Abstract
Membrane cofactor protein (MCP; CD46) is a 50–60 000 MW glycoprotein, expressed on a wide variety of cells and tissues in man, which plays an important role in regulating complement activation. Human MCP has also been shown to be the receptor for measles virus. We have recently identified the pig analogue of MCP and demonstrated that pig MCP has cofactor activity for factor I-mediated cleavage of C3b when these components are derived either from pig or human. As a consequence, pig MCP is an efficient regulator of the classic and alternative pathways of human and pig complement. In order to define the potential importance of MCP in protecting against complement activation in the pig, we have conducted a comprehensive survey of its distribution in pig cells and organs. As in humans, MCP in the pig is broadly and abundantly distributed. Pig MCP is highly expressed on all circulating cells, including erythrocytes, in contrast to its absence on human erythrocytes. Multiple isoforms of MCP are found on cells and in tissues, probably representing products of alternative splicing analogous to those found in man. MCP is abundantly expressed throughout all tissues examined with particularly strong staining on the vascular endothelium. Connective tissue elements within liver and testis are also strongly stained by anti-pig MCP antibodies. Pig MCP is expressed only weakly on skeletal muscle cells and expression is absent from smooth muscle cells in the lung and vessel walls, sites at which human MCP is expressed. Of particular note, MCP is not expressed in B-cell areas of the germinal centres of lymph nodes.
INTRODUCTION
Human membrane cofactor protein (MCP or CD46) is an important membrane bound regulator of complement (C) activation. MCP serves as cofactor for the plasma serine protease factor I in the degradation of C3b and C4b deposited on self tissues.1 It is expressed on a wide variety of cells but it is absent from erythrocytes. Structural analysis reveals that MCP is a glycoprotein consisting of four homologous short consensus repeats (SCR), a serine/threonine/proline (STP) rich region, and transmembrane and cytoplasmic domains. The SCRs are characteristic of the RCA family of C-regulators to which MCP belongs.2 In human MCP, SCR 3 and 4 are necessary for cofactor activity for the cleavage of C4b and C3b.3 Alternative splicing of the STP and cytoplasmic domains results in expression of multiple isoforms of MCP.4 On peripheral blood cells, individuals may express predominantly a 65 000-MW isoform, express predominantly a 45 000-MW isoform or express equal amounts of the two isoforms and this characteristic is stable and inherited in an autosomal codominant fashion.5 In addition to its role in C regulation, human MCP is of interest in reproductive immunology because of its expression on sperm and at the maternal–fetal interface,6 to tumour immunology because of its high expression on malignant cells,7 and to microbiology because of its role as a receptor for measles virus8 and for M protein of group A streptococci.9
Regulation of C in the pig has become a subject of interest because of the planned use of pig organs for transplantation to humans. In order to circumvent C-mediated hyperacute rejection, an inevitable consequence of pig–human transplants, pigs are now bred that express human C regulators on endothelium.10 However, the contribution of the endogenous pig inhibitors to C regulation remains unassessed. We have undertaken to characterize membrane regulators of C in the pig. We have recently described the purification and characterization of the pig analogues of human CD59 and MCP.11 Pig MCP purified from erythrocytes is a 50–60 000 MW glycoprotein with cofactor activities similar to those of the human protein.12 Molecular cloning of pig MCP revealed a 43% amino acid identity with human MCP and a very similar predicted protein structure.13 Pig MCP was an efficient regulator of the classic and alternative pathways of pig and human C, leading us to propose that the presence of a resident MCP on pig cells capable of acting as a cofactor in the control of human C activation has consequences for the use of pig organs in xenotransplantation.12
In order to analyse the contributions of endogenous pig MCP and to define which pig cells and organs are best protected from the activation of C, we set out to study the cellular expression and organ distribution of the pig analogue of MCP.
MATERIALS AND METHODS
Cell preparation
Fresh pig blood was obtained from the UWCM animal facility or from the local abbatoir, collected into 0·38% sodium citrate as anticoagulant, and served as a source of pig leucocytes and erythrocytes (PgE). Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation on Ficoll–Paque (Pharmacia, Uppsala, Sweden). Granulocytes were recovered from the lower Ficoll phase and residual erythrocytes were lysed by hypotonic treatment. Platelet-rich plasma (PRP) was obtained from pig blood by centrifugation at 750 g for 10 min and replaced with an equal volume of phosphate-buffered saline (PBS). Porcine platelets were obtained by centrifugation of the PRP at 1500 g for 10 min. Porcine endothelial cells were obtained from pig aorta (obtained from the local abbatoir) as described previously14 and were a kind gift of Dr C. W. Van den Berg (Department of Pharmacology, UWCM, Cardiff, UK). The porcine cell line ST from fetal testis was from the European Collection of Animal Cell Cultures (ECACC; Porton Down, UK) and IB-RS-2 from pig kidney was from the American Type Tissue Culture Collection (ATTCC, Bethesda, MD, USA).
