Abstract
We have examined the role of endogenously produced interleukin-4 (IL-4) in the contact hypersensitivity (CH) reaction to the haptene trinitrochlorobenzene (TNCB). The CH reaction was abolished in IL-4 genetically deficient mice (IL-4 KO), when compared to wild-type (wt) mice. The CH reaction was restored by treatment with IL-4 and further analysis revealed that IL-4 exerted its action both at the induction and effector stages of the CH reaction. Despite failure to develop a CH reaction, IL-4 KO mice developed a T helper type 1 (Th1) response to TNCB, in terms of lymphokine production in vitro. Furthermore, the number of Vγ3+ cells accumulating in the lymph nodes of TNCB-immune IL-4 KO mice was normal. The recruitment of mononuclear cells and vascular leakage at the challenge site were consistently reduced in IL-4 KO mice and were restored by injection of IL-4. This suggests that IL-4 acts as a proinflammatory mediator in CH, perhaps favouring the accumulation of mononuclear cells at the site of inflammation. Among Th2-type cytokines, IL-13, but not IL-10, was shown to restore the CH reaction to TNCB in IL-4 KO mice. However, IL-4 KO mice developed a normal CH response to oxazolone, indicating that IL-4 was required for the CH reaction to TNCB, but not for that to oxazolone.
INTRODUCTION
The contact hypersensitivity (CH) reaction represents one of the most widely studied in vivo examples of cell-mediated immune responses, which follows epicutaneous application of chemically reactive haptenes.1 During the induction or sensitization phase of the CH reaction, Langherhans’ cells migrate from the sensitized area of the skin to draining lymph nodes and present haptene–major histocompatibility complex (MHC) complexes to T lymphocytes, resulting in activation of the latter. The elicitation of the CH reaction occurs principally as a result of the passage of haptene-specific T lymphocytes to the site of antigen deposition (challenge), with the subsequent production of proinflammatory cytokines. This induces a variety of bystander cells, most notably macrophages, to be recruited to the antigen challenge site, where they give rise to the characteristic lesions perhaps through the production of tumour necrosis factor-α (TNF-α).2 Although the CH reaction has been used in many laboratories as a relatively simple experimental model with which to examine T-lymphocyte response to haptenes, studies to define the role of CD4+ and CD8+ T lymphocytes in CH have provided conflicting results. Several groups have demonstrated that CH is mediated by CD4+ T lymphocytes,3,4 leading to the idea that CH is equivalent to the classical delayed-type hypersensitivity (DTH) reaction, which is primarily mediated by interferon-γ (IFN-γ)-producing T helper type 1 (Th1) CD4+ T lymphocytes. However, treatment with neutralizing antibodies to IFN-γ has been shown to cause modest inhibition or enhancement of the CH reaction.5–7 Studies by Gocinski and Tigelaar8 showed that both CD4+ and CD8+ T lymphocytes mediate CH to dinitrofluorobenzene (DNFB) and data from two laboratories9–11 have demonstrated the requirement for αβ+ and γδ+ T lymphocytes in the CH reaction to trinitrochlorobenzene (TNCB). Xu and coworkers12 have recently provided evidence that CH reactions to DNFB and oxazolone (OX) are mediated by IFN-γ-producing CD8+ T lymphocytes of the Tc1 type. In contrast, interleukin-4- (IL-4) and IL-10-producing CD4+12 or CD8+13 T lymphocytes inhibit CH to DNFB. Gautam and coworkers14 showed that high doses of IL-4 partially reduced the elicitation phase of CH but had no influence when given at the induction phase. Furthermore, anti-IL-4 mAb given at the time of challenge increased the ear swelling response. Based on indirect evidence, the authors suggested that IL-4 inhibited the production of proinflammatory cytokines by macrophages.
However, these results pointing to a down-regulatory role of IL-4 in the CH reaction are in contrast with three recent observations. Berg and coworkers15 showed a reduced ear swelling response to OX in IL-4 gene-targeted (IL-4 knock-out; IL-4 KO) mice, although the difference with the response in wild-type (wt) mice was not statistically significant. Weigmann and coworkers16 showed significant reduction of the CH response to DNFB in IL-4 KO mice and we have reported that IL-4 is a critical cytokine during the elicitation phase of the CH reaction to TNCB.17,18 All together, the above results point to an important role of IL-4 in the CH reaction to different haptenes.
The availability of IL-4 KO mice generated by Kuhn et al.19 by gene targeting in embryonic stem cells provides a useful tool to explore the role of IL-4 and other Th2-type cytokines in the CH reaction to TNCB. In fact, these mice fail to produce typical Th2 cytokines and consequently do not mount immune responses dependent on these cytokines.20 Therefore, we decided to study further the CH reaction to TNCB in IL-4 KO mice.
