Abstract
The memory/activated T cells, which mediate the long-lived host response against tuberculosis, in mice immunized with either bacillus Calmette–Guérin (BCG) or mycobacterium heat-shock protein 65 (hsp 65) antigen expressed from plasmid DNA (DNA-hsp 65), were characterized. Protection against Mycobacterium tuberculosis challenge by DNA-hsp 65 vaccination was associated with the presence of lymph node T-cell populations in which CD8+/CD44hi interferon-γ (IFN-γ)-producing/cytotoxic cells were prominent even after 8 or 15 months of plasmid DNA-mediated immunizations, whereas after BCG vaccination the majority were CD4+/CD44lo IFN-γ-producing T cells. When the cells were separated into CD4+CD8− and CD8+CD4− and then into CD44hi and CD44lo types, CD44lo cells were essentially unable to transfer protection in adoptive transfer experiments, the most protective CD44hi cells were CD8+CD4− and those from DNA-vaccinated mice were much more protective than those from BCG-immunized mice. The frequency of protective T cells and the level of protection were increased up to 8 months and decreased after 15 months following DNA or BCG immunizations.
INTRODUCTION
New generation vaccines against tuberculosis are being developed, but there is a need for improved ways of measuring their immunological effectiveness. Although adoptive transfer of protection with T lymphocytes from infected or immunized rats into naive animals established over 20 years ago that acquired immunity against tuberculosis is cell-mediated,1 attempts to define the phenotype and function of the protective T cells either after infection or after vaccination have given conflicting results. CD4, CD8 and γδ-T-cell receptor (TCR) T cells have all been implicated, as have interferon-γ (IFN-γ) and cytotoxicity, without establishing how they actually contribute to protection.2–4 The accepted paradigm has been that protection is mainly due to antigen-specific CD4 T helper type 1 (Th1) cells, which produce IFN-γ to activate macrophages, which then kill the mycobacterium during phagocytosis.5 A subsidiary function is then served by cytotoxic CD8 T cells that release intracellular bacteria from infected cells so that they can be killed during phagocytosis by activated macrophages.6–8 We have found that immunization procedures that present mycobacterium heat-shock protein 65 (hsp 65) to the immune system as an endogenous antigen (transfected macrophages, liposomes, DNA-vaccination) generate strong protection against tuberculosis challenge and that this is associated with the presence of a splenic T-cell population in which CD8+/CD44hi IFN-γ-producing cytotoxic cells are prominent,9–15 whereas protein in adjuvant did not protect and only increased CD4 cells selectively.6 Moreover, cells with a type 2 profile are also present in substantial numbers following infection with Mycobacterium tuberculosis3,5,15–17 or bacillus Calmette–Guérin (BCG) vaccination.18 Thus, the features that distinguish a protective immune response to mycobacterium antigens from a non-protective response are not known with certainty. In this study we investigate phenotypes, IFN-γ production, expression of CD44 and cytotoxicity as markers of immunological memory/activation of T cells which could mediate the long-lived host response against tuberculosis in mice vaccinated with either BCG or DNA-hsp 65.
MATERIALS AND METHODS
Immunization procedures
Young adult BALB/c mice were obtained from the vivarium of the School of Medicine of Ribeirão Preto, University of São Paulo, and were maintained under standard laboratory conditions. For DNA vaccination a 3·1-kilobase (kb) Xmn I fragment of the M. leprae genome carrying the hsp 65 gene was cloned into the Eco RV site downstream of the hydroxymethylglutaryl-CoA-reductase promoter in plasmid pHMG.9 Intramuscular injection of 50 μg plasmid DNA in 50 μl saline into each quadriceps muscle was done on four occasions at 2-week intervals.10 For protein immunization, recombinant M. leprae hsp 65 antigen (25 μg) emulsified in Freund’s incomplete 11 was injected subcutaneously (100 μl), then 25 μg in saline was injected intravenously twice at weekly intervals. BCG (Pasteur strain) was given as a single subcutaneous injection of about 105 live bacteria in 50 μl saline. Additional control animals received saline only or plasmid pHMG without an insert.
Frequencies of antigen-responsive CD4+CD8− and CD8+CD4− T cells
Two weeks after completion of the DNA-immunization procedures or 30 days after BCG vaccination, antigen-reactive αβ-T cells with CD4+CD8− or CD8+CD4− phenotypes were purified from popliteal and mesenteric lymph nodes by negative selection with specific monoclonal antibodies.12 In brief, each group contained six mice that were killed and the cells from homogenized lymph nodes were purified by centrifugation in complete RPMI-1640 medium (RPMI-13) on lympholyte M, depletion of adherent cells on tissue culture plastic, then by two passages through nylon wool columns. Selection for subpopulations was by sequential steps of complement-mediated lysis. The phenotype and purity of the T-cell subpopulations were checked with a fluorescence-activated cell sorter (FACScan) by using fluorescein isothiocyanate immunofluorescence with rat monoclonal antibody L3T4 or Lyt2 against surface markers CD4 and CD8 and hamster monoclonal antibody (mAb) H57-597 or GL3 (Pharmingen, Uppsala, Sweden) against TCR-αβ and -γγ, respectively.12 Cells purified from BCG-vaccinated animals were found to be free from cultivatable bacteria. Limiting dilution analysis was used to determine the frequencies of hsp 65-reactive lymphocytes.12 The transfected J774 macrophages (J774-hsp 65 cells, endogenously expressing hsp 65 antigen) were irradiated (40 Gy) and used as antigen-presenting 11,12 in RPMI-C with 5×104 cells per V-bottomed well in 96-well plates. Dilutions of subpopulations of splenocytes in RPMI-C were added to give final concentrations of 2–4096 T cells per well in 0·2 ml with 24 replicates. The cells were cultured in the presence of recombinant interleukin-2 (rIL-2; 1 ng/ml) at 37° with 5% CO2 in air for 12–14 days then pulsed with [3H]thymidine for 18 hr and assessed for radiolabel incorporation.12 Wells giving counts that exceeded the counts from control (unstimulated) wells by three standard deviations were scored as positive. Frequencies of antigen-responsive T cells were estimated from plots of the percentage of wells that were negative against T-cell concentration according to Poisson distribution using the minimum χ2 method. Cell concentrations at which all of the wells were positive or negative were not included in the calculations.