Proteins and antibodies
Pig MCP was purified from pig erythrocyte ghosts by immunoaffinity purification, as previously described.12 Monoclonal antibody (mAb) JM4C8 (IgG1) anti-pig MCP was obtained as described previously.12 MAb 6D1 (IgG1) anti-rat CD59 was used as an isotype-matched negative control.
Flow cytometry
Pig cells (106 cells/ml in PBS) were incubated on ice with mAb JM4C8 (50 μg/ml), or an irrelevant mAb as negative control, for 30 min. Cells were washed three times in flow cytometry buffer (FCB: PBS, 0·1% bovine serum albumin (BSA) and 0·1% NaN3). Cells were incubated for 30 min at 4° with 50 μl fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG; Sigma, Poole, UK), diluted 1/500 in FCB. After three washes in FCB, cells were fixed with 0·5 ml 1% paraformaldehyde in PBS. Fluorescence was analysed using a Becton-Dickinson FACScalibur™ (Oxford, UK) equipped with CellQuest™ software. Cells were identified and gated by their characteristic forward and side scatter. Platelet acquisition was performed at logarithmic scale and with the pressure selector in the low position.
Western blotting
Pig leucocytes, erythrocytes, granulocytes and platelets were obtained as described above. Cells from lung, spleen, liver, kidney and brain were released from the corresponding tissue by perfusion and gentle disaggregation to obtain a cell suspension. Residual erythrocytes from organ cell suspensions were lysed by hypotonic treatment. Where possible, all blood and tissue samples were collected from the same pig. Cells and tissue extracts were solubilized in lysis buffer, consisting of 10 mm Tris–HCl, 150 mm NaCl, 1 mm ethylenediamine tetra-actetic acid (EDTA), 1% NP-40, 1 mm phenylmethylsulphonyl fluoride (PMSF), for 1 hr at room temperature. After centrifugation at 12 000 g for 15 min, supernatants were mixed with sample buffer and run on a 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) gel under non-reducing conditions. The separated proteins were transferred to nitrocellulose membrane (Sartorius AG, Göttingen, Germany). Nitrocellulose was blocked with PBS–5% non-fat powdered milk and incubated with the mAb JM4C8, or an irrelevant isotype-matched mAb (6D1) as negative control, for 1 hr at room temperature, followed by a 1-hr incubation with a peroxidase-labelled rabbit anti-mouse immunoglobulin (Sigma). Peroxidase activity was visualized using SuperSignal (Pierce & Warriner, Chester, UK) as a substrate, following the recommendations of the manufacturer.
Densitometric analysis
Protein concentration in cell and tissue lysates produced as described above was measured using the micro-bicinchoninc acid (micro-BCA) protein assay (Pierce). Equal amounts of protein (20 μg) from each lysate were loaded, onto 10% SDS–PAGE gels; purified pig erythrocyte MCP (2 μg) was loaded as an internal standard. Following electrophoresis, gels were blotted onto nitrocellulose and probed with mAb JM4C8 as described above. Quantitative analysis of MCP for each lysate was performed using a Bio-Rad GS-690 imaging densitometer, and Molecular Analyst v.1.4.1 software (Bio-Rad, Hercules, CA). Regions were selected around the visualized bands and subjected to volume analysis. Background signal was subtracted from positive areas and results were expressed as the mean of the optical density (OD). Linearity in the assay was confirmed using various amounts of purified MCP.
Immunohistochemistry
Tissue samples were obtained fresh from English White pigs either at the local abbatoir or from laboratory-maintained animals from the UWCM animal facility, mounted onto cork boards with OCT (EMS Laboratories Ltd. Ashford, UK) and snap frozen in isopentane. Frozen sections were cut on an OFT Cryostat (Bright Instruments Ltd, Cambridge, UK) and placed onto chromium gel-coated glass slides. Sections were fixed in acetone for 10 min, endogenous peroxidase removed by incubation with PBS/0·02% H2O2, then washed in PBS. MAb JM4C8 anti-pig MCP or 6D1 isotype-matched negative control was added at an appropriate dilution for 1 hr at room temperature. Sections were washed three times in PBS and binding of antibody to tissue sections was detected using peroxidase-conjugated goat anti-mouse IgG (Sigma) diluted 1:100 in PBS/1%BSA. Peroxidase activity was developed by incubation in 0·5 mg/ml 3,3′diaminobenzidine-tetrahydrochloride (DAB, Sigma) and 0·02% H2O2 in PBS for 5 min, washed in water and counterstained with haematoxylin for 20 s. Sections were dehydrated with three washes in 100% ethanol (5 min each), then three washes in xylene (5 min each) and mounted in a neutral mounting medium (XAM, BDH, Poole, UK). Staining for pig MCP was quantified by independent researchers with a scale scoring from +++, indicating very strong staining, to ±, which indicated little staining and –, as no staining.
