Abstract
Unlike other immunoglobulin G (IgG) subclasses, IgG4 antibodies in plasma have been reported to be functionally monovalent. In this paper we show that the apparent monovalency of circulating IgG4 is caused by asymmetry of plasma IgG4. A large fraction of plasma IgG4 molecules have two different antigen-binding sites, resulting in bispecificity. Sera from patients with IgG4 antibodies to both house dust mite and grass pollen induced cross-linking of Sepharose-bound grass pollen antigen to radiolabelled house dust mite allergen Der p I. This bispecific binding activity was not observed in sera with IgG4 antibodies to either grass pollen or house dust mite exclusively. Depletion of IgG4 antibodies resulted in disappearance of the bispecific activity. By size exclusion chromatography we excluded the possibility that bispecific activity was caused by aggregation of IgG4 antibodies. These results indicate that circulating (polyclonal) IgG4 antibodies have two different antigen-binding sites and therefore are functionally monovalent antibodies.
INTRODUCTION
The interaction of an antibody with an antigen initiates several immunological responses. The consequences of this antibody–antigen interaction are determined by the isotype of the antibody (mediating through the interaction with immunoglobulin receptors) and by the valency of the antibody–antigen interaction. Bivalency of an immunoglobulin G (IgG) antibody is an important aspect of most effector functions.
Human IgG4 antibody is unable to precipitate purified antigens.1, 2 This inability to precipitate allergen was not due to a difference in affinity of the IgG4 antibodies, but was caused by the inability of IgG4 antibodies to cross-link two antigens: human serum IgG4 antibodies are functionally monovalent.1, 2 In addition, IgG4 antibodies react poorly with Fc-receptors compared to the IgG1 antibody and do not efficiently activate complement.3 Nevertheless, in some individuals chimeric IgG4 antibody was shown to be as potent as IgG1 in antibody-dependent cell-mediated cytotoxicity.4 This unexpected effect, presumably due to a polymorphism, was also seen in an in vivo mouse model of immunotherapy.5
Generally, however, IgG4 antibodies are considered to be less efficient in inducing inflammatory responses and may even inhibit the inflammatory effects of other antibodies.6–11 Prolonged antigenic stimulation with high doses of soluble protein antigen preferentially induces IgG4 antibodies. IgG4-dominated responses have been reported in allergic patients receiving immunotherapy,1, 12, 13 in beekeepers,1 in patients with chronic parasitic infections, such as schistosomiasis and filariasis,8–11 and in haemophiliac patients receiving factor VIII.14 IgG4-mediated inhibition of immune inflammation may be advantageous in some instances, but may be undesirable in infections.
In this report we compared circulating and chimeric IgG4 antibodies. Our results indicate that the apparent monovalency of naturally occurring IgG4 antibodies is due to bispecificity.
MATERIALS AND METHODS
Monoclonal chimeric antibodies and sera
The mouse/human chimeric monoclonal IgG4 antibody (hIgG4-Dp2A) has been described previously.15 This chimeric antibody is directed against the house dust mite allergen Der p II and originates from the mouse hybridoma cell line 2B12.16 The rabbit hyperimmune antiserum to Der p I has been described elsewhere.17
The sera and plasma samples used were obtained from the Allergy Diagnostics Department (CLB, Amsterdam, the Netherlands). Nine sera were used in the heterologous binding assay (described below), plasma #741B (obtained by plasmapheresis) and serum #741 were derived from the same patient; plasma #178 (obtained by plasmapheresis) was used in the valency tests. This plasma contained 1400 ng of Der p II-specific IgG4 antibodies/ml as was determined by the method described previously.15 The IgG4 antibodies of this plasma were affinity purified using monoclonal antibody MH164-4M (CLB) coupled to CNBr-activated Sepharose (Pharmacia, Uppsala, Sweden).