MATERIALS AND METHODS
Mice
Male IL-4 KO mice bred onto C57BL/6 background were obtained from B & K Universal (Essex, UK) through the courtesy of Drs K. Rajewsky and W. Muller (Institute for Genetics, University of Cologne, Cologne, Germany). Wild-type (wt) littermate C57BL/6 mice were obtained from Nossan (Correzzana, Italy). They were maintained in our animal facility at the Institute of General Pathology. Eight- to twelve-week-old mice were used in each experiment and each experimental group consisted of five to eight mice.
Cytokines
Murine recombinant (r) IL-2 (3·6×106 U/mg), rIL-5 (1·5×107 U/mg), rIL-10 (107 U/mg) and rIFN-γ (106 U/mg) were obtained from Genzyme (Cambridge, MA). Murine rIL-4 (107 U/mg) was obtained from R&D Systems (Minneapolis, MN). Human rIL-13 (107 U/mg) was the generous gift of Dr A. Minty (Molecular Biology Department, Sanofi Recherche, Labege, France).
Contact hypersensitivity
Mice were immunized by application of 0·15 ml 5% TNCB (2,4,6-trinitrochlorobenzene, BDH, Poole, UK) or 3% OX (4-ethoxymethylene-2-phenyloxazolone, BDH) in acetone:ethanol (1:9) to the clipped thorax, abdomen and forepaws. Five days later mice were challenged by applying 10 μl of 1% TNCB or OX in olive oil to both sides of both ears.
Ear swelling was assessed 24, 48 and 72 hr later, using an engineer’s micrometer and was expressed in units of 10−3 cm±standard deviation (SD). Where indicated, recombinant murine IL-4 was injected intravenously (i.v.) at the time of immunization or challenge. In experiments reported in Fig. 5, mice were injected with 10 ng of each recombinant cytokine both at the time of immunization and challenge.
Figure 5.
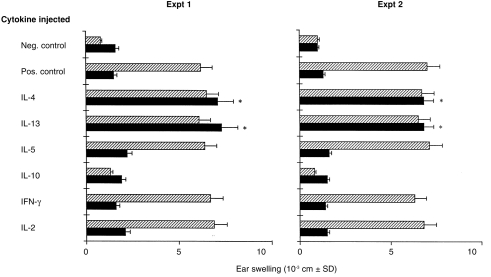
Both IL-4 and IL-13 restore CH in IL-4 KO mice. IL-4 KO (closed columns) and wt (hatched columns) mice were immunized with TNCB and challenged 5 days later. Mice were injected i.v. with 10 ng of several different recombinant cytokines both at the time of immunization and at the time of challenge. The increase in ear thickness was measured 24 hr after challenge. Positive control refers to mice sensitized with TNCB and challenged with TNCB, while negative control refers to mice which were not sensitized with TNCB, but were only challenged with TNCB. *P < 0·001 when compared to IL-4 KO mice sensitized and challenged with TNCB but not injected with IL-4.
Antigen-specific proliferation assay
Mice were immunized with TNCB as described above. Four days later draining lymph node cells were harvested and suspended in medium RPMI-1640 (Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS, Life Technologies), streptomycin, penicillin, glutamine and 2-mercaptoethanol (2×10−5 m). Then 2×105 cells in 100 μl were incubated in flat-bottomed 96-well microtitre plates with irradiated (3000 rads from a caesium source) TNCB-modified spleen cells (4×105 in 100 μl) as antigen-presenting cells (TNCB-APC), prepared as described previously21 and cultured at 37° in the presence of 5% CO2. The wells were pulsed with 1 μCi [3H]thymidine ([3H]Tdr-Amersham, Buckinghamshire, UK) on day 3 of culture and were harvested 18 hr later. Radioactivity was determined using a multichannel cell harvester (Skatron, Lier, Norway). Results are expressed as mean counts per minute (c.p.m.) of triplicate wells±SD. The SD of the means were usually less than 10%.
Production and measurement of cytokines
Four-day TNCB-immune lymph node cells (107/ml) were cultured in 24-well Costar plates with or without an equal volume of irradiated TNCB-APC (107/ml) for 24 hr at 37° in 5% CO2·22 The supernatants were collected, filtered and tested for IL-2, IL-4, IL-10, IFN-γ and TNF-α activities. IL-2 was measured by its ability to support growth of cytotoxic lymphocytes (CTLL). Briefly, CTLL cells (5×104/50 μl) were added to 50 μl of serial dilutions of supernatants in flat-bottomed microwell plates and incubated for 24 hr at 37°. MTT [20 ml of 3-(4,5-dimethylthyazol-2-γl)-2,5-diphenyl-tetrazolium bromide, 5 mg/ml)] was added and the reaction was stopped after 4 hr with 100 μl 10% sodium dodecyl sulphate (SDS). After overnight incubation, the absorbance (570–630 nm) was measured with an ELISA reader. Because IL-4 also allows survival of CTLL cells, supernatants were absorbed twice in microtray wells coated with the anti-IL-4 mAb 11B11 (a kind gift of Dr W. E. Paul, National Institute of Allergy and Infectious Diseases, Bethesda, MD) at the final concentration of 20 μg/ml (100 μl/well). This absorption procedure reduced the rIL-4 activity of 100 U/ml to < 5 U/ml. IL-2 activity was expressed in U/ml, i.e. the reciprocal of dilution giving 50% maximal response. Detection limit for the IL-2 assay was 0·1 U/ml. IL-4, IL-10, IFN-γ and TNF-α were measured by a two-monoclonal antibody (mAb) sandwich capture enzyme-linked immunosorbent assay (ELISA) technique, using commercially available mAbs (Pharmingen, San Diego, CA), according to the manufacturers’ recommendations. Detection limits were 15 pg/ml for IFN-γ, IL-4 and IL-10, and 3 pg/ml for TNF-α.
