Abstract
Mannan-binding lectin (MBL) is a C-type serum lectin that is believed to play an important role in innate immunity. It is one of the collectin family, which is characterized by having a collagen-like sequence and a carbohydrate recognition domain. MBL can bind to sugar determinants of several micro-organisms, neutralize them and inhibit infection by complement activation through the lectin pathway and opsonization by collectin receptors. Bovine conglutinin and mouse MBL inhibit the infective and haemagglutinating activities of influenza A viruses. To identify the direct antiviral activity of human MBL against influenza A viruses that does not depend on complement activation or opsonization, we isolated native MBL from human serum and produced a recombinant MBL in Chinese hamster ovary (CHO) cells using a pNOW/CMV-A expression vector system. Native and recombinant human MBL exhibited neutralization activity against A/Ibaraki/1/90 (H3N2), with the plaque focus reduction assay at the viral attachment phase. Their activities were inhibited by EDTA, mannose and anti-human MBL antibody. Furthermore, at the viral expansion phase both MBL in culture medium prevented viral spreading from primary infected cells to neighbour cells. A virus recovery study using EDTA indicated that interaction between MBL and virus was reversible and non-damaging to the virus. Lectin blot and immunohistochemistry assays showed that these antiviral activities involved binding between MBL and two viral envelope proteins, haemagglutinin and neuraminidase. These findings suggest that human MBL can play an important role in innate immunity by direct viral neutralization and inhibition of viral spread, as well as an indirect role through opsonization and complement activation.
INTRODUCTION
Collectins are characterized by having a collagen-like domain and a carbohydrate recognition domain (CRD)1 that binds to side chains on glycoconjugates rich in d-mannose and N-acetylglucosamine. Collectins are members of the C-type lectin family, which include mannan-binding lectin (MBL),2 conglutinin,3 surfactant protein A (SP-A),4 surfactant protein D (SP-D)5 and collectin 43.6 Human MBL, first identified by Kawasaki,2 seems to play a role in innate immunity, by destroying bacteria via a complement-mediated mechanism and as an opsonin by binding to the C1q receptor. Genetic deficiency of MBL is associated with recurrent infections in children and adults. The concentration of MBL in serum varies according to its allelic forms owing to gene mutations and sequence polymorphism in the promoter region.13
Human and animal sera contain various inhibitors that can neutralize the infectivity or inhibit the haemagglutination of influenza viruses.14 Three classes of such inhibitors have been reported. Two, α and γ inhibitors, are sialylated glycoproteins that inhibit viral haemagglutination by behaving like receptor analogues. The third, so-called β inhibitors, which are not receptor analogues do not contain sialic acid. Bovine conglutinin and mouse mannan-binding lectin (MBL) were found to be β inhibitors because they inhibited the infectivity and haemagglutinating activity of the H1 and H3 subtypes of influenza A viruses.
Human MBL is also a β inhibitor that binds to influenza A virus or inhibits its haemagglutination.17 However, the antiviral activities of human MBL against influenza A viruses are not well characterized.
In this study, we investigated the characteristics of the direct anti-influenza virus activities of human MBL without complement activation or opsonization. We show that human MBL inhibits influenza A virus infection by two mechanisms. First, it blocks viral attachment to host cells, and second, it prevents viral spreading to contiguous cells by interfering with the budding process and viral release. Although other mechanisms are also involved in viral inhibition by MBL, it is probable that these two primitive neutralizing activities of human MBL play important roles in human defence against influenza virus infection.
MATERIALS AND METHODS
Cells, viruses and reagents
MDCK (Madin–Darby canine kidney) cells were grown in modified Eagle’s medium (MEM) (Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum and penicillin–streptomycin. The viral growth medium was MEM supplemented with 0·2% bovine serum albumin (Fraction V), 0·1% glucose, 3% tissue culture vitamins (Flow Laboratories, Inc. Mclean, VA), 2 mg/1 acetyltrypsin and 5 mg/1 amphotericin B. A/Suita/1/89 (H1N1), A/Adachi/2/57 (H2N2), A/Kumamoto/1/65 (H2N2), A/Ibaraki/2/90 (H3N2) and A/Osaka/1/70 (H3N2, a mouse-adapted strain) are laboratory strains and were grown in allantoic cavities of 10-day-old embryonated hen eggs. The allantoic fluid was harvested and stored at −70°. The clinical strains were isolated in MDCK cells in Osaka University as described previously.20 A/Beijing/352/89(H3N2), A/Kitakyushu/159/93 (H3N2) and A/Yamagata/32/89 (H1N1) are vaccine strains kindly provided by Dr H. Kumihashi (The Research Foundation for Diseases of Osaka University, Kannonji, Japan). Anti-influenza A virus monoclonal antibody was purchased from Chemicon International Inc. (Temecula, CA) and peroxidase–goat anti-peroxidase complex was from Organon Teknika Corp. (Boxtel, the Netherlands). Anti-human MBL antibody was kindly provided by Japan Chemical Research (Kobe, Japan). Murine anti-haemagglutinin (HA) and anti-neuraminidase (NA) polyclonal antibodies were prepared by immunizing mice with the HA and NA bands from sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). Their identities were confirmed by N-terminal amino acid sequence analyses.
Preparation of two human MBL
Native human MBL was purified from human serum. Human serum was adjusted to 10 mm calcium with CaCl2 and was applied to mannan–agarose (Sigma Chemical Co., St Louis, MO) that had been equilibrated with TBS (10 mm Tris–HCl, 150 mm NaCl) containing 10 mm CaCl2, and kept overnight at 4°. Recombinant human MBL was produced in a high-level expression system using pNOW/CMV-A expression vector.21 The culture supernatant of stable transformants was dialysed against TBS and TBS/Ca (TBS containing 10 mm CaCl2). The dialysed culture solution was applied to a column (10 ml) of mannan–agarose that was washed with TBS/Ca extensively. The columns bound with both MBL were eluted with TBS/E (TBS containing 10 mm EDTA). The EDTA eluates were adjusted to 10 mm calcium and were applied to mannan–agarose again, and then gels were washed with TBS/Ca. Pure MBL were eluted with TBS/Ca containing 100 mm d-mannose and kept at 4°. The MBL were quantified by SDS–PAGE and Coomassie brilliant blue staining.