Isolation of T-cell clones
CD4+CD8− and CD8+CD4− T-cell subpopulations were prepared by negative selection as described above, seeded at 256 cells per well into V-bottom 96-well plates, and cultured for 14 days in the presence of irradiated (40 Gy) J774-hsp 65 feeder cells (5×104 in 0·1 ml) and rIL-2 (1 ng/ml). The cells were then two-colour-stained with anti-CD44 and Lyt-2 or L3T4 mAb and sorted by FACSort (Becton Dickinson, San Jose, CA) into of CD44hi and CD44lo populations and cloned at 0·3 cells/well in round-bottom 96-well plates in the presence of 105 feeder cells and 2 ng rIL-2/ml. Growing clones were restimulated every 2–3 weeks with phytohaemagglutinin (0·5 μg/ml) in the presence of feeder cells. Twelve strongly growing CD4+CD8− clones (three CD44hi and three CD44lo from DNA-immunized mice; three CD44hi and three CD44lo from BCG-vaccinated mice) and 12 CD8+CD4− clones (three CD44hi and three CD44lo from DNA-immunized mice; three CD44hi and three CD44lo from BCG-vaccinated mice) were selected for characterization. All were TCR-αβ-positive by FACScan analysis.14
Characterisation of bulk CD4+CD8− and CD8+CD4− T cells
The CD4+CD8− and CD8+CD4− T cells had the features of antigen processing and presentation expected of these phenotypes when assessed as previously described.12,15 In brief, antigen specificity was checked with rhsp 65, bovine serum albumin, purified protein derivative and concanavalin A; the antigen-processing pathway was tested with chloroquine and brefeldin A; the antigen-presentation complex was probed with the mAb L3T4, Lyt-2, anti-I-Ad, I-Ab and anti-H-2Kd. Secretion of cytokines IFN-γ and IL-4 was stimulated with phorbolmyristic acid (PMA, 10 ng/ml) and anti-CD3 mAb (YCD3-1 [Gibco-BRL, Gaithersburg, MD]; 50 ng/ml) to achieve maximal 12 measured by enzyme-linked immunosorbent assay (ELISA).12 Antigen-specific cytotoxicity and toxicity for cells infected with M. tuberculosis were measured by 51Cr release from appropriate J774 or J774-hsp 65 cells or thioglycollate-elicited peritoneal macrophages from BALB/c mice in the presence and absence of chloroquine and brefeldin A.12 The antimycobacterial activities of the T cells and their supernatants were tested against M. tuberculosis in bone marrow-derived macrophages in the presence and absence of anti-IFN-γ mAb.12
FACS analysis of CD44 expression
CD4+CD8− and CD8+CD4− T-cell subpopulations were prepared by negative selection as described above. The cells were then stained immediately with fluorescein isothiocyanate (FITC)-labelled anti-CD44, Lyt-2, or L3T4 mAb (Pharmingen) and analysed by FACScan.14
Cytokine ELISPOT assays
CD4+CD8− and CD8+CD4− T cells prepared by negative selection from lymph nodes as described above were separated by FACS into subpopulations of CD44hi and CD44lo cells.15 These T-cell subpopulations were assayed for the frequency of IFN-γ- and IL-4-producing cells by ELISPOT.16 In brief, 96-well nitrocellulose-bottomed plates (Millititre HA, Millipore Inc. Bedford, MA) were coated with mAb specific for IFN-γ or IL-4 (R4-GA2 or 11B11; Pharmingen) by overnight incubation at 4°. T cells (2×104, 1×104 and 5×103 per 200-μl well in triplicate) were incubated with 5×104 irradiated J774-hsp 65 cells as a source of hsp 65 antigen or in the presence of J774 cells and whole BCG antigen or PMA (10 ng/ml) and anti-CD3 mAb [YCD3-1 (Gibco-BRL); 50 ng/ml] to achieve maximal stimulation.12 After overnight incubation, cells were washed off and IFN-γ and IL-4 bound to the nitrocellulose were detected with biotinylated rat anti-mouse IFN-γ (Pharmingen) and rat anti-mouse IL-4 (BVD6-24G2; Pharmingen) followed by streptavidin–alkaline phosphatase as substrate. Spots were counted by light microscopy. The frequency of IFN-γ- and IL-4-producing cells for each T-cell concentration was calculated by averaging the number of spots for triplicate wells.
Adoptive transfer of immunity
Mice were γ-irradiated (9·5 Gy15) then injected intravenously with 5×106 T cells that were bulk-purified from lymph nodes of DNA-hsp 65-immunized or BCG-vaccinated mice as described above. Control mice received T cells purified from lymph nodes of normal mice or were untreated. Mycobacterium tuberculosis [105 colony-forming units (CFU)] was immediately injected intravenously, the mice were killed after 4 weeks and the numbers of CFU in the lungs were determined.11
Duration of protection
At 1, 4, 8 and 15 months after completion of immunization, T cells were purified from the lymph nodes of groups of six mice by depletion of adherent cells on tissue culture plastic then by two passages through nylon wool columns as described above and assessed for CD44 expression by two-colour cytofluorimetry; lymphoproliferation, cytotoxicity, cytokine production and protection as described above.
Lymphoproliferation assay
Lymph nodes of groups of six mice challenged 1, 4, 8, or 15 months after completion of immunization were purified by depletion of plastic-adherent and nylon wool-adherent cells.12 After a 3-day incubation in triplicate 96-well microtitre wells that contained 3×105 T cells and 1×105 irradiated J774-hsp 65 cells (or J774-vector) per well, uptake of [3H]thymidine during 18 hr was assessed. Results are expressed as stimulation index (SI), calculated as the radioactivity counts in the presence of antigen (J774-hsp 65 cells) divided by the counts in the absence of antigen (J774-vector cells).
Statistics
The statistical significance of the data was determined by Student’s t-test and P <0·05 was considered significant. In normal protection experiments we considered data to be significant when CFU in vaccinated mice was 2 logs lower than in control mice. For protection in adoptive transfer experiments we considered significant when P <0·01.