TNCB-specific precursor frequency analysis
For limiting dilution (LD) analysis we used lymph node cells from IL-4 KO and wt mice that had been immunized with TNCB 4 days before. Cells were plated at 1×104 cells/well to 1 cell/well in 100 μl into round-bottomed microtitre plates in groups of 24 wells for each cell concentration. TNCB-modified, irradiated syngeneic spleen cells (4×105/100 μl) and 20 U/ml (final concentration) of murine rIL-2 (R&D Systems) were added to each well and the cultures were incubated for 10 days at 37° with 5% CO2 in air. Every three days, plates were centrifuged and 100 μl of culture medium was removed from each well and substituited with fresh medium containing rIL-2. On day 10, 0·5 μCi [3H]thymidine was added to each well and the next day the cultures were harvested. Wells were scored positive if the mean c.p.m. of cultures in the presence of antigen was higher than the mean c.p.m. of cultures without antigen plus three standard deviations. Estimation of the frequency of antigen responder cells was performed by applying the Poisson Formula:23
| (1) |
where Fr is the probability of obtaining r-specific responder cells in a well when the average number of responder cells per well is u at a given concentration. The fraction of negative wells per total number of wells is given by F0=e−u; when u = 1, F0 = 0·37. Therefore, theoretically, when the average number of precursor cells per well is one, 37% of the wells will be scored as negative. Extrapolation to this point in limiting dilution gives a number of cells, the reciprocal of which represents the frequency of the antigen-specific responder cells.
Measurement of mononuclear cell infiltration and edema formation at the antigen challenge site
To estimate cellular infiltrate at the antigen challenge site we used the iododeoxyuridine (IUdR) uptake method.24 Briefly, mice were sensitized with TNCB and 5 days later were challenged with TNCB on the left ear and vehicle on right ear. Eight hours after the challenge, 2 mCi [125I]IUdR (5·65 mCi/mg, Amersham, Bucks) in 0·5 ml phosphate-buffered saline (PBS) were injected into the tail vein. Alternatively, the mice received 0·2 mCi [125I]bovine serum albumin ([125I]BSA, 1·59 mCi/mg, ICN Biomedicals Inc., Costa Mesa, CA) in 0·5 ml PBS, 16 hr after the challenge.25 Mice were killed 24 hr after challenge, ears were cut off at the hairline and counted in a γ-counter. These time-points were chosen to reflect specific recruiment of inflammatory cells and edema formation in response to contact allergen. Results are expressed as mean c.p.m.±SD of eight ears per group.
Statistical analysis
Significant differences between experimental groups were evaluated by double-tailed Student’s t-test.
RESULTS
IL-4 KO mice fail to develop CH to TNCB
In order to test the role of IL-4 in the CH response, we examined responsiveness in wt and IL-4 KO mice after epicutaneous sensitization with TNCB. Figure 1(a) shows that sensitization of wt mice with TNCB induces antigen-specific swelling that peaked at 24 hr after challenge with TNCB into the ears. Ear swelling response was still evident at 48 hr after challenge and thereafter declined at 72 hr after challenge. In contrast, IL-4 KO mice showed virtually no ear swelling following sensitization and challenge with TNCB (Fig. 1a). Furthermore, as shown in Fig. 1(b) IL-4 KO mice failed to develop CH even if they were immunized with TNCB doses ranging from 0·1% to 10%. Identical results were obtained in eight different experiments. Together, these data demonstrate a significant defect in the ability of IL-4 KO mice to manifest a CH reaction to TNCB.
Figure 1.