RESULTS
Expression of pig MCP on blood cells
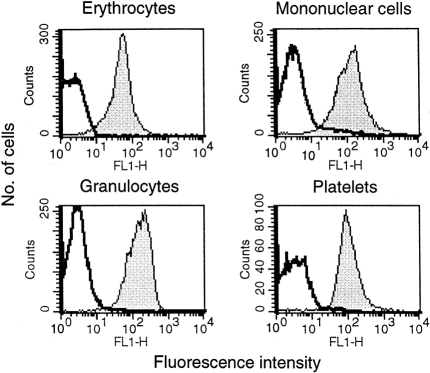
Flow cytometric analysis revealed the presence of pig MCP on all circulating cells. Leucocytes, erythrocytes, granulocytes and platelets all strongly and uniformly expressed pig MCP (Fig. 1). Isotype-matched control antibody was negative on all cell types. All leucocyte populations expressed similar levels of MCP.
Figure 1.
Flow cytometric profiles of pig blood cells. Pig erythrocytes, leucocytes, granulocytes and platelets were separated as described in Materials and Methods, stained with mAb JM4C8 and analysed by flow cytometry. Clear profiles correspond to background staining with an irrelevant isotype-matched mAb.
Western blot analysis
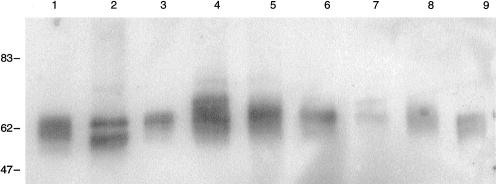
A panel of cell and tissue samples was subjected to SDS–PAGE and western blotting in order to detect the presence and molecular forms of pig MCP in the tissues. MCP was present on all cells and tissues tested (Fig. 2). As in humans, several isoforms of different apparent MW were identified. As shown by the distinct banding pattern, three major isoforms were present, expression of which varied between tissues (Fig. 2). Among blood cells, mononuclear cells (lane 1) expressed a broad band of 60–65 000 MW within which at least two bands were discernable, erythrocytes (lane 2) displayed a clear two-band pattern (55 000 MW and 65 000 MW), platelets (lane 3) expressed predominantly the 65 000 MW isoform and granulocytes (lane 4) contained the broad 60–65 000 MW band seen in mononuclear cells and also a unique, strong band of apparent MW 70 000 MW. Traces of a 75-000 MW band were also seen in erythrocytes and granulocytes. In tissues, lung (lane 5) expressed a broad band of MW 60–65 000 MW together with small amounts of a 75-000 MW band, liver (lane 6) expressed a weaker band of 65 000 MW, kidney (lane 7) a faint doublet of 65 and 70 000 MW, spleen (lane 8) a broad band of 60–65 000 MW and brain (lane 9) a broad band of 55–60 000 MW.
Figure 2.
Western-blotting of pig MCP. Blots were probed with mAb JM4C8 to show the distribution of pig MCP in several tissues. 1. Leucocytes, 2. Erythrocytes, 3. Platelets, 4. Granulocytes, 5. Lung, 6. Liver, 7. Kidney, 8. Spleen, 9. Brain. MW markers are shown on the left.
Densitometric analysis
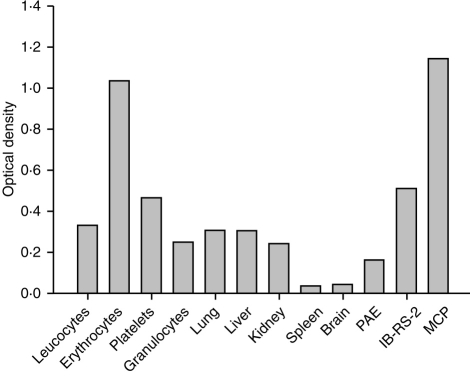
In order to assess the relative abundance of MCP among the different tissues, protein concentrations were measured in tissue and cell lysates and gels run in which protein loading for each tissue or cell was equal. After western blotting, probing with the mAb JM4C8 and ECL development, the relative amounts of MCP in each lysate were assessed by densitometry analysis (Fig. 3). As described above, different isoforms of MCP were found in each tissue. For analysis, the sum of the optical density in all visible bands was used. By this measure, pig MCP was most abundantly expressed in erythrocytes, platelets and the kidney cell line IB-RS-2 (OD values between 0·5 and 1·0). Pig MCP was moderately expressed in leucocytes, granulocytes, lung, liver and kidney with OD values between 0·2 and 0·3 (Fig. 3). Spleen and brain expressed the least pig MCP by this measure, with OD values less than 0·1.
