Abstract
We previously reported that complement-dependent haemolysis of sheep erythrocytes (E) coated with mannan (M) and sensitized with human mannan-binding lectin (MBL) via the lectin pathway in man occurs in Mg-EGTA and requires alternative pathway amplification. Calcium was required for MBL binding to E-M, but once the E-M-MBL intermediate was formed, MBL was retained and haemolysis occurred in the absence of calcium. Comparable or greater lectin pathway haemolysis in the absence of calcium was observed upon incubation of E-M-MBL in guinea-pig, rat, dog and pig sera, and was further investigated in the guinea-pig, in which titres were much higher (∼14-fold) than in man, and in contrast to humans, greater than classical pathway haemolytic activity. As in human serum, no lysis was observed in C4- or C2-deficient guinea-pig serum until purified C4 or C2, respectively, were restored. However, lectin pathway haemolytic activity in the guinea-pig did not require the alternative pathway. Removal (>98%) of factor D activity by three sequential passages through Sephadex G-75, resulting in serum which retained a normal classical pathway but no alternative pathway haemolytic activity, did not reduce the ability of guinea-pig serum to mediate haemolysis via the lectin pathway. Further, the C3-convertase formed via the lectin pathway (E-M-MBL-C4,2) lysed in C2-deficient guinea-pig but not human serum chelated with EDTA, a condition which precludes alternative pathway amplification. Thus, lectin pathway haemolysis occurs efficiently in guinea-pig serum, in the absence of calcium and without requirement for alternative pathway amplification. The guinea-pig provides a model for studying the assembly and haemolytic function of a lectin pathway which contrasts with the lectin pathway of man, and allows for comparisons that may help clarify the role of this pathway in complement biology.
INTRODUCTION
Mannan-binding lectin (MBL) is an acute phase reactant 2 which plays an important role in innate immunity.3 MBL deficiency results in impaired host defence and severe infections.4 MBL has been characterized in many species, including the rabbit,5 rat,6,7 human,8 cow,9 chicken,10 mouse,11 12 and guinea-pig,13,14 and MBL-related serine proteases have been traced back to the invertebrates.15–17 MBL binds to a number of pathogenic micro-organisms, and thus can initiate neutralization, opsonization and cytotoxic reactions;14,18,19 these functions require, or are enhanced by, complement. MBL activates complement through two new serum serine proteases designated MASP-1 and MASP-2.20,21 This lectin pathway requires C4 and C2 for activation of C3 and the terminal components,22 although direct activation of C3 by MASP-1 has been reported,23 and occurs in Mg-EGTA. In humans, alternative pathway amplification also is required for haemolysis via the lectin pathway.24,25 We report here that lectin pathway haemolytic activity can be measured in four other species (guinea-pig, rat, dog and pig) by haemolysis in Mg-EGTA using mannan-coated sheep E sensitized with human MBL, and because of a high titre in the guinea-pig, further characterized in this species.
MATERIALS AND METHODS
Buffers
Veronal-buffered saline, pH 7·4 (VBS), VBS containing 0·1% gelatin, 5 mm Mg2+ and 10 mm Ca2+ (GVB2+), GVB containing 10 mm EDTA (EDTA-GVB), and GVB containing 5 mm Mg2+ and 10 mm EGTA (Mg-EGTA) were prepared as described.26 VBS with 10 mm Ca2+ was used as ‘binding buffer’, and VBS with 20 mm EDTA was used as ‘elution buffer’, in the preparation of MBL.
Reagents
Sheep erythrocytes (E), rabbit erythrocytes (RaE) and rabbit anti-E anti-serum were purchased from Colorado Serum Inc (Denver, CO). Yeast mannan and CrCl3 were purchased from Sigma Chemical Co. (St Louis, MO). Normal guinea-pig sera were purchased from Harlan Inc. (Indianapolis, IN); Sigma Chemical Co. and Colorado Serum Inc. C4- and C2-deficient, as well as normal, guinea-pig sera were generous gifts from Dr Michael M. Frank (Duke University, Durham, NC). Purified human C4 and cobra venom factor (CoVF) were purchased from Calbiochem Inc. (La Jolla, CA). Guinea-pig C2 was purified as previously described.24 Normal rat and dog sera were kindly provided by Dr Thomas Welsh (Rush Medical College), and normal pig sera were a generous gift from Dr Yoon Berm Kim (Chicago Medical School). Normal human sera were donated by healthy laboratory personnel. Outdated human plasma was obtained from the Blood Bank of Rush-Presbyterian-St Luke’s Medical Center.
