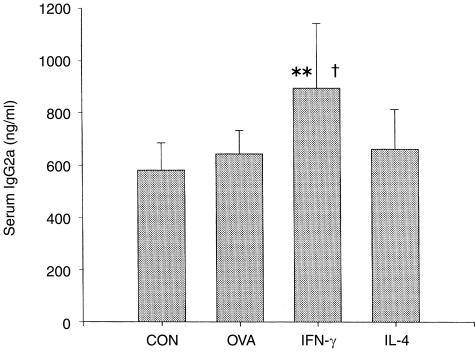
The IgG2a levels of the four groups were also measured to determine the effect of OVA antigen, IFN-γ cDNA, and IL-4 cDNA on the Th1-cell immune response. The IgG2a levels of OVA and IL-4-treated groups were not significantly different from those of the control group (Fig. 3). Only the IFN-γ group, which was injected intramuscularly with OVA–alum plus IFN-γ cDNA, showed an elevated IgG2a level when compared with the control group. This result is in agreement with previous reports.15–19
Figure 3.
Effect of OVA–alum (ovalbumin absorbed onto 100 μg/ml Al(OH)3 adjuvant) and concomitant administration of interferon-γ (IFN-γ) and interleukin-4 (IL-4) cDNAs on serum IgG2a level. The animal treatments were performed as described in the legend to Figure 1. Blood samples were collected on the 14th day after the fourth injection.**P < 0·01 compared with the control (CON) group; †P < 0·01 compared with the OVA group.
DISCUSSION
As previously reported, the OVA–alum treatment caused an elevation in serum IgE level and this was accompanied with an increase in serum IgG1 level. Meanwhile, the serum IgG2a level was not affected by OVA–alum treatment but was increased by concomitant treatment with IFN-γ cDNA. These results suggest that OVA–alum treatment mainly activated the Th2 pathway (as IgG1, but not IgG2a, was elevated) leading to an increase in the level of serum IgE. Concomitant administration of IFN-γ cDNA activated the Th1 pathway but suppressed the Th2 pathway (serum IgG2a was increased, serum IgG1 was decreased) and the increase in serum IgE reverted almost to the control level in the IFN-γ cDNA group. Therefore, not only antagonizing Th2-cell immune responses but also stimulating Th1-cell immune responses in OVA-induced mice probably causes an inhibitory effect of IFN-γ cDNA on the serum IgE level.
Concomitant administration of IL-4 cDNA strongly inhibited the OVA-induced increase of serum IgE. It did not affect the level of serum IgG2a but decreased the serum IgG1 level. The effects of IL-4 cDNA treatment on the OVA-induced elevation of serum IgE were rather unexpected. It was our initial thought that the level of serum IgE would be further increased by IL-4 cDNA treatment because IL-4 is a critical regulator responsible for the isotype switching of IgG to IgE. Our results suggest that IL-4 did not affect the Th1 pathway (the IgG2a level was not changed) but rather it suppressed Th2 pathway (the IgG1 level was decreased). However, the effects of IFN-γ and IL-4 DNA on the distribution of the Th-cell population seem to be complicated and depend on many factors, including the exposure period and the amount of antigen. In these experimental conditions where the cDNA of IL-4 was administrated to the atopic allergic animal but not to the normal animal, IL-4 might have induced the down-regulation of the Th2-cell immune response. Even though we do not have any clear explanation for these IL-4 results at present, our results strongly suggest that both IFN-γ and IL-4 could be potential targets for the gene therapy of atopic allergic diseases.
Acknowledgments
The Korean Ministry of Health and Welfare Grant HMP-97-D-4-0021 supported this work.