Figure 3.
Relative abundance of MCP in cells and tissues. Equal amounts of total protein were loaded for each tissue/cell extract, separated on SDS–PAGE, blotted onto nitrocellulose and probed with mAb JM4C8. Expression was quantified by densitometric analysis as described in methods. Results are expressed in optical density units for each sample. Lane labelled ‘MCP’ contained 2 μg purified MCP as standard. Result representative of two separate experiments from different donors which gave similar results.
Tissue distribution of pig MCP
Frozen sections of pig tissues were stained with the mAb JM4C8 (Fig. 4). Negative controls using an isotype-matched mAb were performed for each tissue and that for lung is shown as an example (Fig. 4a). In controls, only occasional dark brown cells stained, which were eosinophils in which endogenous peroxidase activity was not fully blocked (not shown). As suggested by western analysis, pig MCP was very broadly expressed in tissues. Pig MCP was present in all the tissues examined. In many respects, the distribution of pig MCP resembled that of human MCP although there were some interesting differences.
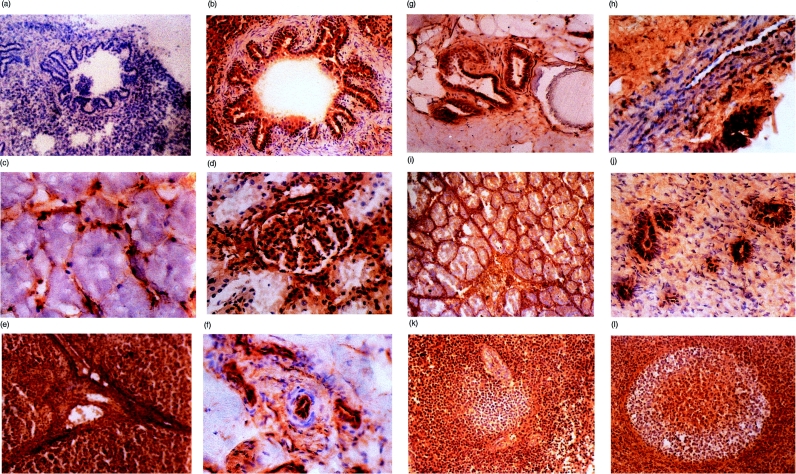
Figure 4.
Frozen sections of pig tissues were examined immunohistochemically for MCP using mAb JM4C8 and isotype control antibody. (a) Lung, negative control. Magnification ×100. (b) Lung bronchiole. Magnification ×200. (c) Skeletal muscle. Magnification ×400. (d) Kidney glomerulus. Magnification ×200. (e) Liver. Magnification ×100. (f) Small arteriole. Magnification ×400. (g) Skin, hair follicle. Magnification ×200. (h) Peripheral nerve. Magnification ×400. (i) Testis. Magnification ×50. (j) Uterus. Magnification ×400. (k) Spleen. Magnification ×200. (l) Lymph node. Magnification ×200.
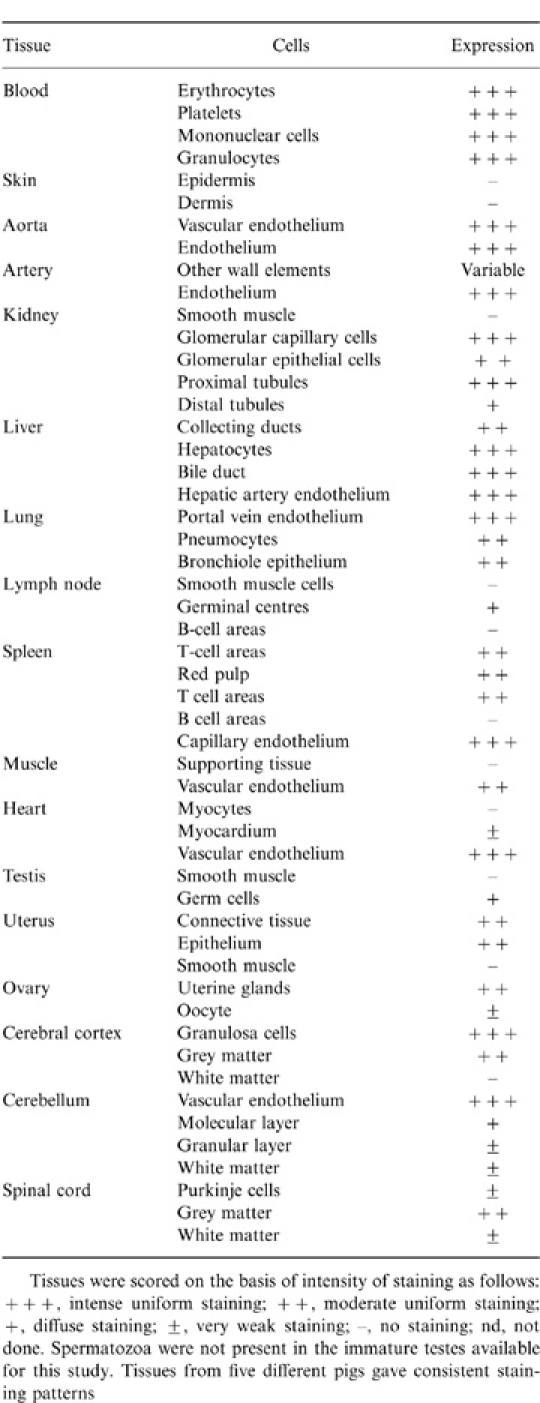
In lung sections, strong staining of pneumocytes and alveolar and bronchial epithelium was found, but no staining was observed in the smooth muscle cells in the walls of the airways (Fig. 4b). Pig MCP was absent from skeletal muscle fibre bundles; however, the intrafassicular capillaries stained for MCP (Fig. 4c). In the kidney cortex, the staining for pig MCP was strong in the glomerular mesangium but staining of glomerular epithelium was weak and inconsistent (Fig. 4d). Proximal convoluted tubules also strongly expressed MCP, whereas distal convoluted tubules and collecting ducts stained weakly. Strong expression of MCP was observed in pig liver, where hepatocytes, all vessels of the portal tract and connective tissue elements were strongly stained (Fig. 4e). In the skin, MCP was absent in epidermis and weak in the dermis; however, expression was strong in the endothelial cells of small arterioles in the dermis (Fig. 4f) and on the epithelium of sweat glands and sebaceous glands of hair follicles (Fig. 4g). In peripheral nerve, MCP was highly expressed on small blood vessels in the perineurium, whereas fibroblasts and Schwann cells in the endoneurium were not stained (Fig. 4h). Tissues of the central nervous system also displayed a remarkable staining pattern. The grey matter in all areas of the brain stained strongly for MCP, while the white matter stained weakly or not at all (not shown).