Purification of human serum mannan-binding lectin
MBL was purified as described,24 with minor modifications. One litre of outdated normal human plasma was recalcified and clotted overnight at 4°. Serum was collected, dialysed in 5 volumes of ‘binding buffer’ at 4° overnight and centrifuged at 10 000 g for 30 min. The supernatant was passed through a 200-ml mannan–Sepharose 4B column equilibrated with binding buffer, and MBL was recovered with elution buffer. The calcium concentration in the eluate was adjusted to 10 mm and this step was repeated on a smaller (25 ml) mannan–Sepharose 4B column. The MBL preparation was further purified by sequential passage through protein A–Sepharose 4B (10 ml) and anti-human IgM (15 ml) affinity columns. The eluate was concentrated and dialysed against the binding buffer.
Assay of classical pathway haemolytic activity
Antibody-sensitized sheep E (EA) were prepared as described.24 Serum dilutions (100 μl) and 100 μl EA (108/ml) in GVB2+ were mixed and incubated at 37° for 60 min, EDTA−GVB (1 ml) was added and, after centrifugation at 3500 g for 5 min, absorbance of supernatant at 414 nm was determined. Classical pathway activity (CH50) was calculated as the dilution that gave 50% lysis.24
Assay of lectin pathway haemolytic activity
Lectin pathway haemolytic activity was assayed as previously described.24 Briefly, equal volumes of 109 E/ml in GVB2+, 100 μg/ml mannan in 0·9% NaCl and 0·5 mg/ml CrCl3 in 0·9% NaCl were mixed and incubated at room temperature for 5 min to prepare E-mannan (E-M). The E-M were washed three times with GVB2+ and sensitized with purified human MBL (10 μg MBL per 109 E-M) and incubated at 37° for 30 min. The E-M-MBL were washed three times with Mg-EGTA and resuspended to 108 cells/ml. Test sera (100 μl) were serially diluted in Mg-EGTA, E-M-MBL (100 μl) were added and the mixture was incubated at 37° for 60 min. Mg-EGTA (1 ml) was added, and after centrifugation, absorbance of the supernatant at 414 nm was measured, and lectin pathway activity (LH50) was calculated as the dilution that gave 50% lysis.
Requirement of C4 for lectin pathway haemolysis in guinea-pig serum
C4-deficient guinea-pig serum (100 μl) was serially diluted in Mg-EGTA. Human C4 (100 μl) and E-M-MBL (100 μl) at 108 cells/ml in Mg-EGTA were added and incubated at 37° for 60 min, and the reconstituted LH50 activity was determined.
Requirement of C2 for lectin pathway haemolysis in guinea-pig serum
C2-deficient guinea-pig serum (100 μl) was serially diluted in Mg-EGTA. C2-deficient guinea-pig serum (100 μl), purified guinea-pig C2 (100 μl) at 20 U/ml and E-M-MBL (100 μl at 108 cells/ml) in Mg-EGTA were added and incubated at 37° for 60 min to determine the reconstituted LH50. Lysis was measured by absorbance at 414 nm.
Preparation of factor D-depleted guinea-pig serum and assay of factor D requirement for lectin pathway haemolysis in guinea-pig serum
Factor D was removed from guinea-pig serum by gel filtration.27 Briefly, serum (2 ml) containing 2 mm EDTA was passed through a Sephadex G-75 column (100 ml) and fractions (2 ml) were collected. Classical pathway and lectin pathway were assayed for each fraction as described above. For assay of factor D activity, aliquots of each fraction (20 μl) were mixed with 50 μl factor D-depleted human serum,28 Mg-EGTA (100 μl) and rabbit E (100 μl at 108 cells/ml), and incubated at 37° for 60 min; Mg-EGTA (1 ml) was added, and after centrifugation the absorbance at 414 nm (A414) was measured and the percent lysis was determined.
Alternative pathway assay in guinea-pig by indirect lysis
Sheep E (100 μl at 108 cells/ml in Mg-EGTA) and serially diluted serum (100 μl) were incubated with 0·1 μg CoVF (100 μl at 1 μg/ml) at 37° for 90 min. EDTA-GVB (1 ml) was added, and after centrifugation, the A414 and the serum dilution yielding 50% lysis (AH50) was determined.
Assay for alternative pathway requirement using the E-M-MBL-C4,2 intermediate
E-M-MBL-C4 were prepared by incubating E-M-MBL (108 cells/ml) with an equal volume of C2D guinea-pig serum (1:50) in Mg-EGTA at 37° for 5 min. The cells were washed three times with Mg-EGTA and incubated with guinea-pig C2 (1:5) at 30° for 5 min. Unbound C2 was removed by two brief washes with Mg-EGTA, and the E-M-MBL-C4,2 were reacted with C2-deficient guinea-pig or human sera in Mg-EGTA or EDTA. The percentage lysis was determined as described above.
Statistical analysis
All experiments were performed in duplicate a minimum of three times; standard deviations are shown in Fig. 1, and representative experiments are shown in Figs. 2–6.
Figure 1.