Haemagglutination inhibition test
The haemagglutination inhibition (HI) test was performed by a standard microtitre assay method in 96-well microplates with 0·5% (v/v) chicken erythrocytes, as described previously.15 Five HA units of viral solution were incubated with dilutions of MBL for 30 min at 37° and then mixed with the chicken erythrocytes. The level of inhibition of virus-mediated agglutination of chicken erythrocytes by human MBL was determined after 1 hr of incubation at 37°. To inhibit the activity of human MBL, some sugars and EDTA were added after incubation of viruses with MBL. The HI titre was expressed as the lowest concentration of human MBL causing HI.22
Neutralization test
The neutralization (NT) test was the rapid focus reduction neutralization test described previously.23 Briefly, MDCK cells (3 × 105 cells/ml) were cultured in a 96-well microplate (Iwaki Glass, Tokyo, Japan) for 3 days. Influenza A virus A/Ibaraki/1/90 (about 100 focus forming units; FFU) was mixed with each diluted human MBL solution at 37° for 60 min, washed with phosphate-buffered saline (PBS), inoculated onto the cell monolayers, and incubated at 37° in a CO2 incubator for 60 min. The cells were then washed with PBS, and cultured with the influenza viral growth medium described above, containing 0·5% tragacanth gum (Wako Chemical Industries, Osaka, Japan). After 24 hr, the cells were washed with PBS and fixed with absolute ethanol. The cells were immunostained as described below using anti-influenza A virus monoclonal antibody, rabbit anti-mouse IgG diluted 1/500, and peroxidase–rabbit anti-peroxidase complex, and the numbers of stained foci were counted using a light microscope. The NT activity is presented as the percentage reduction of FFU compared with FFU from infection without MBL. FFU are the means of triplicates from a single experiment. The inhibition of NT using anti-human MBL antibody was performed as above with the rapid focus reduction test. The antibody was prepared by immunizing rabbits with native MBL. Preimmunization serum from the same animal was used as a control.
Recovery test by detaching influenza virus from human MBL with EDTA
Influenza A virus A/Ibaraki/1/90 (3000 FFU) and human MBL (3 μg in 300 μl) were mixed and incubated at 37° for 60 min. EDTA at final concentrations of 0, 1 or 10 mm was then added. The HI test was performed as above by using 50 μl of this solution (equivalent to 5 HA=500 FFU). Approximately 30 μl of diluted solution (equivalent to 30 FFU) containing detached virus was used for an infection assay after addition of MgCl2 (1 mm final concentration). The virus infection assay was the rapid focus forming assay described above. MDCK cells (3 × 105 cells/ml) were cultured in a 96-well microplate for 3 days. The detached influenza A virus solution was inoculated onto the cell monolayer after washing with PBS, and for viral adsorption the cells were incubated in a CO2 incubator for 60 min. The remaining EDTA did not affect the cell monolayer. After incubation, cells were washed with PBS, and then were cultured with the influenza viral growth medium described above, containing 0·5% tragacanth gum. After 24 hr, cells were washed with PBS and fixed with absolute ethanol. The cells were then stained in same way and the numbers of stained foci were counted and compared.
The immunoplaque assay for viral growth inhibition
After viral inoculation (50 FFU) onto monolayers of MDCK cells in 24-well microplates (Iwaki Glass), cells were incubated at 37° in a CO2 incubator for 60 min, washed with PBS, and incubated with influenza viral growth medium containing 0·5% tragacanth gum and several dilutions of human MBL. After 3 days of culture the cells were washed, fixed with absolute ethanol and incubated with anti-influenza A virus monoclonal antibody diluted 1/500, rabbit anti-mouse IgG diluted 1/500, and peroxidase–rabbit anti-peroxidase complex. Each treatment was for 40 min and was followed by washing with PBS. Finally, a peroxidase reaction was allowed to develop for about 5 min by the method of Graham & Karnovsky,24 in which 0·0l% H2O2 and 0·3 mg/ml 3,3′-diaminobenzidine tetrahydrochloride (Wako Chemical Industries) in PBS were used. The cells were then rinsed with tap water and dried. The infected areas were scanned using HP DeskScan II software by Scanjet IIcx (Hewlett-Packard Co., Palo Alto, CA) and traced and calculated using Color-it (MicroFrontier Inc., Des Moines, IA) and NIH Image 1.60 software.
Immunostaining and lectin-staining of the cells infected with influenza A viruses
After viral infection, the fixed cells were incubated and stained with anti-influenza A virus monoclonal antibody diluted 1/500, rabbit anti-mouse IgG diluted 1/500 and peroxidase–rabbit anti-peroxidase complex, and 0·0l% H2O2 and 0·3 mg/ml 3,3′-diaminobenzidine tetrahydrochloride in PBS were used as described above. The cells were then rinsed with tap water and dried. For lectin-staining of infected cells in a 96-well microplate, 50 μ1 of human MBL (1 μg/ml) was added per well after washing with PBS. Following incubation at 37° for 60 min, cells were washed with PBS and fixed with absolute ethanol. The cells were stained with rabbit anti-human MBL serum diluted 1/20, goat anti-rabbit IgG serum diluted 1/1000 and peroxidase–rabbit anti-peroxidase complex as described above.