The testis was strongly stained for MCP, particularly the Leydig cells and surrounding connective tissue; the germinal epithelium surrounding the seminiferous tubules was also strongly positive (Fig. 4i). No spermatozoa were visible in the immature testes used in this study. The ovary was stained strongly in granulosa cells from follicles and in endothelium and reticular epithelium, but MCP was only weakly expressed on the immature oocytes (data not shown). Uterine epithelium was strongly stained, but the myometrium was negative. In the endometrium, the epithelium of the uterine glands stained strongly but the surrounding endometrial tissue was not stained (Fig. 4j). In the spleen, MCP was abundant in the red pulp and in the T-cell areas from the white nodules, but in the white pulp, B-cell areas of the germinal centres were negative (not shown). In the periarteriolar lymphoid sheaths, MCP was abundant in the T-cell areas surrounding the arteriole, but was weak or absent in the adjacent B-cell areas (Fig. 4k). MCP was also absent from the abundant splenic supporting tissue (not shown).
In lymph nodes, the staining pattern in lymphoid follicles was similar to that in the white pulp of the spleen. The core of the germinal centres, corresponding to the T-cell areas were positive for MCP, whereas the B-cell areas from the germinal centre were negative (Fig. 4l).
The expression pattern of pig MCP is summarized in Table 1. Similar expression patterns were found in five different pigs, all of the common English White strain.
Table 1.
Distribution of MCP in normal pig tissues
DISCUSSION
In humans, MCP is a major component in the control of C activation on self-cells.1 No individuals deficient in MCP have been described, perhaps illustrating the importance of MCP. Analogues of MCP have so far been described only in primates, pigs, guinea-pigs, mice;15,13,16 and rats (our unpublished data). Preliminary evidence from mRNA studies indicates that each of the rodent analogues are preferentially expressed on testicular cells, suggesting a specific role in reproduction. However, the distribution of MCP in species other than man has not been thoroughly examined. Here we provide a detailed analysis of the distribution of MCP in the pig. The distribution of MCP in pig blood cells and tissues was, in most respects, very similar to that of human MCP with just a few notable exceptions.