RESULTS
Both vaccinations with BCG or with plasmid DNA expressing hsp 65 caused substantial increases in the frequency of hsp 65-reactive T cells in lymph nodes (Table 1). The increase occurred equally in cells with CD4+CD8− and CD8+CD4− phenotypes and this contrasted with the response to immunization with the protein in adjuvant, where the increase was almost entirely in the CD4+CD8− cells. Both the vaccination procedures, BCG and DNA immunization, increased the frequency of CD44hi-activated or memory phenotype seen in freshly harvested lymph node T cells (Table 2).
Table 1.
Effect of immunization with BCG or DNA expressing hsp 65 on hsp 65-reactive T-cell frequencies in lymph nodes*
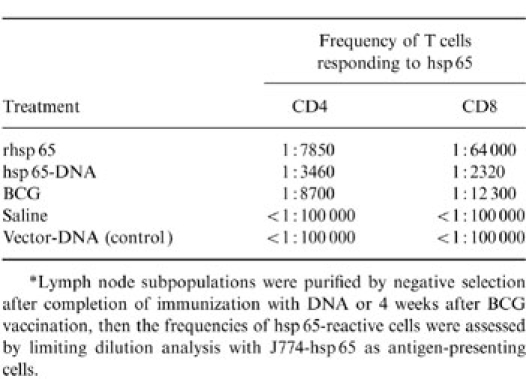
*Lymph node subpopulations were purified by negative selection after completion of immunization with DNA or 4 weeks after BCG vaccination, then the frequencies of hsp 65-reactive cells were assessed by limiting dilution analysis with J774-hsp 65 as antigen-presenting cells.
Table 2.
Expression of CD44 on CD4+CD8− and CD8+CD4− lymph node T cells freshly purified from hsp 65-DNA-immunized or BCG-vaccinated mice, or from control mice
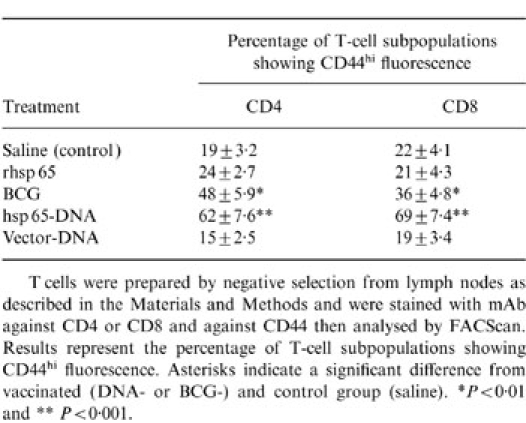
T cells were prepared by negative selection from lymph nodes as described in the Materials and Methods and were stained with mAb against CD4 or CD8 and against CD44 then analysed y FACScan. Results represent the percentage of T-cell subpopulations showing CD44hi fluorescence. Asterisks indicate a significant difference from vaccinated (DNA- or BCG-) and control group (saline). *P < 0·01 and **P < 0·001.
The lymph node T cells from DNA-vaccinated mice more often produced IFN-γ than IL-4 in response to either hsp 65, whole BCG antigen, or stimulation with PMA and anti-CD3, irrespective of whether they were of the CD4+CD8− or CD8+CD4− phenotypes (Fig. 1). The same level of interleukin production in response to hsp 65 was observed for cells from BCG-immunized mice, again irrespective of whether they were CD4+CD8− or CD8+CD4, and it was twofold less effective than that observed for DNA immunization (Fig. 1a). However, BCG immunization induced more IFN-γ in CD4+CD8− and CD8+CD4− T cells in response to whole BCG antigen when compared in response to hsp 65 (Fig. 1b). A similar level of interleukin production was also observed in both T-cell populations under stimulation with PMA plus anti-CD3 (Fig. 1c). When the cells were further subdivided into CD44lo and CD44hi prior to assessing the relative frequencies of IL-4- and IFN-γ-producing cells it was seen that IL-4 production was particularly associated with CD44lo cells and IFN-γ production was particularly associated with CD44hi cells (Table 3). This was the case whether the cells had CD4+CD8− or CD8+CD4− phenotypes, came from BCG- or DNA-vaccinated mice, or those stimulated with hsp 65, whole BCG antigen, or PMA plus anti-CD3. Taken together, these FACSort and ELISPOT assays indicated that in DNA- immunized mice the CD4+CD8− or CD8+CD4− T cells were more frequently CD44hi IFN-γ producers, whereas in BCG-vaccinated mice the T cells produced similar levels of CD44lo/IL-4 and CD44hi/IFN-γ phenotypes.
Figure 1.