Lectin blotting and immunoblotting of viral proteins
After ultracentrifugation of A/Ibaraki/1/90 at 74 000 g for 120 min, virions (10 μg) were dissolved in SDS sample buffer, separated by SDS–PAGE (4–20% gradient gel) and transferred to BioBlot-NC membranes (Corning Costar Corp.; Cambridge, MA) by standard procedures.25 After blocking the membranes with Block Ace (Dainippon Pharmaceutical Co. Ltd, Osaka, Japan) in TTBS (20 mm Tris–HCl, 140 mm NaCl, 0·05% Tween-20), they were incubated with human MBL for 60 min, washed in TTBS with or without CaCl2, EDTA (10 mm) or d-mannose (1 m), and then incubated with rabbit anti-human MBL in TTBS for 60 min. After washing in TTBS, the membranes were incubated with goat anti-rabbit IgG-conjugated biotin (Vector Laboratories Inc., Burlingame) and alkaline phosphatase-conjugated streptavidine (Gibco BRL), and stained with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium (BCIP-NBT system; Gibco BRL). Lectin blotting using bovine conglutinin was performed as above. To confirm the HA and NA glycoproteins, we immunostained the above membranes using murine anti-HA and anti-NA polyclonal antibody. These antibodies were prepared by directly immunizing mice with HA and NA bands from SDS–PAGE, which were confirmed by N-terminal amino acid sequence analyses.
RESULTS
HI activity of human MBL against influenza A viruses
Human MBL was examined for its HI activity against influenza A viruses. Table 1 shows the minimum concentrations of human native MBL causing HI against laboratory and vaccine strains passaged in embryonated hen eggs. The results show that A/Yamagata/32/89 (H1N1), A/Ibaraki/1/90 (H3N2) and A/Beijing/352/89 (H3N2) were more sensitive to HI by human MBL than A/Adachi/2/57 (H2N2), A/Kumamoto/ 1/65 (H2N2) and mouse-adapted A/Osaka/1/70 (H3N2). A/Kitakyushu/159/93 (H3N2) had intermediate sensitivity. Furthermore, among 67 clinical isolates (60 of subtype H3N2 and seven of subtype H1NI) that were isolated from 1990 to 1995 and passaged less than six times in MDCK cells, the average concentration of human MBL causing HI was 0·31±0·67 μg/ml (mean±SD). The distribution of clinical isolates with respect to sensitivity to human MBL is shown in Fig. 1. The modal peak of HI occurred at an MBL concentration of 0·15 μg/ml (46·3%). All isolates showing a titre over 1·25 μg/ml were H1N1 subtypes. Fresh isolates of subtype H3N2 had high sensitivity. On the other hand, there were a few insensitive strains of subtype H1N1 in fresh clinical isolates.
Table 1.
The HI activity of human native MBL against influenza A viruses*
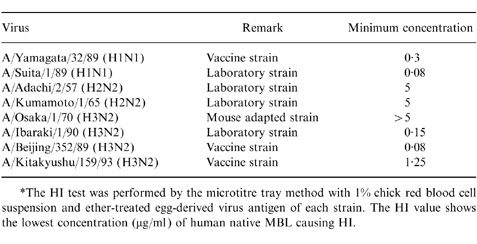
*The HI test was performed by the microtitre tray method with 1% chick red blood cell suspension and ether-treated egg-derived virus antigen of each strain. The HI value shows the lowest concentration (μg/ml) of human native MBL causing HI.
Figure 1.

The distribution of HI titres by human native MBL against clinical isolates of influenza viruses from 1990 to 1995 in Osaka, Japan. The HI test was performed by the microtitre tray method with 1% chick red blood cell suspension, ether-treated egg-derived virus antigen of each strain, and dilutions of MBL. The HI value shows the lowest concentration (μg/ml) of human MBL causing HI.
To determine whether the HI activity of human MBL is the result of it being a lectin, each of four sugars (mannose, GlcNac, glucose, maltose) or EDTA was added to a mixture of human MBL and influenza A virus A/Ibaraki/1/90 (H3N2). The range of sugar concentrations was 0, 50, 100 and 500 mm and the range of EDTA concentrations was 0, 1, 5 and 10 mm. The HI activity of human MBL was inhibited by 100 mm mannose, 50 mm GlcNAc, 500 mm glucose and 1 mm EDTA (data not shown).
NT activity of human MBL against influenza A virus
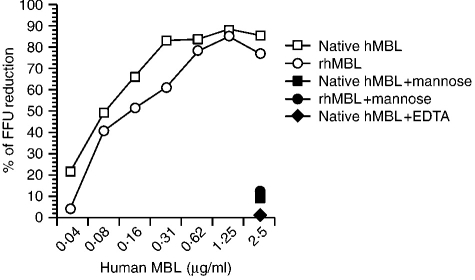
To investigate whether human MBL can neutralize influenza A virus, the NT test with native and recombinant human MBL was performed using the rapid focus reduction assay.23 Both MBL neutralized the infectivity of influenza A virus A/Ibaraki/1/90 (H3N2). It has been found previously that this strain is sensitive to conglutinin and MBL.15 The rapid focus reduction assay showed that human MBL apparently suppressed influenza A virus attachment at a concentration of more than 0·3 μg/ml and that this activity was inhibited by 100 mm mannose or 10 mm EDTA (Fig. 2). Therefore, the NT activity of MBL is also dependent on its lectin activity. Furthermore, anti-human MBL antibody inhibited the NT activity of human MBL against influenza A virus at dilutions of 1:8 or 1:16 (Fig. 3). These results indicate that human MBL can suppress influenza A virus infectivity without the activation of complement.
Figure 2.