Radioimmunoassays (RIA)
Mite-extract. Dermatophagoides pteronyssinus mites were obtained from Commonwealth Serum Laboratories (Melbourne, Australia), 1·5 g of mites were extracted in phosphate-buffered saline (PBS; 3% w/v) supplemented with Tween-20 (0·1% v/v) and sodium azide (0·1% w/v) for 4 hr. After filtration (black ribbon filter, Schleicher & Schüll, Dassel, Germany), the extract was coupled to 10 g CNBr-activated Sepharose 4B (Pharmacia). Per test, 1·5 mg Sepharose in 250 μl was used, equivalent to 170 KU (0·17 μg) Der p II/test. This was also the optimal condition for the two-site assay described below.
Grass pollen. Phleum pratenseDactylis glomerata, obtained from Diephuis Laboratories (Groningen, the Netherlands) were extracted as previously described18 and coupled to CNBr-activated Sepharose. Per test 1 mg Sepharose was used.
Iodination of allergens
Recombinant Der p II was used (kindly supplied by G. A. Mueller19). Der p II was purified by affinity chromatography using monoclonal antibody αDpX.17
The Der p II was iodinated by the chloramine-T method with carrier free 125I (Amersham Life Science, Amersham, UK) as was described previously1 with minor modifications, necessary for iodinating Der p II (20 μg in 100 μl PBS): 1-propanol was added to a final concentration of 30% (v/v) prior to labelling. The labelling was performed with 1 mCi of 125I. Free label was separated from 125I-labelled Der p II by dialysis against PBS containing 0·01% (w/v) PEG-4000. Der p I, affinity purified with anti-P117 from Dermatophagoides pteronyssinus mite extract was labelled by the standard chloramine-T method.1
Two-site assay: testing the valency of the antibodies
To test whether serum or chimeric antibodies were able to cross-link radiolabelled antigen to immunosorbent bound antigen, a two-site assay was performed. In this assay the amount of capturing antigen coupled to Sepharose has to be optimized in order to avoid bivalent binding of antibodies to adjacent antigens on the immunosorbent. To determine this, the amount of mite extract used for coupling to CNBr-activated Sepharose was titrated.
Sepharose-coupled mite-extract (described above) was incubated with 50 μl serum or chimeric antibody (1–20 ng antibody per test) in a total volume of 300 μl in PBS-AT (PBS supplemented with 0·3% bovine serum albumin, 0·1% Tween-20, 0·05% (w/v) NaN3). The ability to cross-link was examined by addition of 125I-labelled Der p II (in the valency tests) or Der p I (in the heterologous cross-linking assay).
Antigen-binding test
For the antigen-binding test 10 μl serum (or serum dilutions) or antibody (1–20 ng antibody/test) was incubated with 1 mg Protein A–Sepharose (Pharmacia) in 500 μl PBS-AT together with 125I-labelled antigen. The results were expressed as the percentage of radioactivity bound relative to the total radioactivity added.
Heterologous cross-linking assay
Sera were tested for bispecific binding activity in the heterologous cross-linking assay. Fifty microlitres of serum (or serum dilutions) were incubated with grass pollen–Sepharose (1 mg/test) in a total volume of 300 μl in PBS-AT. Antibodies bound were detected using radiolabelled mite-allergen Der p I. The results were expressed as the percentage of radioactivity bound relative to the total radioactivity added.
Size-exclusion chromatography
Serum #741 (200 μl) was applied to a ACA34 column (Ultrogel LKB, Villeneuve-la-Garenne, France), 1·5-cm diameter and 35-cm length, 62-ml material, equilibrated in PBS-AT. Fractions of 450 μl were collected and analysed for total IgE (180 000 MW), Der p I-specific IgG (tested in the antigen-binding test for IgG) and for grass pollen/Der p I-bispecific binding activity (tested in the heterologous cross-linking assay). The void volume was determined using Dextran Blue 2000 (Pharmacia). The results were expressed as percentage of starting material.