Pig MCP was broadly expressed on all circulating cells, and was particularly abundant on erythrocytes, in contrast to the situation in humans where erythrocytes are MCP-negative (Fig. 1). Although human and guinea-pig erythrocytes do not express MCP, in the orangutan MCP is expressed on erythrocytes;17,16 These species differences raise interesting questions about how erythrocytes are protected from lysis and the role of MCP in immune complex binding and processing by erythrocytes in different species. In humans, immune complexes are transported on erythrocytes bound to CR1,18 whereas in the pig, there is little or no binding of immune complexes to erythrocytes and the lung is the major site of immune complex elimination.19 Expression of MCP might interfere with erythrocyte immune complex handling by catalysing cleavage of C3b, thereby interfering with the retention of immune complexes on MCP-positive erythrocytes.
Western blotting of pig blood cells, cell lines and cells harvested from solid organs demonstrated the broad expression of MCP and revealed the presence of multiple isoforms. Although in all cases bands in the MW range 55–65 000 were detected, the precise banding pattern was characteristic of the particular cell or tissue. The two-band pattern (55 000 MW and 65 000 MW) in erythrocytes closely resembled that in humans where MCP is characterized by a two-band pattern (55 000 MW and 63 000 MW); the presence of a unique 75 000 MW band in granulocytes also mirrored the pattern in human granulocytes;5,20 The different isoforms of human MCP have been shown to be generated by alternative splicing in the STP region and cytoplasmic tail and display tissue-specific expression.21 The multiple isoforms of pig MCP revealed here are likely to be generated by a similar process of alternative splicing, although this has yet to be confirmed at the mRNA level. Densitometric scanning of Western blots in which sample loading had been equalized for total protein illustrated the abundance of MCP on pig erythrocytes and the low expression in brain and spleen (Fig. 3).
Immunohistology using a well-characterized anti-pig MCP monoclonal antibody12,22 confirmed that MCP was abundantly distributed in pig tissues, particularly on cells of epithelial and endothelial origin (Fig. 4; Table 1). The vascular endothelium in all organs examined was strongly positive for pig MCP. As in humans, staining of MCP was weak in skeletal and cardiac muscle.23 In the kidney, pig MCP was expressed in the juxtaglomerular apparatus, the glomerular capillaries, epithelial cells and mesangial cells and on proximal tubules, a very similar expression pattern to MCP in human kidney.24,25 The abundant staining in liver also closely resembled the pattern of MCP in human liver.26 In lymphoid organs (spleen and lymph nodes) pig MCP was abundantly expressed in most areas but was weak or absent in B cell areas in these organs. Circulating leucocytes were uniformly positive for pig MCP, indicating that circulating B cells do express the protein. The data suggest that immature B cells in the lymphoid organs express little or no MCP, whereas mature B cells in the periphery express abundantly. Human MCP is broadly expressed in cells of fibroblast origin,27 in particular, MCP is present in cultured keratinocytes28 and in all cells in the skin.29 In contrast, pig MCP was absent from skin epidermis and dermis, including smooth muscle cells in the dermis. Expression of human MCP has been reported in brain and in cultured astrocytes.30 In pig, grey matter stained strongly but the identity of the cells expressing was not ascertained. No gross difference in tissue expression, assessed by immunohistochemistry, was seen between at least five pigs of the English White strain used in the study.
An understanding of the regulation of C on pig tissues is particularly important in the context of recent strategies for xenotransplantation.31 Transgenic pigs expressing human C inhibitors on their tissues are being bred for use as organ donors in the expectation that their organs will be less susceptible to hyperacute rejection in the human recipient.32 Our data demonstrate that pigs express MCP abundantly and in a similar pattern to MCP in humans, notably on vascular endothelium. Recent data indicate that pig MCP is at least as efficient as human MCP as cofactor for human factor I cleavage of human C3b.33 Pig cells and tissues are thus intrinsically protected from human C at the C3-convertase stage. Pigs also express CD59 abundantly on endothelium34 and pig CD59 inhibits lysis by human C.11,35 No information has yet been published on the expression or function of pig decay-accelerating factor (DAF). The enhanced resistance to human serum of pig organs expressing human C regulators is thus likely to be caused by the increased availability of regulators; our data indicate that increased expression of porcine C regulators will achieve a similar outcome. We have not yet examined whether differences in expression of MCP exist between different strains of pig. Identification of strains which express MCP and other C regulators at particularly high levels on endothelium would aid the identification of the ideal donor for xenotransplantation studies.
Human MCP and DAF have both been implicated as receptors for pathogens;9,6,37 It has been suggested that transgenic pig organs hyperexpressing human C regulators present a particular risk of cross-species infection for viruses which utilize human C regulators either as receptors or as resistance factors.38 It is possible that overexpression of the endogenous regulators on pig organs will circumvent this risk.
Acknowledgments
JMPL is a recipient of a ‘Marie Curie’-TMR fellowship, contract ERB-FMBICT-972590. BPM is a Wellcome Trust Senior Fellow. We thank Prof. G. T. Williams for help with the histology analysis.