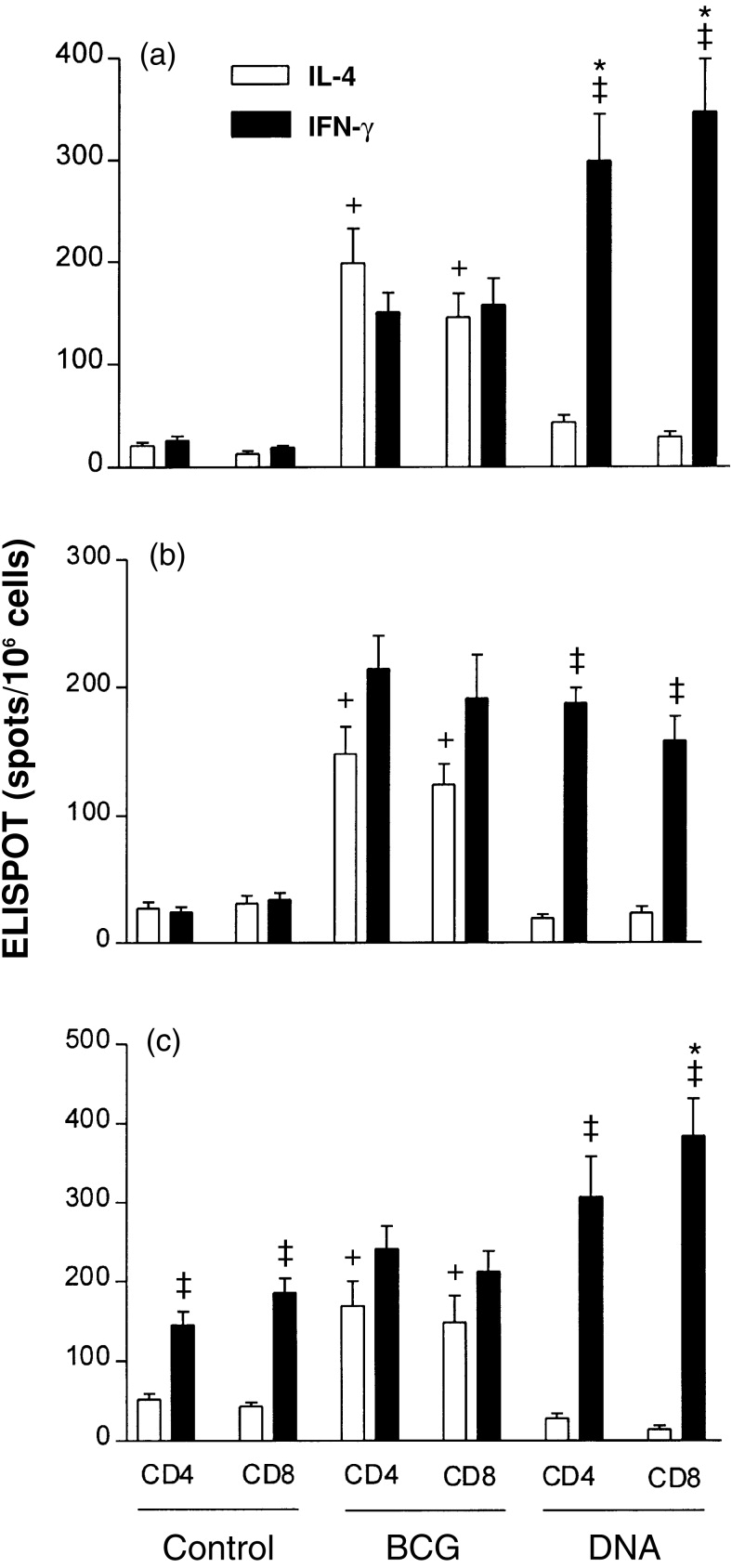
ELISPOT estimates of the frequencies of cells that produced IL-4 or IFN-γ in response to hsp 65 (a) (assay in the presence of J774-hsp 65 cells as feeder cells); whole BCG antigen in the presence of J774 cells (b) and PMA plus anti-CD3 (c) among lymph node T cells from hsp 65-DNA-immunized or BCG-vaccinated mice. CD4+CD8− and CD8+CD4− T cells were prepared by negative selection from lymph nodes as described in the Materials and Methods. The results shown are mean estimates (±SD) from triplicate wells. * indicates statistically significant differences for IFN-γ production from DNA-immunized and from BCG-vaccinated mice (P <0·01); † indicates significant difference for IL-4 production from DNA-immunized and from BCG-vaccinated mice mice (P <0·01); and ‡ indicates a significant difference from frequencies of cells that produced IL-4 or IFN-γ in the same experimental group (P <0·01).
Table 3.
Association of IFN-γ production with CD44hi cells
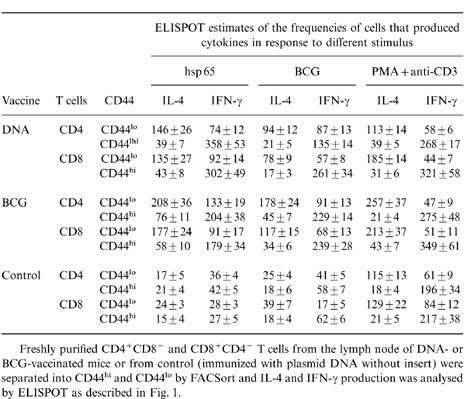
Freshly purified CD4+CD8− and CD8+CD4− T cells from the lymph node of DNA- or BCG-vaccinated mice or from control (immunized with plasmid DNA without insert) were separated into CD44hi and CD44lo by FACSort and IL-4 and IFN-γ production was analysed by ELISPOT as described in Fig. 1.
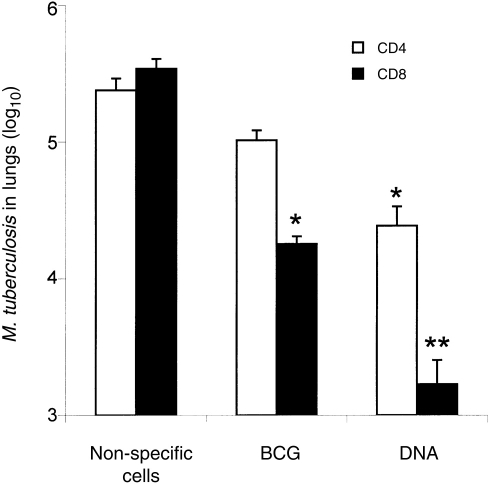
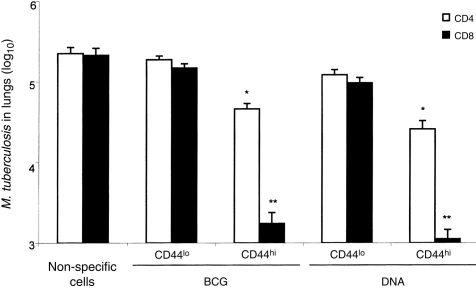
When tested for their ability adoptively to transfer protective immunity to naive mice, bulk CD4+CD8− and CD8+CD4− cells purified from lymph nodes of either BCG- or DNA-immunized mice were effective. Cells from DNA-immunized mice were more effective than those from BCG-vaccinated mice and CD8+CD4− cells were more effective than CD4+CD8− cells (Fig. 2). Statistical analysis showed that these differences were significant (P <0·01 and 2 logs difference in CFU counts). When the cells for adoptive transfer experiments were further subdivided into CD44lo and CD44hi it was seen that protection was associated with the CD44hi phenotype (Fig. 3). CD44hi cells from DNA-immunized mice were more protective than CD44hi cells from BCG-vaccinated mice, and the most protective cells were the CD8+CD4−/CD44hi cells from DNA-immunized mice. Statistical analysis showed that these differences were highly significant (P ≤0·01 and 2 logs difference in CFU counts).