IgG4 and IgE depletion
Serum #741 was depleted for IgG4 and IgE antibodies. Anti-IgG4 monoclonal antibody MH164-4M (CLB), 10 mg, was coupled to 1 g CNBr-activated Sepharose (Pharmacia). For the depletion of IgE antibodies polyclonal sheep antihuman IgE serum coupled to Sepharose (10 ml antiserum was coupled to 20 g CNBr-activated Sepharose) was used. As a control for the depletion experiment, serum was incubated with CNBr-activated Sepharose that had been quenched by the addition of glycine. For depletion of 40 μl serum #741, 16 mg anti-IgG4, anti-IgE, or inactivated CNBr-activated Sepharose was used. The depletion was performed by overnight incubation (end over end at room temperature) in a final volume of 500 μl PBS-AT.
RESULTS
Valency of natural and chimeric IgG4 antibodies directed to the house dust mite allergen Der p II
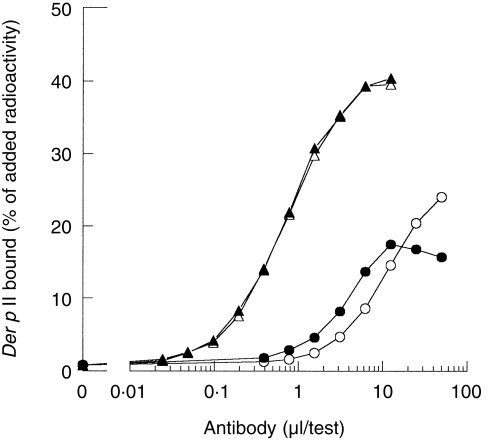
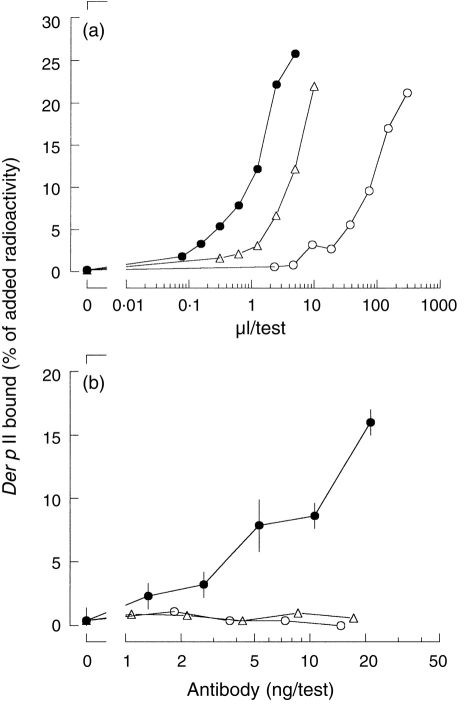
The amount of Der p II-specific IgG antibodies (or, more accurately, IgG124=IgG1+IgG2+IgG4 antibodies) in plasma #178 was determined in the antigen-binding test for Der p II, using as solid phase either Sepharose-coupled Staphylococcal protein A or Sepharose-coupled anti-IgG4; chimeric IgG4 antibody was used as a reference (Fig. 1). From the data presented in Fig. 1 the contribution of IgG4 antibody relative to IgG124 antibody in this serum can be calculated to be 56%. Next, the bivalent reactivity of naturally occurring IgG4 antibodies (purified from plasma #178) was compared to the bivalent reactivity of the mouse/human chimeric IgG4 monoclonal antibody directed to the house dust mite allergen Der p II. Bivalent reactivity was measured as the ability to cross-link radiolabelled Der p II and Sepharose-bound Der p II (Fig. 2). Chimeric IgG4 induced cross-linking of radiolabelled Der p to Sepharose-bound Der p II. In contrast, neither plasma #178 nor affinity purified plasma #178-derived IgG4 antibodies induced significant binding in this assay. Chimeric IgG1 and IgG4 antibodies were equally effective in cross-linking (not shown). These observations confirmed that naturally occurring (polyclonal) IgG4 antibodies behave as monovalent reagents towards purified Der p II in contrast to the bivalent behaviour of chimeric IgG4 antibodies.
Figure 1.
The relative contribution of IgG4 to the total IgG124 antibody activity with respect to the binding of 125I-labelled Der p of plasma #178 was deduced from the comparison between Sepharose-coupled anti-IgG4 (○) and Sepharose-coupled Staphylococcal Protein A (•); the results obtained with the chimeric IgG4 antibody, 17 μg/ml, are also shown (anti-IgG4, ▵, Protein A, ▴).