References
- 1.Seya T, Turner JR, Atkinson JP. Purification and characterization of a membrane protein (gp45) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986;163:837. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hourcade D, Holers VM, Atkinson JP. The regulators of complement activation (RCA) gene cluster. Adv Immunol. 1989;45:381. doi: 10.1016/s0065-2776(08)60697-5. [DOI] [PubMed] [Google Scholar]
- 3.Manchester M, Valsamakis A, Kaufman R, et al. Measles virus and C3 binding sites are distinct on membrane cofactor protein (CD46) Proc Natl Acad Sci USA. 1995;92:2303. doi: 10.1073/pnas.92.6.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liszewski MK, Post TW, Atkinson JP. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Ann Rev Immunol. 1991;9:431. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 5.Seya T, Ballard LL, Bora NS, Kumar V, Cui W, Atkinson JP. Distribution of membrane cofactor protein of complement on human peripheral blood cells. An altered form is found on granulocytes. Eur J Immunol. 1988;18:1289. doi: 10.1002/eji.1830180821. [DOI] [PubMed] [Google Scholar]
- 6.Cervoni F, Oglesby TJ, Adams EM, et al. Identification and characterization of membrane cofactor protein of human spermatozoa. J Immunol. 1992;148:1431. [PubMed] [Google Scholar]
- 7.Seya T, Hara T, Matsumoto M, Akedo H. Quantitative analysis of membrane cofactor protein (MCP) of complement. High expression of MCP on human leukemia cell lines, which is down-regulated during cell differentiation. J Immunol. 1990;145:238. [PubMed] [Google Scholar]
- 8.Naniche D, Varior-Krishnan G, Cervoni F, et al. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada N, Liszewski MK, Atkinson JP, Caparon M. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A streptococcus. Proc Natl Acad Sci USA. 1995;92:2489. doi: 10.1073/pnas.92.7.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCurry KR, Kooyman DL, Alvarado CG, et al. Human complement regulatory proteins protect swine-to-primate cardiac xenografts from humoral injury. Nature Med. 1995;1:423. doi: 10.1038/nm0595-423. [DOI] [PubMed] [Google Scholar]
- 11.van den Berg CW, Harrison RA, Morgan BP. A rapid method for the isolation of analogues of human CD59 by preparative SDS–PAGE: application to pig CD59. J Immunol Methods. 1995;179:223. doi: 10.1016/0022-1759(94)00288-8. [DOI] [PubMed] [Google Scholar]
- 12.van den Berg CW, Perez de la Lastra JM, Llanes D, Morgan BP. Purification and characterization of the pig analogue of human membrane cofactor protein (CD46/MCP) J Immunol. 1997;158:1703. [PubMed] [Google Scholar]
- 13.Toyomura K, Fujimura T, Murakami H, et al. Molecular cloning of a pig homologue of membrane cofactor protein (CD46) Int Immunol. 1997;9:869. doi: 10.1093/intimm/9.6.869. [DOI] [PubMed] [Google Scholar]
- 14.Cattan P, Zhang B, Braet F, et al. Comparison between aortic and sinusoidal liver endothelial cells as targets of hyperacute xenogeneic rejection in the pig to human combination. Transplantation. 1996;62:803. doi: 10.1097/00007890-199609270-00018. [DOI] [PubMed] [Google Scholar]
- 15.Murakami Y, Seya T, Kurita M, Nagasawa S. Molecular cloning of a complementary DNA for a membrane cofactor protein (MCP, CD46)/measles virus receptor on Vero cells and its functional characterization. Biol Pharm Bull. 1996;19:1541. doi: 10.1248/bpb.19.1541. [DOI] [PubMed] [Google Scholar]
- 16.Hosokawa M, Nonaka M, Okada N, Nonaka M, Okada H. Molecular cloning of guinea pig membrane cofactor protein: preferential expression in testis. J Immunol. 1996;157:4946. [PubMed] [Google Scholar]
- 17.Nickells MW, Atkinson JP. Characterization of CR1- and membrane cofactor protein-like proteins of two primates. J Immunol. 1990;144:4262. [PubMed] [Google Scholar]
- 18.Pascual M, Schifferli JA. The binding of immune complexes by the erythrocyte complement receptor 1 (CR1) Immunopharmacology. 1992;24:101. doi: 10.1016/0162-3109(92)90016-6. [DOI] [PubMed] [Google Scholar]
- 19.Davies KA, Chapman PT, Norsworthy PJ, et al. Clearance pathways of soluble immune complexes in the pig: insights into the adapatative nature of antigen clearance in humans. J Immunol. 1995;155:5760. [PubMed] [Google Scholar]