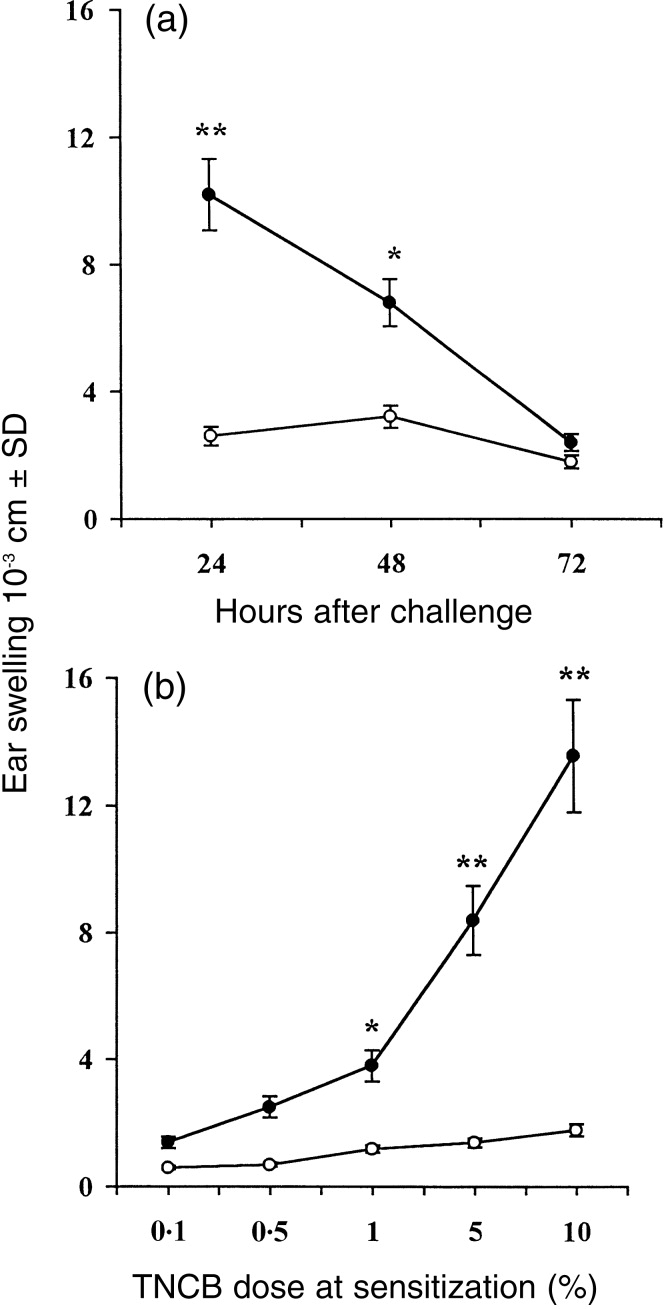
CH response in IL-4 KO and wt mice. In (a), IL-4 KO (○) and wt (•) mice were sensitized with TNCB and challenged 5 days later. The increases in ear thickness were measured 24, 48 and 72 hr after challenge. In (b), IL-4 KO (○) and wt (•) mice were sensitized with different TNCB concentrations and were challenged with a single dose of TNCB. The increase in ear thickness was measured 24 hr after challenge. Data presented are the mean values±SD of eight mice per data point. Mice that were not sensitized, but only challenged with TNCB (challenge only), gave the following results: IL-4 KO 24 hr, 2·1±0·4; 48 hr, 2·5±0·3; 72 hr, 1·7±0·6; and wt 24 hr, 2·0±1·1; 48 hr 1·9±0·5; 72 hr, 1·5±0·2. *P < 0·01 and **P < 0·001 as compared to the values detected in IL-4 KO mice.
To investigate at which stage of the CH reaction IL-4 exerts its action, we injected murine recombinant IL-4 intravenously immediately at sensitization or before challenge with TNCB. Figure 2 shows that injection of IL-4 resulted in a dose-dependent restoration of the CH response to TNCB. Note from a comparison of the upper and lower panels of Fig. 2 that IL-4 given to mice at the time of challenge with TNCB caused an increase in ear swelling response that was greater than the increase caused by IL-4 given at the time of sensitization. Injection of IL-4 in wt mice caused a modest increase in ear swelling response (Fig. 2). This apparent failure of IL-4 to influence CH in wt mice is likely to be due to the fact that this reaction is already maximal. In fact, when wt mice were painted with 1% TNCB (instead of 5%), injection of IL-4 increased the ear swelling response significantly (4·8±0·9 in untreated mice versus 11·2±2·2 in IL-4-treated mice, P < 0·001). In no case did intravenous or local injection of IL-4 alone cause swelling in either wt or IL-4 KO mice (data not shown). Similar results were obtained in five different experiments
Figure 2.