The neutralizing activity of native and recombinant human MBL against the influenza A virus A/Ibaraki/1/90 (H3N2) was determined by the rapid focus reduction test. The NT activity is shown as the percentage reduction of FFU compared with FFU from infection without MBL. FFU is the mean of triplicate results in a single experiment. About 100 FFU of virus was incubated with each dilution of native human (h) MBL, recombinant (r) human MBL, native human MBL with 100 mm d-mannose, recombinant human MBL with 100 mm d-mannose, or native human MBL with 10 mm EDTA.
Figure 3.
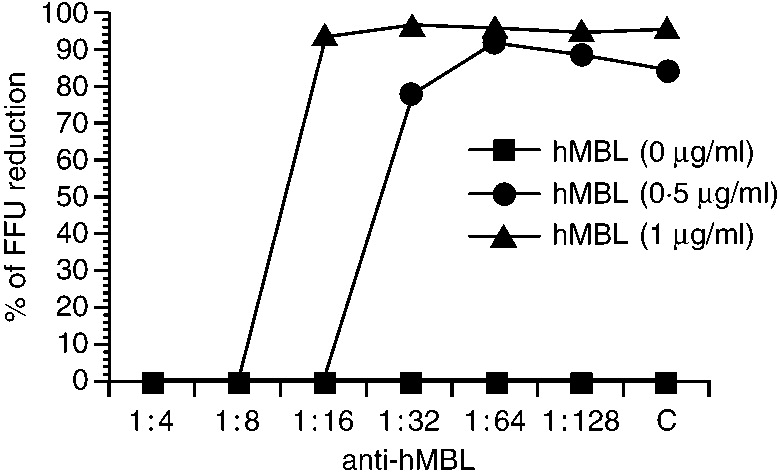
The inhibition of the neutralizing activities of human native MBL by rabbit anti-human MBL antibody. About 100 FFU of the influenza A virus A/Ibaraki/1/90 (H3N2) was incubated with different concentrations of human MBL and each dilution of anti-human MBL antibody. C indicates control rabbit antibody diluted, 1:4.
Recovery of haemagglutination and infection by influenza virus detached from human MBL
We were interested in seeing if MBL injured the virus by binding to it. We prepared virus detached from MBL by EDTA. The study showed that the virus detached from MBL by 10 mm EDTA recovered haemagglutination activity (Fig. 4a) and infectivity to their original levels (Fig. 4b). EDTA only without MBL could not affect the haemagglutination and infection by the virus.
Figure 4.
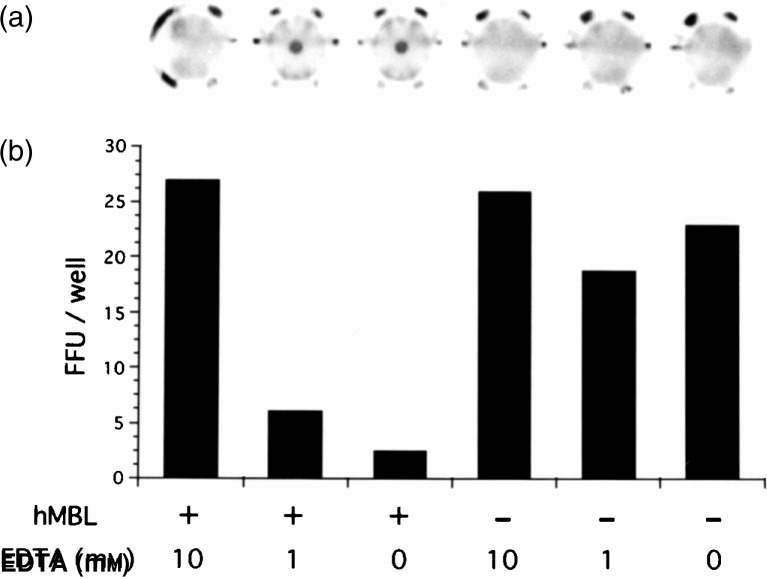
Recovery test by detaching influenza virus from human native MBL with EDTA. The influenza A virus A/Ibaraki/1/90 (3000 FFU) and human native MBL (0·3 μg) in 300 μl were mixed and incubated for 60 min. EDTA was added at the end of the incubation. The final concentrations of EDTA were 0, 1 or 10 mm. The HI test (a) was performed as in Fig. 1 by using 50 μl of this solution (equivalent to 5 HA=500 FFU). The virus infection assay (b) was performed by the rapid focus forming assay using 30 μl of the diluted solution (equivalent to 30 FFU). The cells were then stained and the numbers of stained foci counted and compared.
Blood glucose might be effective against MBL activity in humans, as there are less monosaccharides such as mannose, GlcNAc and calcium chelators in human serum. We examined the effect of glucose on the NT activity of human MBL. Concentrations of glucose in the normal range (less than 10 mm) had no influence on the NT activity of human MBL. However, high concentrations (around 50 mm) of glucose inhibited the activity of human MBL (data not shown).
Viral growth inhibition by human MBL
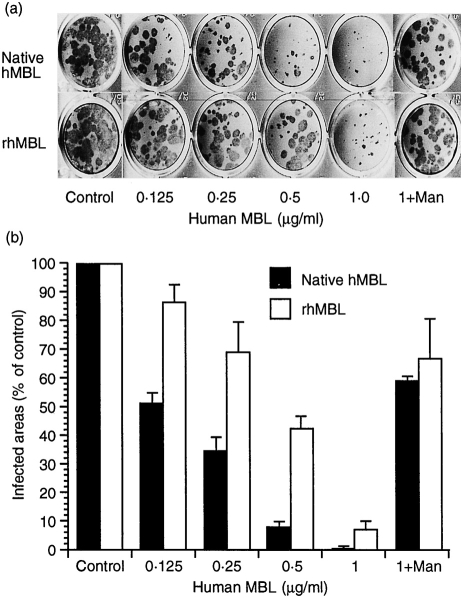
To determine whether MBL could inhibit viral growth in host cells, immunohistochemistry assays were performed. The areas infected with influenza A virus decreased when a gelling medium containing human MBL was overlaid on MDCK cells after infection (Fig. 5a). We found that 0·5 μg/ml of human native MBL reduced the infected areas to less than 10% of the controls and that even 0·125 μg/ml reduced them to 50% of controls (Fig. 5b). Recombinant MBL also inhibited viral growth in a dose-dependent manner. When 100 mm mannose was added to the overlay medium containing 1 μg/ml of either of the human MBL, the infected areas recovered to about 60% of controls.