Figure 2.
The functional valency of chimeric IgG4, plasma #178 and affinity-purified IgG4 from plasma #178. (a) Binding of 125I-labelled Der p II to serial dilutions of chimeric IgG4 (17 μg/ml, •), plasma #178 (▵) and affinity purified IgG4 from plasma #178 (○) to Sepharose-coupled Staphylococcal Protein A were tested and used to determine the amount of Der p II-specific IgG124 in the plasma. (b) The cross-linking of Sepharose-coupled mite-extract to 125I-labelled Der p II by chimeric IgG4 (•), plasma #178 (▵) or affinity-purified IgG4 from plasma #178 (○) was expressed as the amount of radioactivity bound relative to the amount of radioactivity added.
Allergen-specific immunotherapy results in bispecific immunoglobulins
Sera from patients receiving therapeutic injections with both house dust mite and grass pollen extract were tested in a heterologous cross-linking assay. In this heterologous cross-linking assay the binding of purified radiolabelled house dust mite allergen Der p I to Sepharose-coupled grass pollen was measured (Table 1). All sera with IgG4 antibodies to both house dust mite allergen Der p I and to grass pollen allergen were able to cross-link mite-allergen Der p I to Sepharose-coupled grass pollen; binding ranging from 4·6 to 34·1% of added radioactivity. Sera of patients with IgG (IgG4) antibodies directed exclusively to house dust mite (represented by Der p I) or grass pollen (represented by Lol p I), were negative in this test (less than 2% binding of the amount of radioactivity added; the binding was indistinguishable from the binding of Der p I to control Sepharose to which an irrelevant ligand, glycine, had been coupled).
Table 1.
.Sera from patients receiving immunotherapy treatment were tested in a heterologous binding test
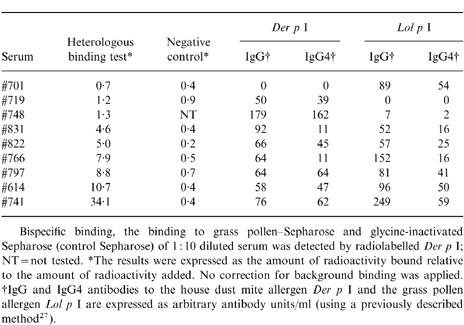
A fresh plasma sample from the patient that donated serum #741 was obtained, coded #741B. This sample showed similar results: 44·2% binding to Sepharose-coupled grass pollen and 0·8% binding to control Sepharose (binding expressed as percentage of labelled Der p I added).
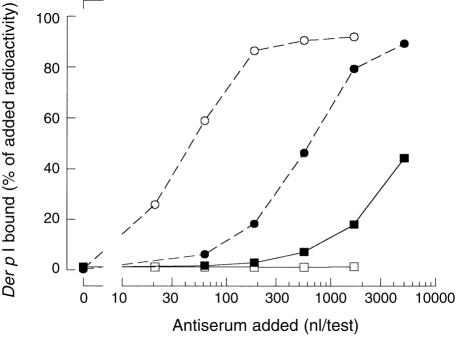
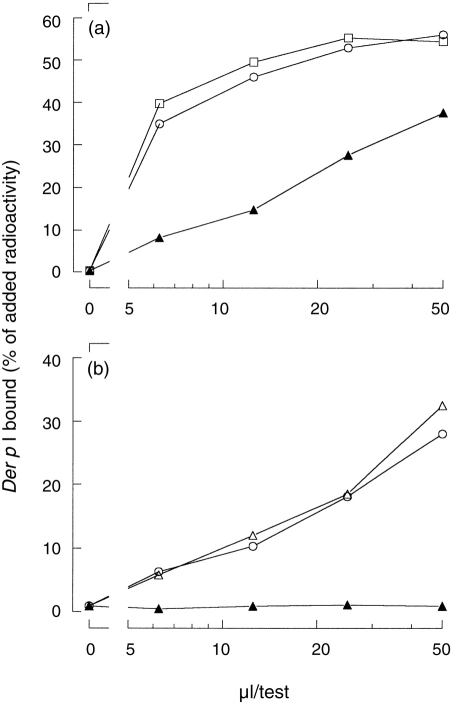
To test whether a Der p I contamination of the allergosorbent (Sepharose-coupled grass pollen) was the cause of the bispecific binding of these sera, hyperimmune rabbit antiserum to Der p I was incubated with Sepharose-coupled grass pollen (Fig. 3). Although Der p I-binding activity of this hyperimmune rabbit antiserum exceeded the binding of plasma #741B, the hyperimmune rabbit antiserum induced less than 1% binding of labelled Der p I to grass pollen–Sepharose.