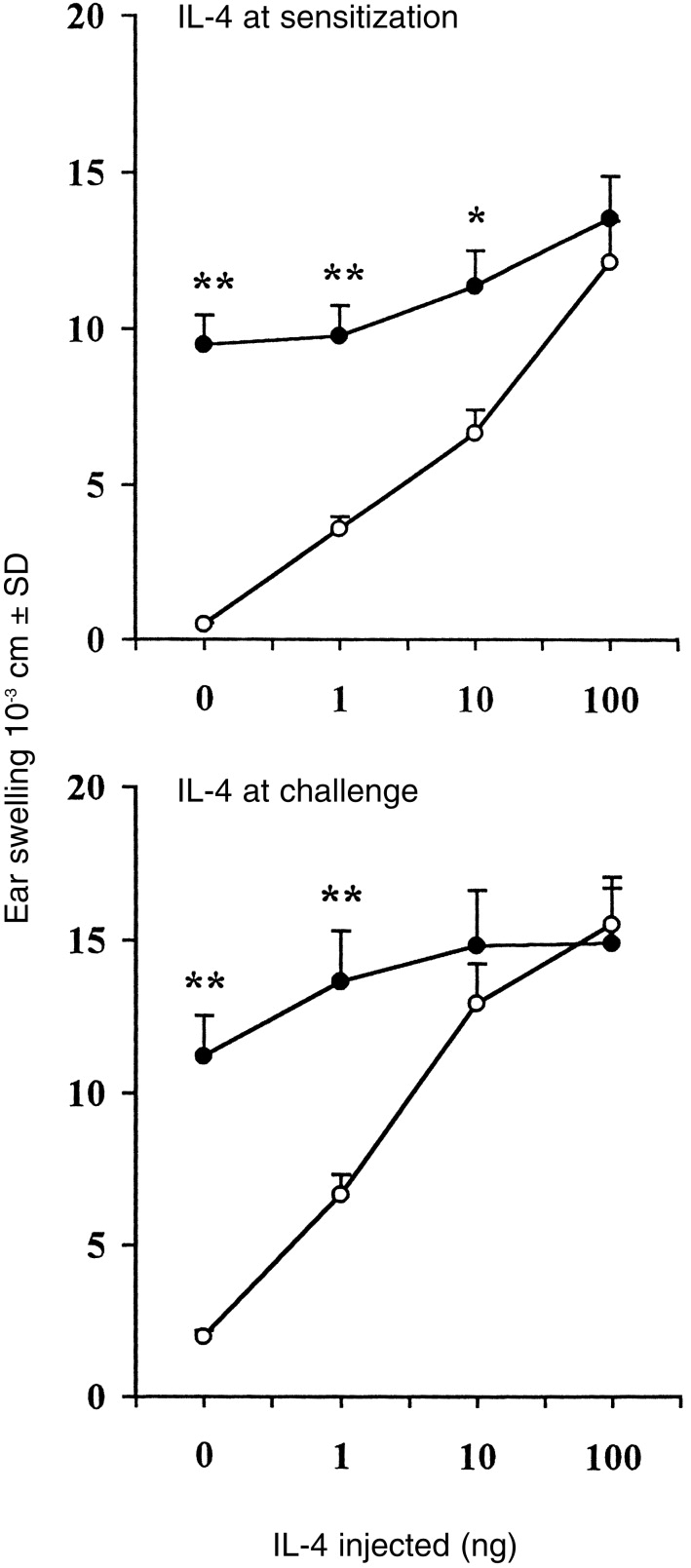
CH response in IL-4 KO mice can be restored by IL-4. IL-4 KO (○) and wt (•) mice were sensitized with TNCB and challenged 5 days later. Some groups of mice also received different amounts of IL-4 immediately before sensitization (upper panel) or immediately before challenge (lower panel). The increases in ear thickness were measured 24 hr after challenge. Mice that were not sensitized, but only challenged with TNCB (challenge only), gave the following results: IL-4 KO, 1·1±0·2; wt, 1·0±0·1. *P < 0·01 and **P < 0·001 as compared to the values detected in IL-4 KO mice.
Despite failure in developing CH to TNCB IL-4 KO mice contain a high TNCB-specific T-cell precursor frequency and produce Th1 cytokines
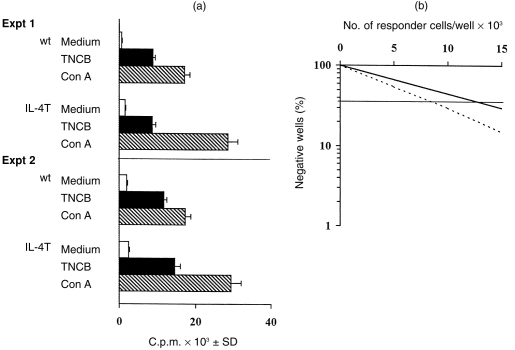
Cells were isolated from the draining lymph nodes of TNCB-immune mice and were stimulated in vitro with TNCB-APC and antigen-specific proliferation was assessed. Lymph node cells from both IL-4 KO and wt immune mice proliferated equally to TNCB-APC in vitro (Fig. 3a). These data are in agreement with limiting dilution analysis of cells responding to TNCB, showing that the TNCB precursor frequency was similar in IL-4 KO and wt mice (10·8±2·9/105 cells in IL-4 KO mice versus 7·6±1·8/105 cells in wt mice, see Fig. 3b). As a control, both mice proliferated equally well to concanavalin A (Con A), although responses of IL-4 KO mice were more vigorous than responses of wt mice. Similar results were obtained in three different experiments.