Figure 5.
Viral growth inhibition by human native and recombinant MBL. MDCK cells inoculated with influenza A virus A/Ibaraki/1/90 (H3N2) (50 FFU/well) were incubated for 60 min, and then incubated with influenza viral growth medium containing 0·5% tragacanth gum and diluted human native and recombinant MBL (0, 0·125, 0·25, 0·5, 1·0 μg/ml) without or with 100 mm d-mannose (Man). The infected foci were stained by using anti-human MBL antibody with the immunohistochemistry assay described in the Materials and Methods (a). The infected area is presented as a histogram of percentages of the control (mean±SD, n = 6) (b).
Lectin staining of cells infected with influenza A virus
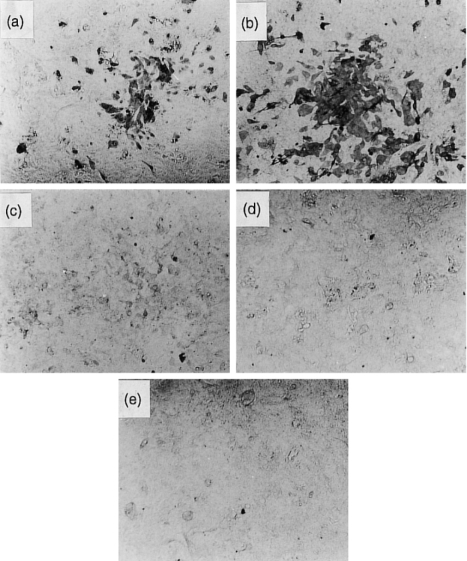
Figure 6a shows a positive control using anti-influenza virus rabbit serum in which the cells infected with influenza A virus were clearly stained by immunostaining. Figure 6b shows that the same infected cells were also positive for lectin staining. Mannose in the culture medium resulted in no lectin staining of infected cells (Fig. 6d). Figure 6e shows no lectin staining in mock infection, and Fig. 6c is a negative control without human MBL. We also found that cells infected with A/Suita/1/89 (H1N1) were clearly stained for lectin but that cells infected with A/Adachi/2/57 (H2N2) were not (data not shown). These results indicate that human MBL can bind only in cells infected with sensitive influenza A viruses (subtypes of H1N1 and H3N2) and that it might prevent viral spreading to adjacent cells.
Figure 6.
The immunohistochemistry and lectin staining analysis of cells infected with influenza A virus A/Ibaraki/1/90 (H3N2). The staining study utilized anti-influenza A virus antibody or human native MBL (1 μg/ml). (a) Anti-influenza A virus antibody as positive control; (b) anti-human MBL with human MBL; (c) anti-human MBL without human MBL; (d) anti-human MBL with human MBL and 100 mm d-mannose; (e) anti-human MBL antibody with human MBL as a negative control of mock infection.
Human MBL can bind both HA and NA of envelope glycoproteins
A lectin blot showed that human MBL could bind to both HA and NA of glycoproteins (Fig. 7, lane 2). A bovine conglutinin (Fig. 7, lane 1) could bind to HA but not to NA, as shown previously.25 HA and NA molecules were confirmed by immunoblotting with murine anti-HA and anti-NA antibodies (Fig. 7, lane 5 and 6). Both positive bands were reduced by treatment with EDTA or mannose (Fig. 7, lane 3 and 4).
Figure 7.
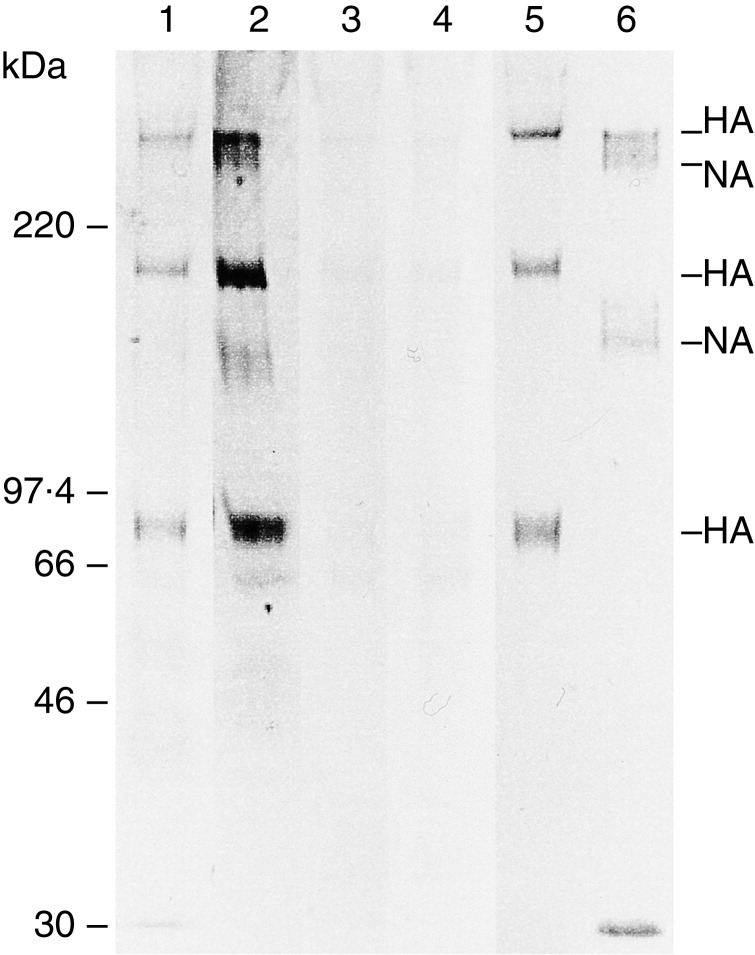
The lectin blotting and immunoblotting of influenza A virus A/Ibaraki/1/90 (H3N2). The viral proteins transferred onto the membrane were treated with human native MBL, bovine conglutinin, murine anti-HA polyclonal antibody or murine anti-NA polyclonal antibody. Lane 1, lectin blotting with bovine conglutinin; lane 2, lectin blotting with human MBL; lane 3, lectin blotting with human MBL and EDTA; lane 4, lectin blotting with human MBL and d-mannose; lane 5, immunoblotting with anti-HA antibody; lane 6, immunoblotting with anti-NA antibody.