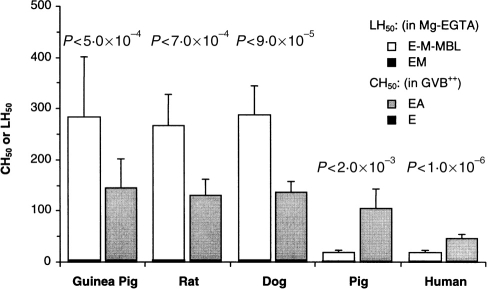
Lectin and classical pathway haemolytic activity in five species. Sera from guinea-pigs (14 samples), rats (nine samples), dogs (seven samples), pigs (six samples) and humans (14 samples) were assayed for lectin (LH50) and classical (CH50) pathway activity, by lysis of E-M-MBL in Mg-EGTA and EA in GVB2+, respectively; lysis of E-M not presensitized with MBL, and of E not presensitized with A, are shown for comparison; each sample was assayed three times. The data shown are the means±1SD. Lectin pathway haemolytic activity was greater than classical pathway activity in the three species, and greatest in the guinea-pig, in which titers were ∼14-fold greater than in humans.
Figure 2.
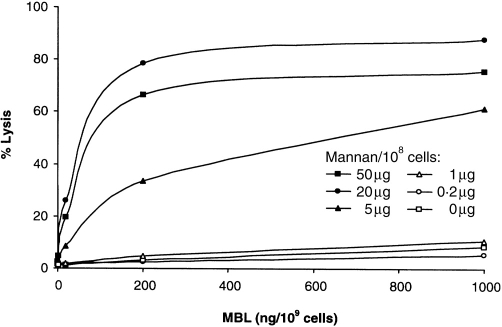
Optimization of lectin pathway haemolytic activity in guinea-pig serum. Sheep E coated with increasing amounts of mannan (up to 50 μg/108 cells) and were sensitized with increasing amounts of human MBL (up to 1000 ng/108 cells) and lysed in 1:80 guinea-pig serum-Mg-EGTA. Maximal lysis was obtained with 20 μg mannan and 200 ng MBL per 108 cells.
Figure 6.
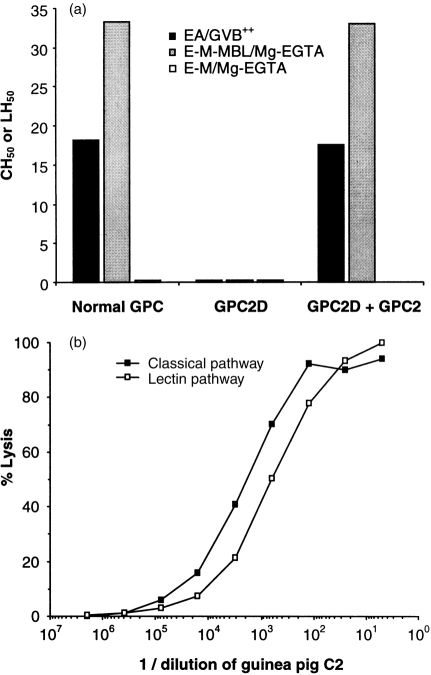
E-M-MBL-C4,2 were subjected to lysis by guinea-pig and human C2-deficient serum in either Mg-EGTA (which permits alternative pathway activity) or EDTA (which does not). Comparable haemolyis was observed in GPC2D in both Mg-EGTA and EDTA; by contrast, haemolysis was observed in HuC2D-Mg-EGTA but not in HuC2D-EDTA, supporting the conclusion that lectin pathway haemolyis occurs without alternative pathway amplification in guinea-pig but not in human serum.
RESULTS
MBL-initiated haemolysis via the lectin pathway in guinea-pig, rat, dog and pig as well as human sera
The ability of guinea-pig, rat, dog and pig sera to lyse E-M sensitized with human MBL in Mg-EGTA was compared with human serum (Fig. 1). Guinea-pig serum had much higher haemolytic activity (LH50 283±118 units/ml; range 122–487 units/ml), about 14-fold greater than that of human serum (LH50 20±4 units/ml; range 13–27 units/ml). The rat and dog LH50 were comparable to that of the guinea-pig (227±62 and 289±55, respectively), while the pig LH50 was comparable to that of man (18±5 units/ml). Whereas the LH50 was less than the CH50 [20 compared to 46 (P < 0·00001) and 18 compared to 104 (P < 0·002) units/ml, respectively] in humans and pigs, the LH50 was much higher than the CH50 in the guinea-pig (283 compared to 146 units/ml; P < 0·0005), rat (227 compared to 130 units/ml; P < 0·00009) and dog (288 compared to 137 units/ml; P < 0·0007). The presence of high titres, along with the historical emphasis upon the guinea-pig as a model for studies of haemolytic complement activity, led to further examination of lectin pathway activity in the guinea-pig.