Figure 2.
Adoptive transfer of protective immunity against tuberculosis by bulk transfer of CD4+CD8− or CD8+CD4− T cells to naive mice. T-cell subsets were obtained by negative selection from lymph nodes of DNA-immunized or BCG-vaccinated mice. Recipient mice were γ-irradiated then injected intravenously with 5×106 T cells and 1×105M. tuberculosis cells. The number of live bacteria in the lungs was determined 4 weeks after infection. Control mice were either untreated or were irradiated and reconstituted with non-specific lymph node T cells enriched from normal mice. Results are shown as mean CFU±SD from groups of five animals. Asterisks indicate a significant difference from vaccinated (DNA- or BCG-) and control group. *P <0·01 and **P <0·001 and 2 logs difference.
Figure 3.
Association of protection with CD8+/CD44hi T cells. Purified CD4+CD8− and CD8+CD4− T cells were separated into CD44hi and CD44lo by FACSort and amplified by culture for 14 days on the presence of J774-hsp 65 feeder cells. The expanded cells were tested for their ability to protect naive recipient mice against challenge with M. tuberculosis as described in Fig. 2. There was no variation in the CD44 and interleukin expression in expanded cells in relation to freshly purified cells. Asterisks indicate a significant difference from vaccinated (DNA- or BCG-) and control group. *P <0·01 and **P <0·001 and 2 logs difference.
Twenty-four hsp 65-reactive T-cell clones representing the CD4, CD8 and CD44 phenotypes were established, 12 from DNA-immunized and 12 from BCG-vaccinated mice, and then characterized and tested for their ability to confer protection to recipient mice in adoptive transfer experiments. The 12 CD8+/CD4− clones recognized hsp 65 processed and presented via the major histocompatibility complex (MHC) class I and not the class II pathway and the 12 CD4+CD8− clones had the converse characteristics. This was established as described in the methods by using mAbs to selectively block CD4, CD8, and specific MHC class I and II haplotypes, and brefeldin A and chloroquine to block processing in lymphoproliferation assays (data not shown) essentially as described for clones from mice immunized with J774-hsp 65 cells.12,15 As expected, most produced either IFN-γ or IL-4 (Table 4). Some of the IFN-γ-producing clones showed antigen-dependent cytotoxicity against M. tuberculosis-infected macrophages (Table 4). Clones that produced IFN-γ and were cytotoxic were the most effective in adoptive transfer of protection (Table 4). Statistical analysis showed that these differences were significant (P ≤ 0·01 and 2 logs difference in CFU counts). These tended to be properties of the CD44hi clones. The CD8+/CD44hi clones with these characteristics were more effective than the corresponding CD4+/CD44hi clones. All three of the CD8+/CD44hi clones from DNA-immunized mice were of this highly protective phenotype, whereas one of the three CD8+CD44hi clones from BCG-immunized mice was almost non-protective, was not cytotoxic and produced IL-4.
Table 4.
Correlation between CD44 expression, cytokine production, cytotoxicity and protection by hsp 65-specific T cell clones from BCG or DNA vaccinated mice
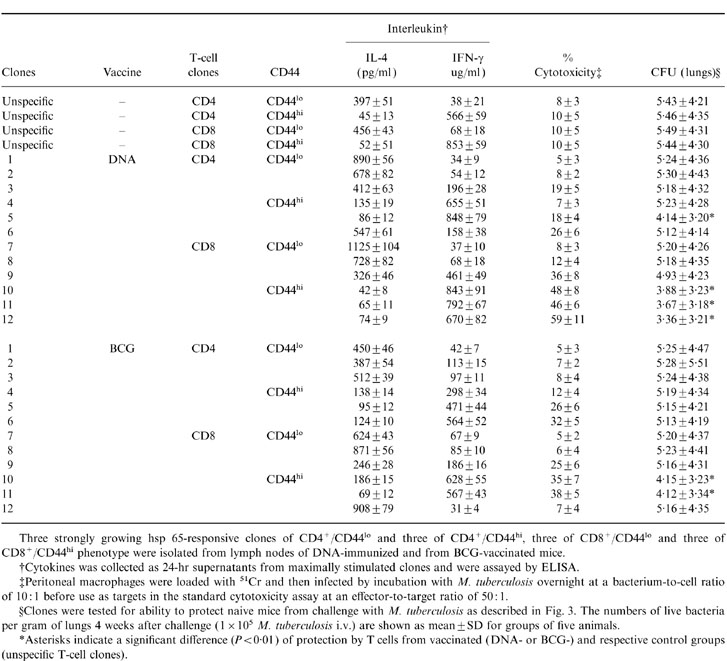
Three strongly growing hsp 65-responsive clones of CD4+/CD44lo and three of CD4+/CD44hi, three of CD8+/CD44lo and three of CD8+/CD44hi phenotype were isolated from lymph nodes of DNA-immunized and from BCG-vaccinated mice.
†Cytokines was collected as 24-hr supernatants from maximally stimulated clones and were assayed by ELISA.
‡Peritoneal macrophages were loaded with 51Cr and then infected by incubation with M. tuberculosis overnight at a bacterium-to-cell ratio of 10: 1 before use as targets in the standard cytotoxicity assay at an effector-to-target ratio of 50: 1.
§Clones were tested for ability to protect naive mice from challenge with M. tuberculosis as described in Fig. 3. The numbers of live bacteria per gram of lungs 4 weeks after challenge (1 × 105 M. tuberculosis i.v.) are shown as mean±SD for groups of five animals.
*Asterisks indicate a significant difference (P < 0·01) of protection by T cells from vaccinated (DNA-or BCG-)and respective control groups (unspecific T-cell clones).
We have now characterized the immune response in terms of duration of response, cytokine profile (IFN-γ and IL-4) and expression of CD44, a marker of immunological 16,17 after, 1, 4, 8 and 15 months after DNA or BCG vaccination. Both immunization procedures generated strong lymphoproliferative responses to hsp 65 (Table 5). These responses were sustained for at least 8 or even 15 months after hsp 65-DNA or live BCG vaccination. With the exception of rhsp65 IFA, all of the immunization procedures elicited substantial protective immunity against virulent M. tuberculosis (Table 5). When challenge infection was given 1 or 4 months after completion of immunization, bacterial numbers in lungs 4 week later were at least 2 logs lower than in lungs of vaccinated controls. Protection was sustained even after 15 months of vaccination with DNA or BCG.