Figure 3.
The bispecific binding is not caused by Der p I contamination of the grass pollen–Sepharose. Serial dilutions of rabbit polyclonal antiserum to Der p (○, □) and plasma #741B (•, ▪) were incubated with protein A–Sepharose (○, •) or with grass pollen–Sepharose (□, ▪). The binding was detected using radiolabelled Der p I. The results were expressed as the amount of radioactivity bound relative to the amount of radioactivity added.
Size fractionation of bispecific binding activity
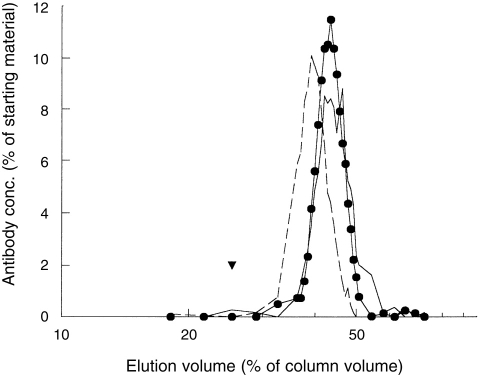
Serum #741 was subjected to gel filtration to exclude the involvement of antibody aggregates. Size-exclusion chromatography was performed using ACA34. Fractions were tested for total IgE, Der p I-specific IgG and the ability to cross-link Sepharose-coupled grass pollen to Der p I (Fig. 4). Der p I-specific IgG and the bispecific binding activity were detected in the same fractions, eluting just behind IgE. This indicates that the heterologous cross-linking activity was due to antibodies with the size of monomeric IgG.
Figure 4.
Gel filtration of serum #741. Serum #741, 200 μl was fractionated on an ACA 34 column. The fractions were tested for total amounts of IgE (180 000 MW, broken line), Der p II-specific IgG (solid line) and for the binding in the heterologous binding assay (•). The void volume is indicated by ▾. The results are expressed as percentage of starting material.
IgG subclass of bispecific binding activity: IgG4
To investigate whether IgG4 antibodies were responsible for the bispecific binding measured in the heterologous binding assay, serum #741 was depleted for its IgG4, using anti-IgG4 antibodies coupled to CNBr-activated Sepharose. As controls, the serum was also depleted for IgE antibodies (using anti-IgE–Sepharose) and was incubated with control Sepharose (glycine-inactivated Sepharose). After depletion, the three samples of manipulated serum #741 were tested in the antigen-binding test for Der p I using protein A–Sepharose, and in the grass pollen/Der p I heterologous cross-linking assay (Fig. 5). Binding of Der p I in the antigen-binding test was decreased (≈10-fold) after depletion of IgG4 antibodies; depletion of IgE, or incubation of the serum with control Sepharose (glycine-inactivated Sepharose), did not decrease Der p I binding. By the removal of IgG4, the bispecific binding activity disappeared. This bispecific binding activity remained when IgE was removed, or when the sample was incubated with glycine-inactivated Sepharose. These observations demonstrate that IgG4 antibodies cause the bispecific binding of allergen in this serum.
Figure 5.
Depletion of IgG4 antibodies from serum #741. Serum #741 was depleted for IgG4 (▴). As control, a sample was also depleted for IgE (□) and incubated with control Sepharose (○). The three samples were tested for the amount of Der p I-specific IgG (a) and for the binding activity in the heterologous binding assay (b).