Optimization of lectin pathway haemolytic activity in the guinea-pig
E-M-MBL were prepared with increasing amounts of mannan (up to 50 μg per 108 cells) and MBL (up to 1000 ng per 109 cells), and tested for lysis in guinea-pig serum in Mg-EGTA. The optimal amount of mannan was 20 μg per 108 cells; higher doses were inhibitory (Fig. 2). MBL was optimal at 500 ng MBL per 109 cells. No haemolysis of E-M was seen unless the cells were sensitized with MBL. Lysis was optimal at neutral pH and at 37°
Requirement of C4 for lectin pathway haemolytic activity in the guinea-pig
Guinea-pig serum deficient in C4 was tested for the ability to lyse E-M-MBL in the presence and absence of purified human C4. No LH50 activity was observed in C4D guinea-pig serum in the absence of human C4 (Fig. 3a), with haemolytic activity restored when purified human C4 was added. The restored LH50 was higher than the restored CH50. (129 compared to 109 units/ml). The ability of C4D guinea-pig serum to lyse EA and E-M-MBL, respectively, was strictly proportional to the amount C4 added (Fig. 3b).
Figure 3.
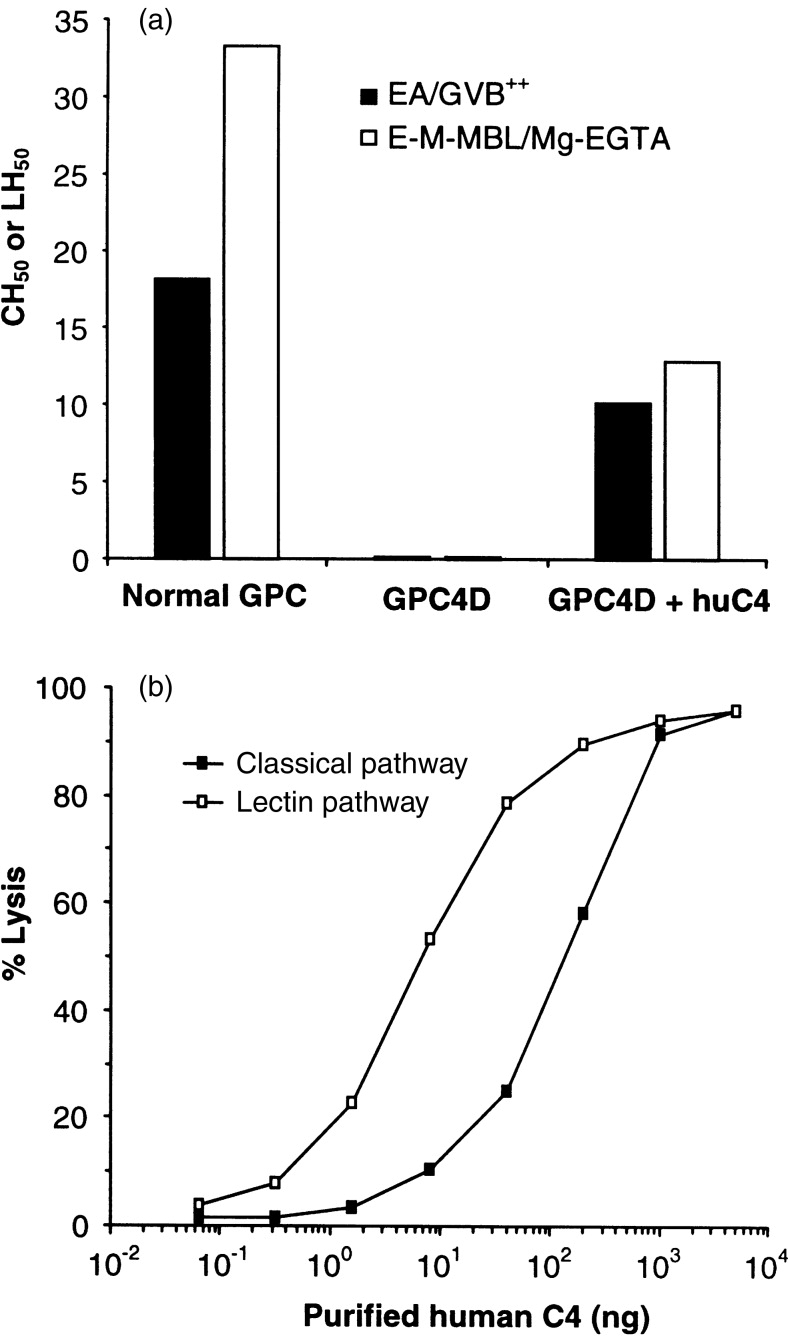
Requirement of C4 for lectin pathway haemolysis in the guinea-pig. E-M-MBL were incubated with C4-deficient guinea-pig serum (GPC4D) in Mg-EGTA in the presence and absence of human C4 to quantify LH50 (a) or with GPC4D and increasing amounts of human C4 (b); lysis of EA via the classical pathway is shown for comparison. No LH50 was observed in GPC4D in the absence of added human C4. Smaller amounts of C4 were required to restore lectin pathway compared to classical pathway haemolytic activity.