Table 5.
Correlation between the duration of protection against tuberculosis challenge infection and development of immunological memory and cytokine production at several intervals after vaccination
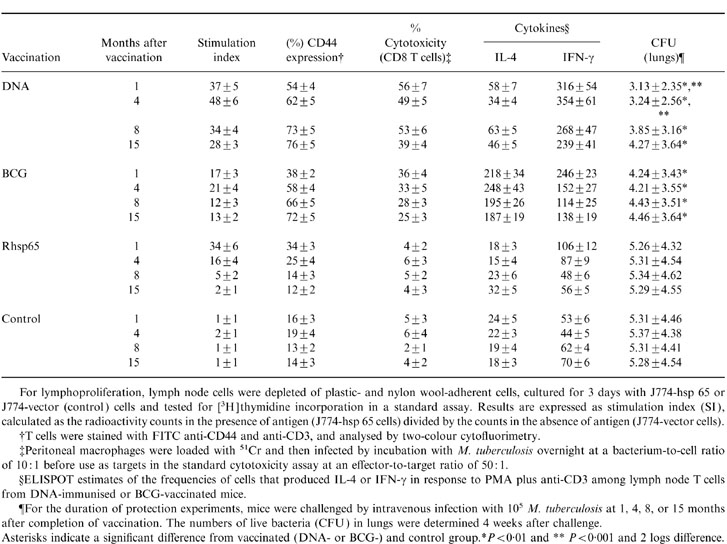
For lymphoproliferation, lymph node cells were depleted of plastic- and nylon woll-adherent cells, cultured for 3 day with J774-hsp 65 or J774-vector (control) cells and tested for [3H]thymidine incorporation in a standard assay. Results are expressed as stimulation index (SI), calculated as the radioactivity counts in the presence of antigen (J774-hsp 65 cells) divided by the counts in the absence of antigen (J774-vector cells).
†T cells were stained with FITC anti-CD44 and anti-CD3, and analysed by two-colour cytofluorimetry.
‡Peritoneal macrophages were loaded with 51Cr and then infected by incubation with M. tuberculosis overnight at a bacterium-to-cell ratio of 10: 1 before use as targets in the standard cytotoxicity assay at an effector-to-target ratio of 50: 1.
§ELISPOT estimates of the frequencies of cells that produced IL-4 or IFN-γ in response to PMA plus anti-CD3 among lymph node T cells from DNA-immunised or BCG-vaccinated mice.
¶For the duration of protection experiments, mice were challenged by intravenous infection with 105 M. tuberculosis at 1, 4, 8, or 15 months after completion of vaccination. The numbers of live bacteria (CFU) in lungs were determined 4 weeks after challenge.
Asterisks indicate a significant difference from vaccinated (DNA- or BCG-) and control grroup. *P < 0·01 and **P < 0·001 and 2 logs difference.
Since we have found that protective immunity is particularly associated with T cells and the development of immunological memory is known to be associated with the persistence of cells expressing high levels of CD44,15 we monitored the expression of CD44hi by lymph node T cells after both immunization procedures (Table 5). Both BCG and DNA at 1 month after vaccination had caused only a modest increase in CD44hi expression but this continued to increase, reaching 66 and 73% of total T cells by 8 months and 72 months and 76% after 15 months. We next assessed the cytokine produced by lymph node T cells after 1, 4, 8 and 15 months of vaccination. The T cells from BCG-vaccinated mice more often produced IL-4 than IFN-γ in all of the time-points analysed (Table 5). The opposite was true for cells from DNA-immunized mice, where threefold more cells produced IFN-γ than IL-4.
DISCUSSION
Following our unexpected observation of the effectiveness of J774-hsp 65 cells in raising protective immunity against tuberculosis,11 and of the prominence of antigen-specific CD8+/CD44hi IFN-γ-producing/cytotoxic cells in expressing protection,12,15 we earlier hypothesized that the endogenous origin of the antigen via the cytosol in antigen-presenting cells could be of critical importance.12 The validity of this interpretation is strengthened by our previous 10,15 and by the results presented here, that an alternative method of introducing antigen into the cytosol, such as plasmid DNA, also generated protective immunity with a typical type 1 immune response. On the other hand, the immune responses to mycobacterium, whether to BCG vaccine, M. leprae, or virulent M. tuberculosis, whether in mouse or man, are well known to show at times strong CD8+CD4−-mediated immune responses which are associated with the later or chronic phases of the infection.15,18–20 To better understand what contribution the different T cells make to protection against tuberculosis we determined here the correlation among the frequency of the different phenotypes, cytokine production, adoptive transfer of protection, memory, cytotoxicity and duration of protection in purified subpopulations or T-cell clones obtained from lymph nodes of hsp 65-DNA-immunized or BCG-vaccinated mice.
The equal increase in CD4+CD8− and CD8+CD4− hsp 65-reactive cells seen in BCG-vaccinated mice (Table 1) resembles that seen in mice protected by immunization with plasmid DNA or with M. tuberculosis infection.15 Although this strong CD8+CD4− response is consistent with the endogenous origin of the antigen and appears to be important for protection,10,12,15 there were marked differences in the profiles of memory/activation (CD44hi) and cytokine production (IL-4, IFN-γ) between the BCG- and DNA-immunized mice. Since the bulk-purified CD8+CD4− cells from DNA-immunized mice were more efficient than that BCG-vaccinated mice in transferring protection to naive animals (Fig. 2), we could assess how these differences might relate to the inadequate immunity in the BCG-vaccinated animals.