Figure 3.
TNCB-specific proliferative response and precursor frequency analysis in IL-4 KO mice. IL-4 KO and wt mice were immunized with TNCB and the draining lymph nodes were harvested 4 days later. In (a), TNCB-immune lymph node cells were re-exposed to medium, TNCB-hapteneized APC or Con A in vitro and proliferation was measured 72 hr later. In (b), the frequency of TNCB-specific precurors in IL-4 KO (—) and wt (—) mice was estimated as described under the Materials and Methods.
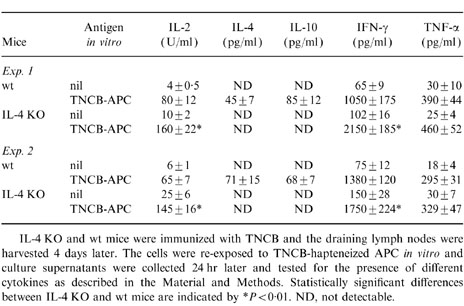
In the next set of experiments, lymph node cells from TNCB-immune mice were stimulated in vitro with TNCB-hapteneized spleen cells as APC and antigen-induced cytokine production was assessed. Table 1 (Exp. 1 and Exp. 2) shows that TNCB-immune lymph node cells from wt mice produced IL-2, IFN-γ and TNF-α, and low amounts of IL-4 and IL-10, upon in vitro stimulation with TNCB-APC, thus confirming previous results from our laboratory.22 TNCB-immune lymph node cells from IL-4 KO mice did not produce IL-4 and IL-10 upon in vitro stimulation with TNCB-APC, thus confirming the finding that these mice fail to produce typical Th2 cytokines. However, TNCB-immune lymph node cells from IL-4 KO mice were fully able to produce the Th1 cytokines IL-2 and IFN-γ, as well as TNF-α, and further analysis shows that both IL-2 and IFN-γ levels were higher than those produced by wt mice (see Table 1). These results were confirmed in four more experiments. We conclude that despite failure to develop a CH reaction to TNCB, IL-4 KO mice are fully able to mount a Th1-type cytokine response to TNCB.
Table 1.
Cytokine production by TNCB-immune lymph node cells from IL-4 KO and wt mice
Infiltration of radiolabelled mononuclear cells and vascular permeability in IL-4 KO mice
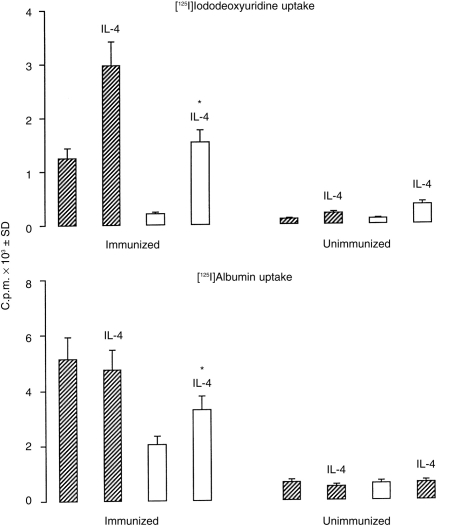
Monocytes and lymphocytes were labelled with [125I]IUdR 8 hr after challenge and infiltration of labelled cells at the site of the CH reaction was determined 24 hr after the challenge by subtracting the counts in non-challenged ears. The IL-4 KO mice had 80% lower infiltration of radiolabelled mononuclear cells than wt mice (Fig. 4, top panel). Mice challenged with contact sensitizers without prior sensitization showed much lower ( < 10%) infiltration than did the sensitized animals (Fig. 4), and there was no statistical difference between wt and IL-4 KO mice. Injection of IL-4 significantly increased infiltration of radiolabelled mononuclear cells in both wt and IL-4 KO mice. Vascular leak in CH can be demonstrated by the local leakage of systemically injected radiolabelled albumin into the tissues.25 The leakage of radiolabelled albumin in IL-4 KO mice was 50% lower that in wt mice (Fig. 4, lower panel) and this difference was statistically significant. Injection of IL-4 increased leakage of radiolabelled albumin in IL-4 KO mice but had no effect in wt mice (Fig. 4). Similar results were obtained in three experiments. These results demonstrate impairment of infiltration of inflammatory cells and edema formation in IL-4 KO mice, during the CH reaction to TNCB.
Figure 4.