Requirement of C2 for lectin pathway haemolytic activity in the guinea-pig
C2D guinea-pig serum was tested for the ability to lyse E-M-MBL in the presence and absence of purified guinea-pig C2. Neither lectin nor classical pathway haemolytic activity was observed in C2D guinea-pig serum in absence of guinea-pig C2 (Fig. 4a), but both were restored to the normal range when guinea-pig C2 was added. The restored LH50 was higher than the restored CH50. Again, the ability of C2D guinea-pig serum to lyse EA and E-M-MBL, respectively, was strictly proportional to the amount of C2 added (Fig. 4b).
Figure 4.
Requirement of C2 for lectin pathway haemolysis in the guinea-pig. E-M-MBL were incubated with C2-deficient guinea-pig serum (GPC2D) in Mg-EGTA in the presence and absence of purified guinea-pig C2 (a), or with GPC2D and increasing amounts of guinea-pig C2 (b); lysis of EA via the classical pathway is shown for comparison. No LH50 was observed in GPC2D in the absence of added guinea-pig C2. Smaller amounts of C2 were required to restore lectin pathway compared to classical pathway haemolytic activity.
Absence of factor D requirement for lectin pathway haemolytic activity in the guinea-pig
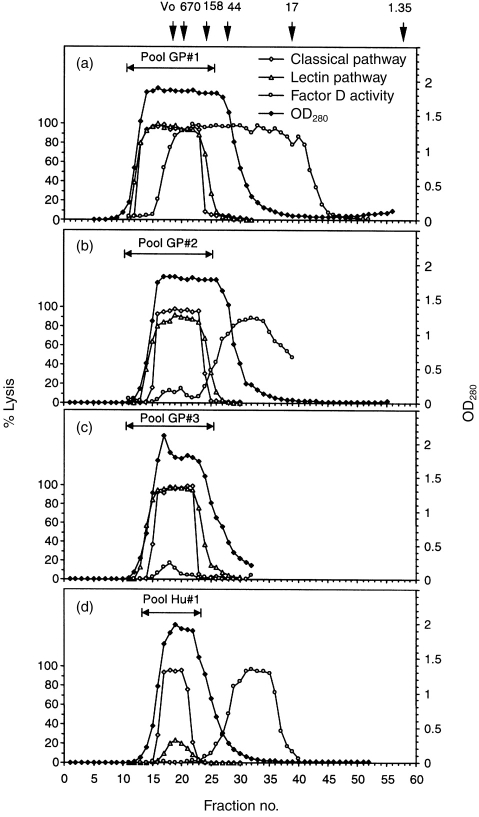
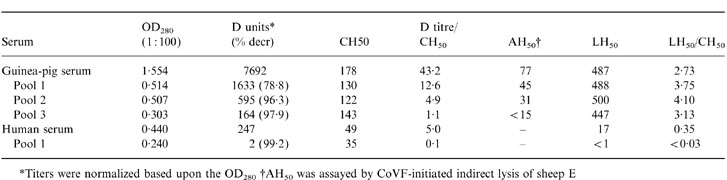
To remove selectively the 22 000 MW factor D, guinea-pig serum was passed through a 100-ml Sephadex G-75 column three times. Total protein and lectin pathway, classical pathway and factor D haemolytic activities were assayed in each fraction, with the major protein peaks eluting with markers >35 000 MW collected into pools designated 1, 2 and 3 after each passage (Fig. 5). Almost all (>98%) of the factor D was removed after the third passage, while normal classical and lectin pathway activities were retained (Fig. 5; Table 1). Removal of alternative pathway activity was confirmed by loss (from 70 to <15 haemolytic units/ml) of the ability of CoVF to induce lysis of bystander sheep 29 (Table 1); this proved to be a suitable assay for guinea-pig alternative pathway activity. Addition of either human or guinea-pig factor D did not increase LH50 activity in the d-depleted pool 3. In contrast to guinea-pig serum, removal of D by one passage of human serum through the Sephadex G-75 column completely eliminated lectin pathway activity, with the LH50 restored to normal when purified human factor D was added (Fig. 5; Table 1).
Figure 5.
Lectin pathway haemolytic activity in factor D-depleted guinea-pig serum. Guinea-pig serum in 2 mm EDTA was passed through a G-75 column equilibrated with VBS-EDTA (a). Lectin pathway, classical pathway, and factor D activities were measured for each fraction. Fractions with lectin pathway and classical pathway activities were pooled (pool #1), concentrated and passaged through the same column (b) to produce pool #2, and the process was repeated (c) to generate pool #3; this resulted in effective removal of factor D activity while both lectin and classical pathway haemolytic activities were retained. For comparison, removal of factor D from human serum by a single passage through the same column (d), resulted in loss of lectin pathway haemolytic activity while classical pathway haemolytic activity was retained.