DISCUSSION
Our results indicated the presence of bispecific antibodies in sera from allergic patients receiving immunotherapy (Table 1). We considered four trivial explanations of the apparent bispecificity:
1. Contamination of the grass pollen–Sepharose with allergen Der p I;
2. Cross-reactivity of a grass pollen allergen with the house dust mite allergen Der p;
3. Antibody aggregation;
4. Non-immune binding of IgG4 to Sepharose-coupled grass pollen.
First, the bispecific binding was not caused by Der p I contamination of the grass pollen–Sepharose. This was tested using a hyperimmune rabbit antiserum (Fig. 3).
Second, if the observed bispecificity of IgG4 was due to cross-reactivity, the bispecific sera would show higher binding to Sepharose-coupled mite extract than to Sepharose-coupled grass pollen extract. Even if 100% of the antibodies that reacted with radiolabelled Der p I were cross-reactive with grass pollen extract, the cross-reactive binding of these Der p -reactive antibodies could not be higher to grass pollen than to the homologous antigen, in this case: to Sepharose-coupled Der p I (or to Sepharose-coupled mite extract). However, we observed that the Der p I-binding activity of plasma #741B was approximately threefold lower to optimally coupled mite-extract than to Sepharose-coupled grass pollen (data not shown). This excludes cross-reactivity as explanation for the observed bispecificity. Furthermore, no grass pollen allergen has been described to be cross-reactive with the house dust mite allergen Der p I; IgE reactivity is absent in the majority of mite allergic sera. The bispecific binding described in this paper was not caused by, but could easily be mistaken for, cross-reactivity of allergens. In addition, in sera from patients treated for grass pollen allergy, bispecific antibodies to two non-homologous grass pollen allergens, Lol p I and Lol p V, were observed (R. van Ree, manuscript in preparation). Bispecific antibodies were also shown in sera from patients receiving immunotherapy for other allergen combinations than grass pollen and house dust mite and were measured to diphtheria and tetanus toxoid (data not shown).
Third, the gel filtration experiment indicated that bispecific antibodies were monomeric IgG molecules, and not antibody aggregates (Fig. 4).
Fourth, the absence of binding of serum #748 (with extremely high levels of Der p I-specific IgG4 but with negligible levels of IgG4 to grass pollen) in the heterologous cross-linking assay, excluded non-immune binding of the antibodies to the grass pollen as explanation. The absence of binding of the tested sera to control Sepharose (Table 1) also showed that the binding in the heterologous two-site assay was not caused by a non-specific interaction with Sepharose.
Depletion of the IgG4 antibodies from the serum clearly showed that the bispecific binding was largely, if not completely, caused by IgG4 antibodies (Fig. 5b). A possible explanation of the bispecificity of IgG4 antibodies can be found in some structural features of IgG4. IgG4 half-molecules (containing one heavy and one light chain when analysed on non-reducing sodium dodecyl sulphate–polyacrylamide gel electrophoresis) were noted by several investigators.20–23 These half-molecules are virtually absent in IgG4 with a mutation of a single amino acid (serine 241 to proline, amino acid numbering according to Kabat et al.24) in the hinge region in IgG425 resulting in a closer resemblance to the IgG1 hinge region. This incomplete formation of interheavy chain disulphide bonds might make IgG4 antibodies susceptible to exchange of half-molecules. Exchange in a pool of polyclonal IgG4 would result in bispecific antibodies that behave as monovalent antibodies towards a single antigen. In contrast, monoclonal chimeric IgG4 antibodies would remain bivalent. The bivalency of monoclonal chimeric IgG4 antibodies was found both in electron microscopic analysis26 and in radioimmunoassay (shown in Fig. 2).
The simplest explanation of the bispecificity of IgG4 is the above-mentioned postsecretion exchange of IgG4 half-molecules. An alternative, but less likely explanation is failure of allelic exclusion. This explanation would require an unprecedented phase in the development of IgG4-producing B cells, but would be in line with the observation that in vitro incubation of mixtures of chimeric antibodies did not result in bispecific reactions. Also, in vitro culturing of a mixture of the nitrophenyl (NP) and Der p II-specific IgG4 hybridoma cell lines did not induce bispecific antibodies (not shown).