The lower protective efficacy of cells from BCG-vaccinated mice in adoptive transfer experiments was probably not due to differential entry of fewer CD8+CD4− than CD4+CD8− cells into the activated/memory state; the CD8:CD4 ratio among CD44hi hsp 65-reactive cells was about 1:1 in both BCG- and DNA-vaccinated mice. However, it might be related to the no preponderance of IFN-γ-producing cells in the BCG-vaccinated animals. This was clearly indicated by the ELISPOT assays of both CD4+CD8− and CD8+CD4− cells responding to either hsp 65, whole BCG antigen or PMA plus anti-CD3 stimulus (Fig. 1). Furthermore, IL-4 production was associated with CD44lo cells (Table 3), suggesting a predominance of CD44lo cells in BCG-vaccinated mice. FACScan of freely isolated lymph nodes CD4+CD8− or CD8+CD4− cells also indicated predominance of CD44lo cells in BCG-vaccinated animals. Thus it appears that the majority of T cells in BCG-vaccinated mice were not memory/activated and produced low levels of IFN-γ, whereas the majority of T cells in DNA-immunized mice were activated and produced IFN-γ. Although hsp 65 is only one of many antigens involved in the immune response, and others may have equal or greater roles in protective immunity, this pattern of activation and cytokine profile is likely to extend to cells with other antigen specificities through the regulatory role of the cytokines in the local environment. CD8+/CD44hi cells of BCG-vaccinated mice, besides being less abundant than the CD8+/CD44hi cells of DNA-immunized animals, were also less efficient per cell in transfer of protective immunity. The basis for this was not established. It may have been related to a lower frequency of either IFN-γ-producing cells or cytotoxic cells or to some other unidentified factor affecting this minority population.
We showed previously that although both cytotoxicity and IFN-γ can come from either CD4+CD8− or CD8+CD4− T cells, the most potent protective cells in our model is CD8+CD4−, produces IFN-γ and is cytotoxic.12,15 This conclusion is now refined by this study of new clones to include the observation that cells with that phenotype are most effective when they also express CD44hi (Table 4). It was striking that CD4+CD8−/CD44lo IL-4-producing or CD8+/CD4−CD44lo IL-4-producing T cells had essentially no effect in our model. They might not be expected to be protective, since they produced little IFN-γ and were not cytotoxic, but their failure to interfere with expression of immunity was remarkable. Since type 2 cytokines down-regulate T-cell differentiation for expression of type 1 cytokines in vivo and in vitro21–23 we may conclude that the low rate of growth of the bacteria in the model of BCG-vaccination is not dependent on such differentiation; some other mechanism is retarding bacterial multiplication in the presence of only limited numbers of CD44hi cytotoxic type 1 cells. The existence of such additional antimycobacterial mechanisms has been indicated in several studies, for example in αβ-T-cell-depleted 24 and gene- deleted (‘knock-out’) mice.24–27 Presumably, if the low growth rate is a consequence of residence within IFN-γ-activated macrophages, the necessary IFN-γ could be coming from alternative sources such as γδ T cells and natural killer 28,29 although there is no evidence that these sources are more resistant to down-regulation by type 2 cytokines than are the CD4+CD8− or CD8+CD4− cells.30,31
Protection by both immunization procedures generated strong lymphoproliferative responses to hsp 65 and protection against M. tuberculosis challenge after 1, 4, 8 and 15 months after vaccination (Table 5). These responses were sustained for at least 8 or even 15 months after DNA or live BCG vaccination. Analysis of the lymph node T-cell populations from DNA-immunized mice indicated that this was due to a progressive increase during the same period in numbers of cells with the memory/activated phenotype (CD8+ CD4−/CD44hi) that produced IFN-γ and antigen-specific cytotoxicity. Plasmid DNA does not integrate into host cell DNA but persists episomally and can express encoded antigen for the lifetime of the injected mouse.32 It may be relevant that the persistence of viable BCG has been argued to make a significant contribution to the persistence of protection after BCG vaccination.33 Presumably the continued synthesis and presentation of endogenous hsp 65 antigen may account for the persistent protection after DNA vaccination.
A striking difference between the immune response to DNA vaccination and the immune response to either BCG vaccination or M. tuberculosis infection is that DNA induces almost entirely a protective CD44hi type 1 cytokine response, whereas the mycobacterium infections have a major component of non-cytotoxic CD44lo T cells that produced low level of IFN-γ.15 The hypothesis that inappropriate type 1/type 2 switching of the immune response might underlay the failure of BCG in some human 34 has already been mentioned. Therefore, a vaccine that works well in populations where BCG fails is obviously a major objective. Unfortunately the basis of BCG failure is not known with certainty so that any animal models and design strategies are necessarily speculative. The consensus view is that a major cause of BCG failure is that the environmental mycobacterium may subvert the immune response so that key elements of protective immunity are no longer evoked by BCG.35 In this case a better vaccine would be one that can switch the protective responses back on, by changing the way the antigens are seen by the immune system rather than necessarily changing the antigen seen.
Acknowledgments
We thank Izaira T. Brandao for technical assistance. Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq) and Finaciadora de Estudos e Projetos (FINEP) supported this study.