Table 1.
Complement pathway activities in guinea-pig and human serum depleted of factor D
*Titers were normalized based upon the OD280 †AH50 was assayed by CoVF-initiated indirect lysis of sheep E
Further evidence for lectin pathway haemolysis in guinea-pig serum in absence of the alternative pathway
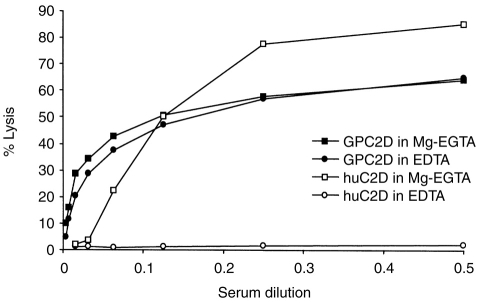
To evaluate further a role for the alternative pathway in lectin pathway haemolysis, E-M-MBL-C4 were prepared by incubation of E-M-MBL in C2D guinea-pig serum in Mg-EGTA, and were converted to E-M-MBL-C4,2 by addition of purified guinea-pig C2. These cells lysed in C2D guinea-pig serum-EDTA (in which alternative pathway amplification was blocked by chelation of magnesium) to the same extent as in C2D guinea-pig serum in Mg-EGTA (in which alternative pathway amplification could occur). By contrast, these E-M-MBL-C4,2 lysed in human C2D serum in Mg-EGTA but not in EDTA (Fig. 6). These results support the conclusion that alternative pathway amplification is not required for lectin pathway haemolysis in guinea-pig, even though it is required in human.
DISCUSSION
We previously reported that lectin pathway activity can be quantified in human serum by lysis in Mg-EGTA of mannan (M)-coated E sensitized with MBL.24,30 Mg-EGTA prevents both classical pathway activation through C1 and binding of endogenous MBL to the indicator cells; E-M-MBL lyse in this buffer in part because MBL added to E-M in the presence of calcium is retained even in the presence of a calcium chelator, rendering these cells suitable for assay of the human lectin pathway in Mg-EGTA. The present studies show this is applicable for assay of lectin pathway activity in other species as well. Haemolysis in guinea-pig, rat, dog and pig sera in Mg-EGTA suggests that reactivity in Mg-EGTA is a characteristic of the lectin pathway generally. It also implies that MASP association with and activation by the MBL in these species also is calcium independent, which is consistent with findings of Tan et al.31 in man. Lectin pathway haemolysis was particularly strong in the guinea-pig, and hence was studied in more detail in this species.
There was ∼14-fold greater lectin pathway haemolytic activity in the guinea-pig than in man, and guinea-pig lectin pathway haemolytic activity was greater than classical pathway haemolytic activity; the reverse was observed in man using identical indicator cells and assays. As in lectin pathway haemolysis in human serum, C4 and C2 were required, but alternative amplification was not. It is likely that the MASPs responsible for cleavage of guinea-pig C4 and C2 were provided by the guinea-pig serum, since comparable lysis with recombinant human MBL occurs in human serum (unpublished observations), but this must be tested directly. In any case, the high degree of lectin pathway haemolytic activity in the guinea-pig raises the question of whether this could account in part for activity previously attributed to the classical pathway.
Since a quantitative assay for guinea-pig alternative pathway haemolytic activity had not previously been reported, indirect fallout haemolysis initiated by cobra venom 29 was adapted for this purpose. Three passages through G-75 removed 98% of D activity from guinea-pig serum, and rendered the treated serum devoid of alternative pathway activity, but there was no reduction at all in haemolysis via the lectin pathway. There was still residual D activity in the pooled serum which, because it corresponded to a size much larger than factor D, most likely was attributable to previously described surrogate proteases.32 By contrast, one passage of human serum through the same column removed 99% of D activity and reduced the LH50 from 17 to <1 units/ml. Alternative pathway independence was further proved with the E-M-MBL-C4,2 intermediate cell bearing the lectin pathway C3 convertase. Thus, E-M-MBL-C4,2 were lysed in GPC2D to the same extent in Mg-EGTA (which permits alternative pathway activity) and EDTA (which does not), while lysis was seen in Mg-EGTA but not EDTA when HuC2D was used as the serum source for the terminal complement components. Whether the lack of requirement for an alternative pathway for guinea-pig haemolysis via the lectin pathway, in contrast to its requirement in humans, is attributable to greater activation or activity of the MASP enzymes, control at the level of the MASPs or the C3 33 or other factors awaits further definition of the interactions between MBL, MASP-1 and MASP-2.