DISCUSSION
Recent papers have shown that human MBL is associated with two serine proteases, MASP-126 and MASP-2,27 and that it is inhibited by α2-macroglobulin.28 IgG and IgM might also bind to MBL, making it difficult to purify MBL from plasma or serum. SDS–PAGE and silver staining showed that MBL purified by only using a mannan-column contains many contaminating proteins. Recently we developed a high-expression system of recombinant bovine conglutinin.29 To avoid the contaminant problem, we developed a high-expression system for recombinant human MBL.21 This recombinant human MBL has an oligomeric structure, sugar specificity and complement activation activity similar to native MBL (data not shown). In this study, we tried to characterize the activity of human MBL that inhibits infection by influenza A virus without complement activation or opsonization. We used two types of neutralization assays with purified native MBL and a recombinant MBL produced in Chinese hamster ovary (CHO) cells. The first was a classical plaque reduction assay to measure the inhibition of viral attachment, and the second was a viral growth inhibition test to investigate the inhibition of budding or viral release. We found two inhibitory activities of human MBL that were independent of complement activation. After tight binding, the virus and the viral-infected cells will be cleared by a phagocytotic mechanism due to collectin receptor, or they will be lysed by complement activation through a ‘lectin pathway.’30.
Previously several animal collectins have been shown to exhibit HI and NT activities. Bovine conglutinin and SP-D showed very high HI and NT activities at 0·04–0·08 μg/ml. Compared with these, human MBL has lower HI (0·31 0·67 μg/ml) and NT (0·3 μg/ml) activities. Terai et al. found that the concentration of MBL in the serum of Japanese is about 1·72±1·15 μg/ml.31 Our preliminary study has shown that NT activities of MBL in rabbit and bovine are 0·2 μg/ml and 0·08 μg/ml, respectively. The concentrations of MBL in animal species, except for human, are usually high (data not shown). Human MBL has a moderate NT value and a low serum concentration.
The neutralizing effect of human MBL without complement activation was not irreversible. MBL could not kill virus, so that after detachment from the lectin by EDTA or mannose the virus could infect cells and cause haemagglutination of chicken red blood cells (Fig. 4). Physiologically, there are neither chelating agents nor high glucose levels in humans. However, the hyperglycaemia (around 50 mm glucose) might inhibit binding of MBL to virus, for example transiently in diabetes mellitus and some viral infections or locally in focus by microbial infections.
Collectin is considered a β-inhibitor against influenza A virus and has HI and NT activity, but it does not act on the H2N2 subtype of influenza A virus.15 This is because the glycosylation pattern of the H2N2 subtype is not of the mannose or hybrid-type sugar, although the H1N1 and H3N2 subtypes have hybrid-type sugar and high mannose glycosylation patterns.32 Human MBL also exhibited two activities against these two subtypes (H1N1 and H3N2). This sensitivity is considered to be mainly dependent on the sugar chain on HA molecules. The high mannose chain at residue 165 of HA is conserved in all H3 subtype influenza A viruses so they are sensitive to animal collectins. A β-inhibitor-resistant mutant virus was characterized by loss of the glycosylation site at residue 165.14 The sugar chain at residue 165 in H3 subtypes extends close to the receptor-binding site on the adjacent HA molecule. This loss of sugar chain can make the receptor binding site easily accessible to the cell-surface receptor without binding between collectin and the sugar chain. Other glycosylation sites are at residues 8, 22, 38 and 285 of HA1 and 154 of HA2, which were located on the HA stalk.33 These sites also might be associated with the sensitivity of H3 subtype viruses to collectin.
NA is an another envelope protein that is glycosylated. Several lectin blot studies have shown that NA is recognized by collectins. Malhotra et al. found that human SP-A and MBL bound to NA but not to HA.17 Our recent lectin blot study revealed that SP-D could bind to NA as well as HA but bovine conglutinin bound mainly to HA.25 This study was confirmed by using recombinant truncated trimer SP-D and conglutinin lacking the collagen domain. The lectin blot in this study showed that human MBL was able to bind to both proteins. Furthermore, truncated MBL was not oligomeric or a very large structure but it was able to bind to both HA and NA, as did oligomeric native MBL.34 This indicates that human SP-D and MBL binds to both HA and NA, and conglutinin only to HA. The role of NA in viral infection and replication is not clear. There are several reports that neutralizing antibody against NA inhibits viral release from infected cells35 and inhibitors of NA prevent viral replication in mice.36 In our experiment, the inhibition of viral spread by MBL indicates that MBL can act like a neutralizing antibody against NA. Preliminary viral growth inhibition studies using rabbit and bovine MBL, bovine conglutinin and human SP-D showed that the collectin group (rabbit and bovine MBL, human SP-D), which can bind to NA as well as HA, exhibited stronger inhibition than bovine conglutinin, which binds only to HA (data not shown). Malhotra et al. and Reading et al. also showed that human MBL and SP-A and rat SP-D inhibit NA activity. Malhotra et al. suggested that this inhibition resulted from direct binding of human MBL and SP-A to NA,17 and Reading et al.37 suggested that the inhibition resulted from steric hindrance of the active site of NA by SP-D binding to adjacent HA molecules on the virions. Further experiments are required to elucidate these biological properties of collectins.