REFERENCES
- 1.Lefford MJ, McGregor DD, Mackaness GB. Immune response to Mycobacterium tuberculosis in rats. Infect Immun. 1973;8:182. doi: 10.1128/iai.8.2.182-189.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harboe M, Andersen P, Colston MJ, et al. European Commission COST/STD Initiative. Report of the expert panel IX. Vaccines against tuberculosis. Vaccine. 1996;14:701. doi: 10.1016/s0264-410x(96)90051-1. [DOI] [PubMed] [Google Scholar]
- 3.Boom WH. The role of T-cell subsets in Mycobacterium tuberculosis infection. Infect Agents Dis. 1996;5:73. [PubMed] [Google Scholar]
- 4.Ladel CH, Blum C, Dreher A, Reifenberg K, Kaufmann SH. Protective role of gamma/delta T cells and alpha/betal T cells in tuberculosis. Eur J Immunol. 1995;25:2877. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- 5.De Libero G, Flesch I, Kaufmann SH. Mycobacterium-reactive Lyt-2+ T cell lines. Eur. J. Immunol. 1988;18:59. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- 6.Lowrie DB, Silva CL, Tascon RE. Genetic vaccination against tuberculosis. Springer Semin, Immunopathol. 1997;19:161. doi: 10.1007/BF00870266. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann SHE. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988;9:168. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- 8.Orme IM. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293. [PubMed] [Google Scholar]
- 9.Gautier C, Mehtali M, Lathe R. A ubiquitous mammalian expression vector, pHMG, based on a housekeeping gene promoter. Nucleic Acids Res. 1989;17:83. doi: 10.1093/nar/17.20.8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowrie DB, Silva CL, Colston MJ, Ragno S, Tascon RE. Protection against tuberculosis by a plasmid DNA vaccine. Vaccine. 1997;15:834. doi: 10.1016/s0264-410x(97)00073-x. [DOI] [PubMed] [Google Scholar]
- 11.Silva CL, Lowrie DB. A single mycobacterial protein (hsp65) expressed by a transgenic antigen-presenting cells vaccinates mice against tuberculosis. Immunology. 1994;82:244. [PMC free article] [PubMed] [Google Scholar]
- 12.Silva CL, Silva MF, Pietro RCLR, Lowrie DB. Characterization of T cells that confer a high degree of protective immunity against tuberculosis in mice after vaccination with tumor cells expressing mycobacterial hsp65. Infect Immun. 1996;64:2400. doi: 10.1128/iai.64.7.2400-2407.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva CL, Palacios A, Colston MJ, Lowrie DB. Mycobacterium leprae 65hrsp antigen expressed from a retroviral vector in a macrophage cell line is presented to T cells in association with MHC class II in addition to MHC class I. Microb Pathog. 1992;12:27. doi: 10.1016/0882-4010(92)90063-t. [DOI] [PubMed] [Google Scholar]
- 14.Silva CL, Silva MF, Pietro RCLR, Lowrie DB. Protection against tuberculosis by passive transfer with T-cell clones recognizing mycobacterial heat-shock protein 65. Immunology. 1994;83:341. [PMC free article] [PubMed] [Google Scholar]
- 15.Bonato VLD, Lima VMF, Tascon RE, Lowrie DB, Silva CL. Identification and characterization of protective T cells in hsp65 DNA vaccinated and Mycobacterium tuberculosis infected mice. Infect Immun. 1998;66:169. doi: 10.1128/iai.66.1.169-175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen P, Andersen AB, Sorensen AL, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359. [PubMed] [Google Scholar]
- 17.Sander B, Skansensaphir U, Damm O, Hakansson L, Anderson J, Andersson U. Sequential production of Th1 and Th2 cytokines in response to live bacillus Calmette-Guerin. Immunology. 1995;86:512. [PMC free article] [PubMed] [Google Scholar]
- 18.Mahanty S, Abrams JS, King CL, Limaye AP, Nutman TB. Parallel regulation of IL-4 and IL-5 in human helminth infections. J Immunol. 1992;148:3567. [PubMed] [Google Scholar]
- 19.Hernandez Pando R, Orozcoe H, Sampieri A, et al. Correlation between the kinetics of Th1/Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26. [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamura M, Wang XH, Ohmen JD, et al. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992;149:1470. [PubMed] [Google Scholar]
- 21.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 22.Dutton RW. The regulation of the development of CD8 effector T cells. J Immunol. 1996;157:4287. [PubMed] [Google Scholar]
- 23.Seder RA, Legros GG. The functional role of CD8 (+) T helper type 2 cells. J Exp Med. 1995;181:5. doi: 10.1084/jem.181.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ladel CH, Blum C, Dreher A, Reifenberg K, Kaufmann SHE. Protective role of gamma/delta T cells and alpha/beta T cells in tuberculosis. Eur J Immunol. 1995;25:2877. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann SHE. Immunity to intracellular microbial pathogens. Immunol Today. 1995;16:338. doi: 10.1016/0167-5699(95)80151-0. [DOI] [PubMed] [Google Scholar]
- 26.Flynn JAL, Goldstein MM, Triebold KJ, Bloom BR. Major histocompatibility complex class I restricted T cells are necessary for protection against Mycobacterium tuberculosis in mice. Infect Agent Dis. 1993;2:259. [PubMed] [Google Scholar]
- 27.Kaufmann SHE, Ladel CH. Role of T cell subsets in immunity against intracellular bacteria. Experimental infections of knock-out mice with Listeria monocytogenes and Mycobacterium. Immunobiology. 1994;191:509. doi: 10.1016/S0171-2985(11)80457-2. [DOI] [PubMed] [Google Scholar]
- 28.Cron RQ, Gajewski TF, Sharrow SO, Fitch FW, Matis LA, Bluestone JA. Phenotypic and functional analysis of murine CD3+, CD4-, CD8-, TCR-gamma/delta-expressing peripheral T cells. J Immunol. 1989;142:3754. [PubMed] [Google Scholar]
- 29.Welsh RM. Natural killer cells and interferon. Crit Rev Immunol. 1984;5:55. [PubMed] [Google Scholar]
- 30.Naume B, Espevik T. Immunoregulatory effects of cytokines on natural killer cells. Scan J Immunol. 1994;40:128. doi: 10.1111/j.1365-3083.1994.tb03441.x. [DOI] [PubMed] [Google Scholar]
- 31.Schlaak JF, Hermann E, Gallati H, Meyer Zun Buschenfelde KH, Fleischer B. Differential effects of IL-10 on proliferation and cytokine production of human gamma/delta and alpha/beta T cells. Scand J Immunol. 1994;39:209. doi: 10.1111/j.1365-3083.1994.tb03362.x. [DOI] [PubMed] [Google Scholar]
- 32.Wolffe JA, Ludtke JJ, Acsadi G. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Human Mol Genetics. 1992;1:363. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 33.Lefford MJ, MacGregor DD. Immunological memory in tuberculosis. I. Influence of persisting viable organisms. Cell Immun. 1974;14:417. doi: 10.1016/0008-8749(74)90192-0. [DOI] [PubMed] [Google Scholar]
- 34.Rook GAW, Onyebujoh P, Stanford JL. TH1/TH2 switching and loss of CD4+ T-cells in chronic infections – Na immunoendocrinological hypothesis not exclusive to HIV. Immunol Today. 1993;14:568. doi: 10.1016/0167-5699(93)90190-V. [DOI] [PubMed] [Google Scholar]
- 35.Stanford JL, Rook GAW. Environmental mycobacteria and immunitation with BCG. In: Easmon CSF, Jeljaszewics J, editors. Medical Microbiology, Immunization Against Bacterial Disease. Vol. 2. London (UK): Academic Press; 1983. p. 43. [Google Scholar]