The lectin pathway in the guinea-pig, which as in humans is haemolytically active in the absence of calcium, contrasts with that of man by its intensity and ability to lyse MBL-coated E without alternative pathway amplification. These characteristics suggest that the guinea-pig can serve as model for studying the assembly and haemolytic function of the lectin pathway which may help clarify the role of this pathway in complement biology.
Acknowledgments
This investigation is presented by Y.Z. in partial fulfilment of the requirements for a Ph.D. from Rush University. C.S. was supported in part by a Thai Royal Government Scholarship. H.G. holds the Thomas J. Coogan Sr. Chair in Immunology/Microbiology established by Marjorie Lindheimer Everett.
Glossary
Abbreviations
- E
sheep erythrocytes
- E-M
mannan-coated E
- E-M-MBL
E-M sensitized with human mannan-binding lectin
- GVB
gelatin-veronal-buffered saline
- GVB2+
gelatin-veronal-buffered saline containing 10 m CaCl2 and 5 m MgCl2
- LH50
titre of serum lysing 50% of mannan-binding lectin-sensitized E-M in Mg-EGTA via the lectin pathway under standardized conditions
- M
mannan
- MASP-1 and MASP-2
mannan-binding lectin-associated serine proteases structurally homologous to C1r and C1s, respectively
- MBL
mannan-binding lectin
- MCF
mean channel fluorescence
- Mg-EGTA
GVB containing 5 m Mg2+ and 10 m EGTA
- VBS
veronal-buffered saline, pH 7·4.
REFERENCES
- 1.Zhang Y, Suankratay C, Jones DR, Zang X-H, Lint TF, Gewurz H. Lectin pathway hemolysis in the serum of the guinea pig and other species (Abstract) Mol Immunol. 1998;35:390. [Google Scholar]
- 2.Thiel S, Holmskov U, Hviid L, Laursen SB, Jensenius JC. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin Exp Immunol. 1992;90:31. doi: 10.1111/j.1365-2249.1992.tb05827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmskov U, Malhotra R, Sim RB, Jensenius JC. Collectins: Collagenous C-type lectins of the innate immune defense system. Immunol Today. 1994;15:67. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 4.Summerfield JA, Sumiya M, Levin M, Turner MW. Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. Br Med J. 1997;314:1229. doi: 10.1136/bmj.314.7089.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kozutsumi Y, Kawasaki T, Yamashina I. Isolation and characterization of a mannan-binding protein from rabbit serum. Biochem Biophys Res Commun. 1980;95:658. doi: 10.1016/0006-291x(80)90836-0. [DOI] [PubMed] [Google Scholar]
- 6.Mizuno Y, Kozutsumi Y, Kawasaki T, Yamashina I. Isolation and characterization of a mannan-binding protein from rat liver. J Biol Chem. 1981;256:4247. [PubMed] [Google Scholar]
- 7.Townsend R, Stahl P. Isolation and characterization of a mannose/N-acetylglucosamine/fucose-binding protein from rat liver. Biochem J. 1981;194:209. doi: 10.1042/bj1940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawasaki N, Kawasaki T, Yamashina I. Isolation and characterization of a mannan-binding protein from human serum. J Biochem (Tokyo) 1983;94:937. doi: 10.1093/oxfordjournals.jbchem.a134437. [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki N, Kawasaki T, Yamashina I. Mannan-binding protein and conglutinin in bovine serum. J Biochem (Tokyo) 1985;98:1309. doi: 10.1093/oxfordjournals.jbchem.a135398. [DOI] [PubMed] [Google Scholar]
- 10.Oka S, Kawasaki T, Yamashina I. Isolation and characterization of mannan-binding proteins from chicken liver. Arch Biochem Biophys. 1985;241:95. doi: 10.1016/0003-9861(85)90366-2. [DOI] [PubMed] [Google Scholar]
- 11.Holt P, Holmskov U, Thiel S, Teisner B, Hojrup P, Jensenius JC. Purification and characterization of mannan-binding protein from mouse serum. Scand J Immunol. 1994;39:202. doi: 10.1111/j.1365-3083.1994.tb03361.x. [DOI] [PubMed] [Google Scholar]
- 12.Storgaard P, Nielsen EH, Andersen O, et al. Isolation and characterization of porcine mannan-binding proteins of different size and ultrastructure. Scand J Immunol. 1996;43:289. doi: 10.1046/j.1365-3083.1996.d01-39.x. [DOI] [PubMed] [Google Scholar]
- 13.Reading PC, Hartley CA, Ezekowitz RA, Anders EM. A serum mannose-binding lectin mediates complement-dependent lysis of influenza virus-infected cells. Biochem Biophys Res Commun. 1995;217:1128. doi: 10.1006/bbrc.1995.2886. [DOI] [PubMed] [Google Scholar]
- 14.Anders EM, Hartley CA, Reading PC, Ezekowitz RA. Complement-dependent neutralization of influenza virus by a serum mannose-binding lectin. J Gen Virol. 1994;75:615. doi: 10.1099/0022-1317-75-3-615. [DOI] [PubMed] [Google Scholar]