Genetic deficiency of MBL has subtle consequences in most cases but sometimes causes severe immunodeficiency in children and adults. A complementation study in MBL-deficient animals, such as MBL knock-out mice, should help in understanding the real function of MBL. However, this is not easy because mice have two MBL genes38 and other collectins such as SP-D and SP-A might be increased to compensate for the lack of MBL.39 Reading et al. showed in a mouse study that SP-D and MBL were related in the inhibition of viral growth in the lung.37 Our preliminary study showed that the viral strains sensitive to collectins could not grow in murine lung in normal or severe combined immunodeficiency disease mice. Several recent clinical isolates of the H1N1 subtype showed resistance to human MBL, while all the clinical isolates of the H3N2 subtypes showed sensitivity in this study. The loss of glycosylation on HA was found among H1N1 subtypes, and similar isolates were recovered from an immunodeficient child with persistent infection.40 These results indicate that some of the pathogenicity and virulence of influenza virus might be affected by collectins binding to mannose or another saccharide on viral envelope glycoproteins.
It is clear that the major impact on the pathogenesis of influenza virus has been attributed to antigenic shift and drift. The major host defence involves neutral antibody and cytotoxic T lymphocytes (CTL). However, the present results suggest that human MBL can also play a role in innate immunity by direct viral neutralization and inhibition of viral spread, as well as by acting indirectly through opsonization and complement activation. Other mechanisms are also involved in viral inhibition by MBL, and further experiments are required to determine the biological roles of human MBL.
This work was supported in part by the Grants-in Aid for Scientific Research (numbers 09672356, 10178210) from the Ministry of Education, Science, Sports, and Culture of Japan (to N. Wakamiya and Y. Suzuki), Fuso Pharmaceutical Industry and the Sankyo Foundation of Life Science.
Acknowledgments
We are grateful to Professor T. Azuma and the late Vt Dr M. Naiki for useful discussions and encouragement. We thank Mr S. Yamamoto for analysis of the aa sequence of human MBL, and Dr H. Schulman for critical reading of the manuscript.
This work was supported in part by the Grants-in Aid for Scientific Research (numbers 09672356, 10178210) from the Ministry of Education, Science, Sports, and Culture of Japan (to N. Wakamiya and Y. Suzuki), Fuso Pharmaceutical Industry and the Sankyo Foundation of Life Science.
Glossary
Abbreviations
- HA
haemagglutinin
- HI
haemagglutination inhibition
- MBL
mannan-binding lectin
- NA
neuraminidase
- SP-A
surfactant protein A
- SP-D
surfactant protein D
REFERENCES
- 1.Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988;263:9557. [PubMed] [Google Scholar]
- 2.Kawasaki N, Kawasaki T, Yamashina I. Isolation and characterization of a mannan-binding protein from human serum. J Biochem Tokyo. 1983;94:937. doi: 10.1093/oxfordjournals.jbchem.a134437. [DOI] [PubMed] [Google Scholar]
- 3.Davis AED, Lachmann PJ. Bovine conglutinin is a collagen-like protein. Biochemistry. 1984;23:2139. doi: 10.1021/bi00305a006. [DOI] [PubMed] [Google Scholar]
- 4.White RT, Damm D, Miller J, et al. Isolation and characterization of the human pulmonary surfactant apoprotein gene. Nature. 1985;317:361. doi: 10.1038/317361a0. [DOI] [PubMed] [Google Scholar]
- 5.Persson A, Chang D, Rust K, Moxley M, Longmore W, Crouch E. Purification and biochemical characterization of CP4 (SP-D), a collagenous surfactant-associated protein. Biochemistry. 1989;28:6361. doi: 10.1021/bi00441a031. [DOI] [PubMed] [Google Scholar]
- 6.Holmskov U, Holt P, Reid KB, Willis AC, Teisner B, Jensenius JC. Purification and characterization of bovine mannan-binding protein. Glycobiology. 1993;3:147. doi: 10.1093/glycob/3.2.147. [DOI] [PubMed] [Google Scholar]
- 7.Kuhlman M, Joiner K, Ezekowitz RA. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989;169:1733. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malhotra R, Sim RB, Reid KB. Interaction of C1q, and other proteins containing collagen-like domains, with the C1q receptor. Biochem Soc Trans. 1990;18:1145. doi: 10.1042/bst0181145. [DOI] [PubMed] [Google Scholar]
- 9.Sumiya M, Super M, Tabona P, et al. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337:1569. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- 10.Summerfield JA, Ryder S, Sumiya M, et al. Mannose binding protein gene mutations associated with unusual and severe infections in adults. Lancet. 1995;345:886. doi: 10.1016/s0140-6736(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 11.Lipscombe RJ, Sumiya M, Hill AV, et al. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet. 1992;1:709. doi: 10.1093/hmg/1.9.709. [DOI] [PubMed] [Google Scholar]
- 12.Madsen HO, Garred P, Kurtzhals JA, et al. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics. 1994;40:37. doi: 10.1007/BF00163962. [DOI] [PubMed] [Google Scholar]
- 13.Madsen HO, Garred P, Thiel S, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155:3013. [PubMed] [Google Scholar]
- 14.Anders EM, Hartley CA, Jackson DC. Bovine and mouse serum beta inhibitors of influenza A viruses are mannose-binding lectins. Proc Natl Acad Sci USA. 1990;87:4485. doi: 10.1073/pnas.87.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wakamiya N, Okuno Y, Sasao F, et al. Isolation and characterization of conglutinin as an influenza A virus inhibitor. Biochem Biophys Res Commun. 1992;187:1270. doi: 10.1016/0006-291x(92)90440-v. [DOI] [PubMed] [Google Scholar]