The postulated physiological mechanism of generation of bivalency in vivo, by IgG4 half-molecule exchange, needs further study.
The presence of bispecific IgG4 antibodies creates two new questions:
1. What is the mechanism of this half-molecule exchange?
2. Is there any (patho-)physiological significance of this bispecificity, for instance in regard to helminth infections and to allergen-specific immunotherapy, or is the generation of bispecific antibodies a mechanism to generate monovalent antibodies. Monovalent antibodies might be beneficial to inhibit continuous immune-inflammation.
Our data indicate that the apparent monovalency of circulating IgG4 antibodies is owing to bispecificity. This indicates that results obtained with IgG4 antibodies have to be analysed with care. The hypotheses proposed, an intracellular failure of allelic exclusion or an extracellular mechanism involving the exchange of IgG4 half-molecules would explain three observations: first, the apparent monovalency of polyclonal IgG4; second, the difference in valency between polyclonal and monoclonal IgG4, and third, the bispecific activity of IgG4 in selected sera.
References
- 1.Aalberse RC, Van Der Gaag R, Van Leeuwen J. Serological aspects of IgG4 antibodies I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983;130:722. [PubMed] [Google Scholar]
- 2.Van Der Zee JS, Van Swieten P, Aalberse RC. Serological aspects of IgG4 antibodies. II. IgG4 antibodies form small, nonprecipitating immune complexes due to functional monovalency. J Immunol. 1986;137:3566. [PubMed] [Google Scholar]
- 3.Brueggemann M, Williams GT, Bindon CI, et al. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987;166:1351. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenwood J, Clark M, Waldmann H. Structural motifs involved in human IgG antibody effector functions. Eur J Immunol. 1993;23:1098. doi: 10.1002/eji.1830230518. [DOI] [PubMed] [Google Scholar]
- 5.Isaacs JD, Clark MR, Greenwood J, Waldmann H. Therapy with monoclonal antibodies. An in vivo model for the assessment of therapeutic potential. J Immunol. 1992;148:3062. [PubMed] [Google Scholar]
- 6.Van Der Zee JS, Van Swieten P, Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol. 1986;64:415. [PMC free article] [PubMed] [Google Scholar]
- 7.Lambin P, Bouzoumou A, Murrieta M, et al. Purification of human IgG4 subclass with allergen-specific blocking activity. J Immunol Methods. 1993;165:99. doi: 10.1016/0022-1759(93)90111-j. [DOI] [PubMed] [Google Scholar]
- 8.Hagan P, Blumenthal UJ, Dunn D, Simpson AJG, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 9.Kurniawan A, Yazdanbakhsh M, Van Ree R, et al. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J Immunol. 1993;150:3941. [PubMed] [Google Scholar]
- 10.Rihet P, Demeure CE, Dessein AJ, Bourgois A. Strong serum inhibition of specific IgE correlated to competing IgG4, revealed by a new methodology in subjects from a S. mansoni endemic area. Eur J Immunol. 1992;22:2063. doi: 10.1002/eji.1830220816. [DOI] [PubMed] [Google Scholar]
- 11.Hussain R, Poindexter RW, Ottesen EA. Control of allergic reactivity in human filariasis. J Immunol. 1992;148:2731. [PubMed] [Google Scholar]
- 12.Van Der Giessen M, Homan WL, Van Kernebeek G, Aalberse RC, Dieges PH. Subclass typing of IgG antibodies formed by grass pollen-allergic patients during immunotherapy. Int Arch Allergy Appl Immunol. 1976;50:625. doi: 10.1159/000231566. [DOI] [PubMed] [Google Scholar]