- 15.Ji X, Azumi K, Sasaki M, Nonaka M. Ancient origin of the complement lectin pathway revealed by molecular cloning of mannan binding protein-associated serine protease from a urochordate, the Japanese ascidian, Halocynthia roretzi. Proc Natl Acad Sci USA. 1997;94:6340. doi: 10.1073/pnas.94.12.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawakami M, Ihara I, Ihara S, Suzuki A, Fukui K. A group of bactericidal factors conserved by vertebrates for more than 300 million years. J Immunol. 1984;132:2578. [PubMed] [Google Scholar]
- 17.Matsushita M, Endo Y, Nonaka M, Fujita T. Complement-related serine proteases in tunicates and vertebrates. Curr Opin Immunol. 1998;10:29. doi: 10.1016/s0952-7915(98)80027-7. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki N, Kawasaki T, Yamashina I. A serum lectin (mannan-binding protein) has complement-dependent bactericidal activity. J Biochem (Tokyo) 1989;106:483. doi: 10.1093/oxfordjournals.jbchem.a122878. [DOI] [PubMed] [Google Scholar]
- 19.Kuhlman M, Joiner K, Ezekowitz RA. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989;169:1733. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiel S, Vorup-Jensen T, Stover CM, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 21.Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji YH, Matsushita M, Okada H, Fujita T, Kawakami M. The C4 and C2 but not C1 components of complement are responsible for the complement activation triggered by the Ra-reactive factor. J Immunol. 1988;141:4271. [PubMed] [Google Scholar]
- 23.Matsushita M, Fujita T. Cleavage of the third component of complement (C3) by mannose-binding protein-associated serine protease (MASP) with subsequent complement activation. Immunobiology. 1995;194:443. doi: 10.1016/S0171-2985(11)80110-5. [DOI] [PubMed] [Google Scholar]
- 24.Suankratay C, Zhang XH, Zhang Y, Lint TF, Gewurz H. Requirement for the alternative pathway as well as C4 and C2 in complement-dependent hemolysis via the lectin pathway. J Immunol. 1998;160:3006. [PubMed] [Google Scholar]
- 25.Zhang Y, Suankratay C, Zhang X-H, Lint TF, Gewurz H. Lysis via the lectin pathway of complement activation: minireview and lectin pathway enhancement of endotoxin– initiated hemolysis. Immunopharmacology. doi: 10.1016/s0162-3109(99)00029-6. in press. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda K, Sannoh T, Kawasaki N, Kawasaki T, Yamashina I. Serum lectin with known structure activates complement through the classical pathway. J Biol Chem. 1987;262:7451. [PubMed] [Google Scholar]
- 27.Lesavre PH, Muller-Eberhard HJ. Mechanism of action of factor D of the alternative complement pathway. J Exp Med. 1978;148:1498. doi: 10.1084/jem.148.6.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Praz F, Barreira MC, Lesavre P. A. one-step procedure for preparation of classical pathway (C1q) and alternative pathway (factor D) depleted human serum. J Immunol Methods. 1982;50:227. doi: 10.1016/0022-1759(82)90229-0. [DOI] [PubMed] [Google Scholar]
- 29.Pickering RJ, Wolfson MR, Good RA, Gewurz H. Passive hemolysis by serum and cobra venom factor: a new mechanism inducing membrane damage by complement. Proc Natl Acad Sci USA. 1969;62:521. doi: 10.1073/pnas.62.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suankratay C, Mold C, Zhang Y, Potempa LA, Lint TF, Gewurz H. Complement regulation in innate immunity and the acute-phase response: inhibition of mannan-binding lectin-initiated complement cytolysis by C-reactive protein (CRP) Clin Exp Immunol. 1998;113:353. doi: 10.1046/j.1365-2249.1998.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan SM, Chung MC, Kon OL, Thiel S, Lee SH, Lu J. Improvements on the purification of mannan-binding lectin and demonstration of its Ca2+-independent association with a C1s-like serine protease. Biochem J. 1996;319:329. doi: 10.1042/bj3190329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brade V, Nicholson A, Bitter-Suermann D, Hadding U. Formation of the C3-cleaving properdin enzyme on zymosan. Demonstration that factor D is replaceable by proteolytic enzymes. J Immunol. 1974;113:1735. [PubMed] [Google Scholar]
- 33.Suankratay C, Mold C, Zhang Y, Lint TF, Gewurz H. Mechanism of complement-dependent hemolysis via the lectin pathway: Role of the complement regulatory proteins. Clin Exp Immunol. 1999 doi: 10.1046/j.1365-2249.1999.00998.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]