REFERENCES
- 1.Heyman B. The immune complex: possible ways of regulating the antibody response. Immunol Today. 1990;11:310. doi: 10.1016/0167-5699(90)90126-t. [DOI] [PubMed] [Google Scholar]
- 2.Kahlert H, Petersen A, Becker WM, Schlaak M. Epitope analysis of the allergen ovalbumin (Gal d II) with monoclonal antibodies and patients’ IgE. Mol Immunol. 1992;29:1191. doi: 10.1016/0161-5890(92)90055-3. [DOI] [PubMed] [Google Scholar]
- 3.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 4.Corrigan CJ, Kay AB. CD4 T-lymphocyte activation in acute severe asthma. Relationship to disease severity and atopic status. Am Rev Respir Dis. 1990;141:970. doi: 10.1164/ajrccm/141.4_Pt_1.. [DOI] [PubMed] [Google Scholar]
- 5.Liblau RS, Singer SM, McDevitt HO. TH1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 6.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 7.Kim TS, Xu WS, Sun T, Cohen EP. Immunization with interleukin-2/interferon-γ double cytokine-secreting allogeneic fibroblasts prolongs the survival of mice with melanoma. Melanoma Res. 1995;5:217. doi: 10.1097/00008390-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Romagnani S. Development of Th1- or Th2-dominated immune responses: what about the polarizing signals? Int J Clin Res. 1996;26:83. doi: 10.1007/BF02592350. [DOI] [PubMed] [Google Scholar]
- 9.Torres CA, Iwasaki A, Barber BH, Robinson HL. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J Immunol. 1997;158:4529. [PubMed] [Google Scholar]
- 10.Hill PN, Liu FT. A sensitive enzyme-linked immunosorbent assay for the quantitation of antigen-specific murine immunoglobulin E. J Immunol Methods. 1981;45:51. doi: 10.1016/0022-1759(81)90093-4. [DOI] [PubMed] [Google Scholar]
- 11.Hirano T, Miyajima H, Kitagawa H, et al. Studies on murine IgE with monoclonal antibodies. I. Characterization of rat monoclonal anti-IgE antibodies and the use of these antibodies for determinations of serum IgE levels and for anaphylactic reactions. Int Arch Allergy Appl Immunol. 1998;85:47. [PubMed] [Google Scholar]
- 12.Oshiba A, Hamelmann E, Haczku A, et al. Modulation of antigen-induced B and T cell responses by antigen-specific IgE antibodies. J Immunol. 1997;159:4056. [PubMed] [Google Scholar]
- 13.Nagai H, Maeda Y, Tanaka H. The effect of anti-IL-4 monoclonal antibody, rapamycin and interferon-γ on airway hyperreactivity to acetylcholine in mice. Clin Exp Allergy. 1997;27:218. [PubMed] [Google Scholar]
- 14.Coffman RL, Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-γ. J Immunol. 1986;136:949. [PubMed] [Google Scholar]
- 15.Kim TS, DeKruyff RH, Rupper R, Maecker HT, Umetsu DT. An ovalbumin–IL-12 fusion protein is more effective than ovalbumin plus free recombinant IL-12 in inducing a T helper cell type 1-dominated immune response and inhibiting antigen-specific IgE production. J Immunol. 1997;158:4137. [PubMed] [Google Scholar]
- 16.Hazenbos WL, Gessner JE, Hofhuis FM, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in FcγIII (CD16) deficient mice. Immunity. 1996;5:181. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 17.Walker C, Virchow JC, Jr, Bruijnzeel PL, Blaser K. T cell subsets and their soluble products regulate eosinophilia in allergic and nonallergic asthma. J Immunol. 1991;146:1829. [PubMed] [Google Scholar]
- 18.Grun J, Maurer PH. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell Immunol. 1989;121:134. doi: 10.1016/0008-8749(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 19.Yang X, Hayglass KT. Allergen-dependent induction of interleukin-4 synthesis in vivo. Immunology. 1993;78:74. [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy E, Shibuya K, Hosken N, et al. Reversibility of T helper 1 and helper 2 populations is lost after long-term stimulation. J Exp Med. 1996;183:901. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]