- 13.Oehling A, Sanz ML, Garcia BE. Immunological parameters in the immunotherapy follow-up. Int Arch Allergy Immunol. 1992;99:474. doi: 10.1159/000236317. [DOI] [PubMed] [Google Scholar]
- 14.Fulcher CA, De Graaf M, Zimmerman TS. FVIII inhibitor IgG subclass and FVIII polypeptide specificity determined by immunoblotting. Blood. 1987;69:1475. [PubMed] [Google Scholar]
- 15.Schuurman J, Perdok GJ, Mueller GA, et al. Mouse/human chimeric IgG1 and IgG4 antibodies directed to the house dust mite allergen Der p 2: Use in quantification of allergen specific IgG. Clin Exp Allergy. 1997;27:1095. doi: 10.1111/j.1365-2222.1997.tb01262.x. [DOI] [PubMed] [Google Scholar]
- 16.Ovsyannikova IG, Vailes LD, Li Y, Heymann PW, Chapman MD. Monoclonal antibodies to group II Dermatophagoides spp. allergens: murine immune response, epitope analysis, and development of a two-site ELISA. J Allergy Clin Immunol. 1994;94:537. doi: 10.1016/0091-6749(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 17.Van Der Zee JS, Van Swieten P, Jansen HM, Aalberse RC. Skin tests and histamine release with P1-depleted D. pteronyssinus body extracts and purified P1. J Allergy Clin Immunol. 1988;81:884. doi: 10.1016/0091-6749(88)90946-3. [DOI] [PubMed] [Google Scholar]
- 18.Van Ree R, Clemens JCJ, Aalbers M, Stapel SO, Aalberse RC. Characterization with monoclonal and polyclonal antibodies of a new major allergen from grass pollen in the group I molecular weight range. J Allergy Clin Immunol. 1989;83:144. doi: 10.1016/0091-6749(89)90489-2. [DOI] [PubMed] [Google Scholar]
- 19.Mueller GA, Smith AM, Williams DC, et al. Expression and secondary structure determination by NMR methods of the major house dust mite allergen Der p 2. Biol Chem. 1997;272:6893. doi: 10.1074/jbc.272.43.26893. [DOI] [PubMed] [Google Scholar]
- 20.Petersen JGL, Dorrington KJ. An in vitro system for studying the kinetics of interchain disulphide bond formation in immunoglobulin G. J Biol Chem. 1974;249:5633. [PubMed] [Google Scholar]
- 21.King DJ, Adair JR, Angal S, et al. Expression, purification and characterization of a mouse-human chimeric antibody and chimeric Fab fragment. Biochem. 1992;281:317. doi: 10.1042/bj2810317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colcher D, Milenic D, Roselli M, et al. Characterization and biodistribution od recombinant/chimeric constructs of monoclonal antibody B72.3. Cancer Res. 1994;49:1738. [PubMed] [Google Scholar]
- 23.Turner MW, Bennich HH, Natvig JB. Pepsin digestion of human G-myeloma proteins of different subclasses II. Immunochemical investigations of the products of pepsin digestion. Clin Exp Immunol. 1970;7:627. [PMC free article] [PubMed] [Google Scholar]
- 24.Kabat EA, Wu TT, Reid-Miller M, Perry HM, Gottesman KS. Sequences of proteins of immunological interest, edn 4. US Department of Health and Human Services, Public Health Service, National Institutes of Health.
- 25.Angal S, King DJ, Bodmer MW, et al. A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human antibody. Mol Immunol. 1993;30:105. doi: 10.1016/0161-5890(93)90432-b. [DOI] [PubMed] [Google Scholar]
- 26.Phillips ML, Tao MH, Morrison SL, Schumaker VN. Human/mouse chimeric monoclonal antibodies with human IgG1, IgG2, IgG3 and IgG4 constant domains: electron microscopic and hydrodynamic characterization. Mol Immunol. 1994;31:1201. doi: 10.1016/0161-5890(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 27.Aalberse RC, Dieges PH, Knul-Bretlova V, Vooren P, Aalbers M, Van Leeuwen J. IgG4 as blocking antibody. Clin Rev Allerg. 1983;1:289. doi: 10.1007/BF02991163. [DOI] [PubMed] [Google Scholar]