Infiltration of radiolabelled mononuclear cells and leakage of radiolabelled albumin in IL-4 KO mice. TNCB-sensitized (immunized) or not sensitized (unimmunized) wt (hatched columns) and IL-4 KO (open columns) mice were challenged 5 days later with TNCB on their left ears. [125I]IUdr was injected 8 hr later to label dividing mononuclear cells (top panel), while [125I]albumin was injected 16 hr later (lower panel). Where indicated (i.e. IL-4) mice were injected i.v. with 10 ng IL-4 immediately before challenge. Ears were cut off 24 hr after challenge (see the Materials and Methods) and radioactivity incorporation was determined. Counts of the non-challenged right ears were subtracted from those of the challenged ears. *P < 0·0001 when compared to IL-4 KO mice not injected with IL-4.
Both IL-4 and IL-13 restore the CH reaction to TNCB in IL-4 KO mice
In the following experiments we investigated whether Th2-type cytokines, other than IL-4, were also involved in the CH reaction to TNCB. IL-4 KO and wt mice were injected with different cytokines both at the sensitization and at the challenge phase and CH to TNCB was assessed. Figure 5 (Exp. 1 and Exp. 2) shows that amongst the Th2 cytokines tested, IL-4 and IL-13 were able to restore the CH reaction to TNCB in IL-4 KO mice, while IL-10 had no effect. Neither IL-2 nor IFN-γ were able to restore the CH reaction to TNCB in IL-4 KO mice. Similar results were obtained in five additional experiments. Note from Fig. 5 that IL-10 caused significant inhibition of the CH reaction to TNCB in wt mice, while all the other cytokines tested did not exert an effect.
Despite failure in developing CH to TNCB IL-4 KO mice normally develop CH to OX
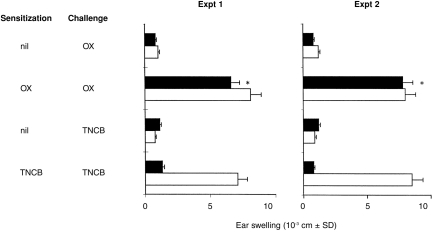
Berg and coworkers15 previously reported reduced ear swelling response to OX in IL-4 KO mice, although the difference with the response in wt mice was not statistically significant. Therefore, we were interested in further testing the CH reaction to OX in IL-4 KO and wt mice. IL-4 KO and wt mice were sensitized with TNCB or OX and were challenged with the specific antigen 5 days later and the ear swelling response was measured after 24 hr. Figure 6 (Exp. 1 and Exp. 2) shows that IL-4 KO mice failed to develop CH to TNCB, but developed a CH response to OX comparable to the CH response detected in wt mice. These data suggest that the CH responses to TNCB and OX are not equivalent and that IL-4 is crucial for the CH response to TNCB but not for that to OX.
Figure 6.
IL-4 KO mice fail to develop CH to TNCB, but not to OX. IL-4 KO (closed columns) and wt (open columns) mice were sensitized with TNCB or OX and challenged 5 days later with the specific antigen. The increase in ear thickness was measured 24 hr after challenge. *P < 0·001 when compared to mice which were not sensitized with OX, but only challenged with OX.
DISCUSSION
Although the CH reaction to chemical haptenes is one of the most widely used experimental models of cell-mediated immune response in vivo, the cellular and molecular mechanisms responsible for its expression and down-regulation are still not well understood. In fact, while some studies have suggested that CH is mainly dependent on the activity of Th1 and/or Tc1 cells and the cytokines they produce,12 other studies have pointed to an important role played by Th2-derived cytokines.16–18 In this paper, we have used IL-4-deficient (IL-4 KO) mice to study the role of Th2 cells and Th2-derived cytokines in the CH reaction to TNCB. In fact, as IL-4 is required for the differentiation of Th2 cells, immune responses dependent on Th2 cytokines are deficient in these mice.19,20
Initial experiments confirm our previous studies on the critical role of IL-4 in the CH reaction to TNCB and show that IL-4 KO mice virtually failed to develop CH upon sensitization and challenge with TNCB. Changing the dose of TNCB used to sensitize mice did not modify the inability of IL-4 KO mice to develop CH. Injection of IL-4 KO mice with recombinant IL-4 restored the CH reaction in a dose-dependent fashion. This effect was evident when IL-4 was injected immediately before either sensitization or challenge, although IL-4 caused a greater ear swelling response when given at the time of sensitization.
In our previous experiments,18 mAb to IL-4 inhibited CH when injected twice (i.e. both before sensitization and before challenge), or when injected at very high doses only before challenge. Moreover, systemic transfer of CH was inhibited by treatement of TNCB-immune cells with IL-4 antisense oligonucleotide or by treatment of recipient mice with mAb to IL-4. Results reported in this paper show that the effect of IL-4 administration was more evident at the time of elicitation of CH, although we observed restoration of the CH reaction also when IL-4 was given at the sensitization phase. A recent paper from our laboratory has shown that development of CD4+ T lymphocytes producing IL-4 following sensitization with TNCB, requires an early IL-4 production which occurs 1 day after sensitization.26 This finding may explanain why IL-4 restores CH in IL-4 KO mice when given either at the sensitization or at the elicitation stage.