- 20.Matsumoto M, Seya T, Nagasawa S. Polymorphism and proteolytic fragments of granulocyte membrane cofactor protein (MCP, CD46) of complement. Biochem J. 1992;281:493. doi: 10.1042/bj2810493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnstone RW, Russell SM, Loveland BE, McKenzie IF. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Mol Immunol. 1993;30:1231. doi: 10.1016/0161-5890(93)90038-d. [DOI] [PubMed] [Google Scholar]
- 22.Perez de la Lastra JM, van den Berg CW, Bullido Domínguez J, Almazán F, Llanes D, Morgan BP. Epitope mapping of 10 monoclonal antibodies against the pig analogue of human membrane cofactor protein (MCP) Immunology. 1999;96:663. doi: 10.1046/j.1365-2567.1999.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnstone RW, Loveland BE, McKenzie IF. Identification and quantification of complement regulator CD46 on normal human tissues. Immunology. 1993;79:341. [PMC free article] [PubMed] [Google Scholar]
- 24.Nakanishi I, Moutabarrik A, Hara T, et al. Identification and characterization of membrane cofactor protein (CD46) in the human kidneys. Eur J Immunol. 1994;24:1529. doi: 10.1002/eji.1830240711. [DOI] [PubMed] [Google Scholar]
- 25.Ichida S, Yuzawa Y, Okada H, Yoshioka K, Matsuo S. Localization of the complement regulatory proteins in the normal human kidney. Kidney Int. 1994;46:89. doi: 10.1038/ki.1994.247. [DOI] [PubMed] [Google Scholar]
- 26.Scoazec JY, Delautier D, Moreau A, et al. Expression of complement-regulatory proteins in normal and UW-preserved human liver [see comments] Gastroenterology. 1994;107:505. doi: 10.1016/0016-5085(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 27.McNearney T, Ballard L, Seya T, Atkinson JP. Membrane cofactor protein of complement is present on human fibroblast, epithelial, and endothelial cells. J Clin Invest. 1989;84:538. doi: 10.1172/JCI114196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayama K, Shiraishi S, Shirakata Y, Kobayashi Y, Seya T, Miki Y. Expression and characterization of membrane co-factor protein (MCP) in human skin. J Invest Dermatol. 1991;97:722. doi: 10.1111/1523-1747.ep12484155. [DOI] [PubMed] [Google Scholar]
- 29.Sayama K, Shiraishi S, Miki Y. Distribution of complement regulators (CD46, CD55 and CD59) in skin appendages, and in benign and malignant skin neoplasms. Br J Dermatol. 1992;127:1. doi: 10.1111/j.1365-2133.1992.tb14814.x. [DOI] [PubMed] [Google Scholar]
- 30.Gordon DL, Sadlon TA, Wesselingh SL, Russell SM, Johnstone RW, Purcell DF. Human astrocytes express membrane cofactor protein (CD46), a regulator of complement activation. J Neuroimmunol. 1992;36:199. doi: 10.1016/0165-5728(92)90051-l. [DOI] [PubMed] [Google Scholar]
- 31.White D. Alteration of complement activity: a strategy for xenotransplantation. Trends Biotechnol. 1996;14:3. doi: 10.1016/0167-7799(96)80906-1. [DOI] [PubMed] [Google Scholar]
- 32.McCurry KR, Diamond LE, Kooyman DL, et al. Human complement regulatory proteins expressed in transgenic swine protect swine xenografts from humoral injury. Transpl Proc. 1996;28:758. [PubMed] [Google Scholar]
- 33.Van den Berg CW, Loveland BE, Morgan BP. Pig MCP is a better cofactor for human factor I in the degradation of human C3 than human MCP. Mol Immunol. 1998;35:380. [Google Scholar]
- 34.Hanna SM, Williams GT, Van den Berg CW, Morgan BP. Characterisation in vitro and in vivo of the pig analog of human CD59 using new monoclonal antibodies. Immunology. 1998;95:450. doi: 10.1046/j.1365-2567.1998.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Berg CW, Morgan BP. Complement-inhibiting activities of human CD59 and analogues from rat, sheep, and pig are not homologously restricted. J Immunol. 1994;152:4095. [PubMed] [Google Scholar]
- 36.Ward T, Pipkin PA, Clarkson NA, Stone DM, Minor PD, Almond JW. Decay-accelerating factor CD55 is identified as the receptor for echovirus 7 using CELICS, a rapid immuno-focal cloning method. EMBO J. 1994;13:5070. doi: 10.1002/j.1460-2075.1994.tb06836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergelson JM, Mohanty JG, Crowell RL, St. John NF, Lublin DM, Finberg RW. Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55) J Virol. 1995;69:1903. doi: 10.1128/jvi.69.3.1903-1906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss R. Transgenic pigs and virus adapatation. Nature. 1998;391:327. doi: 10.1038/34772. [DOI] [PubMed] [Google Scholar]