- 16.Hartley CA, Jackson DC, Anders EM. Two distinct serum mannose-binding lectins function as beta inhibitors of influenza virus: identification of bovine serum beta inhibitor as conglutinin. J Virol. 1992;66:4358. doi: 10.1128/jvi.66.7.4358-4363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malhotra R, Haurum JS, Thiel S, Sim RB. Binding of human collectins (SP-A and MBP) to influenza virus. Biochem J. 1994;304:455. doi: 10.1042/bj3040455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anders EM, Hartley CA, Reading PC, Ezekowitz RA. Complement-dependent neutralization of influenza virus by a serum mannose-binding lectin. J Gen Virol. 1994;75:615. doi: 10.1099/0022-1317-75-3-615. [DOI] [PubMed] [Google Scholar]
- 19.Malhotra R, Sim RB. Collectins and viral infection. Trends Microbiol. 1995;3:240. doi: 10.1016/s0966-842x(00)88932-5. [DOI] [PubMed] [Google Scholar]
- 20.Tobita K, Sugiura A, Enomote C, Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol Berl. 1975;162:9. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]
- 21.Ohtani K, Suzuki Y, Eda S, et al. High-level and effective production of human mannan-binding lectin (MBL) in Chinese hamster ovary (CHO) cells. J Immunol Methods. 1999;222:135. doi: 10.1016/s0022-1759(98)00190-2. [DOI] [PubMed] [Google Scholar]
- 22.Eda S, Suzuki Y, Kase T, et al. Recombinant bovine conglutinin, lacking the N-terminal and collagenous domains, has less conglutination activity but is able to inhibit haemagglutination by influenza A virus. Biochem J. 1996;316:43. doi: 10.1042/bj3160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okuno Y, Tanaka K, Baba K, Maeda A, Kunita N, Ueda S. Rapid focus reduction neutralization test of influenza A and B viruses in a microtiter system. J Clin Microbiol. 1990;28:1308. doi: 10.1128/jcm.28.6.1308-1313.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham RC, Jr, Karnovsky MJ. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966;14:291. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- 25.Eda S, Suzuki Y, Kawai T, et al. Structure of a truncated human surfactant protein D is less effective in agglutinating bacteria than the native structure and fails to inhibit haemagglutination by influenza A virus. Biochem J. 1997;323:393. doi: 10.1042/bj3230393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiel S, Vorup Jensen T, Stover CM, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 28.Storgaard P, Holm Nielsen E, Skriver E, Andersen O, Svehag SE. Mannan-binding protein forms complexes with alpha-2-macroglobulin. A protein model for the interaction. Scand J Immunol. 1995;42:373. doi: 10.1111/j.1365-3083.1995.tb03670.x. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki Y, Eda S, Kawai T, et al. Characterization of recombinant bovine conglutinin expressed in a mammalian cell. Biochem Biophys Res Commun. 1997;238:856. doi: 10.1006/bbrc.1997.7402. [DOI] [PubMed] [Google Scholar]
- 30.Holmskov U, Malhotra R, Sim RB, Jensenius JC. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol Today. 1994;15:67. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 31.Terai I, Kobayashi K, Fujita T, Hagiwara K. Human serum mannose binding protein (MBP): development of an enzyme-linked immunosorbent assay (ELISA) and determination of levels in serum from 1085 normal Japanese and in some body fluids. Biochem Med Metab Biol. 1993;50:111. doi: 10.1006/bmmb.1993.1052. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K, Compans RW. Host cell-and virus strain-dependent differences in oligosaccharides of hemagglutinin glycoproteins of influenza A viruses. Virology. 1979;95:8. doi: 10.1016/0042-6822(79)90397-0. [DOI] [PubMed] [Google Scholar]
- 33.Wilson IA, Skehel JJ, Wiley DC. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981;289:366. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 34.Eda S, Suzuki Y, Kawai T, et al. Characterization of truncated human mannan-binding protein (MBP) expressed in Escherichia coli. J Virol. 1998;62:1326. doi: 10.1271/bbb.62.1326. [DOI] [PubMed] [Google Scholar]
- 35.Kilbourne ED, Laver WG, Schulman JL, Webster RG. Antiviral activity of antiserum specific for an influenza virus neuraminidase. Nature. 1968;2:281. doi: 10.1128/jvi.2.4.281-288.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Itzstein M, Wu WY, Kok GB, et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 37.Reading PC, Morey LS, Crouch EC, Anders EM. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol. 1997;71:8204. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sastry K, Zahedi K, Lelias JM, Whitehead AS, Ezekowitz RA. Molecular characterization of the mouse mannose-binding proteins. The mannose-binding protein A but not C is an acute phase reactant. J Immunol. 1991;147:692. [PubMed] [Google Scholar]
- 39.Korfhagen TR, Bruno MD, Ross GF, et al. Altered surfactant function and structure in SP-A gene targeted mice. Proc Natl Acad Sci USA. 1996;93:9594. doi: 10.1073/pnas.93.18.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rocha E, Cox NJ, Black RA, Harmon MW, Harrison CJ, Kendal AP. Antigenic and genetic variation in influenza A (H1N1) virus isolates recovered from a persistently infected immunodeficient child. J Virol. 1991;65:2340. doi: 10.1128/jvi.65.5.2340-2350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]