These results extend data recently reported by Berg et al.15 and Weigmann et al.16 Berg and coworkers15 reported that IL-4 KO mice showed a slightly reduced CH response to OX, but the difference with the OX response in wt mice was not statistically significant. Accordingly, we herewith show that IL-4 KO mice have a normal CH response to OX (see Fig. 6). Weigmann et al.16 showed that IL-4 KO mice had a significantly reduced CH response to a different haptene, DNFB. These data therefore indicate a discrepancy between the DNFB and TNCB systems on the one side and the OX system on the other and suggest that the cutaneous responses to these haptenes are not equivalent. A similar finding has been reported in IL-1β KO mice, that failed to mount a CH response to TNCB, while being fully able to develop CH to OX.27 Although the mechanisms remain undefined, differences in the relative potency of haptenes, the density of haptene determinants on the APC and/or the different kind of APCs,28 may underline the different results obtained. Additionally, the cellular constituents responsible for TNCB and OX reactivity may be different.
Altogether, these results clearly indicate an important, yet variable, role of IL-4 and/or Th2-derived cytokines as a mediator of the CH reaction to different haptenes. A similar effect might also be played by another Th2 cytokine, IL-13, and results reported in Fig. 5 clearly point to an important role of IL-13 in the CH reaction to TNCB. This interpretation is in agreement with results obtained by Muller et al.29 who demonstrated that short-term alloreactive Th2 cell lines caused swelling when injected into the footpads of allogeneic mice and this response was shown to be dependent on the production of IL-4. Similarly, Th2-type T lymphocytes are the effector cells of the CH reaction to nickel in humans.30
Despite failure to develop a CH response to TNCB, IL-4 KO mice are fully able to develop a Th1 response to TNCB in terms of cytokine production, thus indicating the lack of correlation between the Th1-type response to TNCB in vitro and the CH reaction to TNCB in vivo.
An important aspect of the CH reaction to TNCB is the accumulation of Vγ3+ T lymphocytes in the draining lymph nodes after sensitization with TNCB.10,31 There is now evidence that Vγ3 and αβ cells are required for the transfer of the CH reaction carried out by TNCB-immune lymph node cells11 or by long-term TNCB-specific T-cell lines.17 The Vγ3 cells involved express IL-4 receptor10,17 and require contact with IL-4 to display functional activities.17 As IL-4 is required for the early stages of Vγ3+ T-cell development in the thymus32 the possibility arises that this cell subset may be affected in IL-4 KO mice, thus explaining the reduced CH reaction. At first sight, this seems not to be the case, as Vγ3+ cells accumulate to a similar extent in the draining lymph nodes of TNCB-immune IL-4 KO and wt mice (data not shown).
Both mononuclear cell localization and edema formation are reduced in the ears of IL-4 KO mice and are reversed by injection of IL-4 (see Fig. 4), although this cytokine induces a modest increase of albumin extravasation. At present, we can only speculate on the mechanisms of action of IL-4 and IL-13, but it is likely that both cytokines induce adhesion of lymphocytes to endothelial cells by up-regulating expression of vascular cell adhesion molecule-1 on endothelial cells,33–35 a prerequisite for transmigration and localization at sites of inflammation.
In summary, our results demonstrate that endogenously produced IL-4 is an important cytokine in the CH reaction to TNCB. Additional evaluation of the CH response in IL-4 KO mice can permit fine definition of the cellular and molecular elements that mediate this effect and the role played by other Th2-type cytokines in the CH reaction. As such, IL-4 KO mice may prove valuable in identifying new targets with clinical relevance to human contact hypersensitivity diseases.
Acknowledgments
We would like to thank Dr W. Muller and Professor K. Rajewsky for their authorization to use IL-4-deficient mice, Drs W. E. Paul, A. Minty, F. Melchers and A. G. Morris for their generous gifts of reagents and Professor K. Rajewsky for reading the manuscript. This work was supported by grants from the Ministry for Education and Scientific and Technological Research (MURST 40% to A.S. and MURST 60% to A.S. and F.D.).
References
- 1.Friedmann PS. Contact hypersensitivity. Curr Opin Immunol. 1989;1:690. doi: 10.1016/0952-7915(89)90043-5. [DOI] [PubMed] [Google Scholar]
- 2.Piguet PF, Grau GE, Hauser C, Vassalli P. Tumor necrosis factor is a critical mediator in haptene-induced irritant and contact hypersensitivity reactions. J Exp Med. 1991;173:673. doi: 10.1084/jem.173.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gautam S, Matriano J, Chikkala N, Edinger M, Tubbs R. L3T4 (CD4+) T cells that mediate contact sensitivity to trinitrochlorobenzene express I-A determinants. Cell Immunol. 1991;135:27. doi: 10.1016/0008-8749(91)90251-6. [DOI] [PubMed] [Google Scholar]
- 4.Miller SD, Jenkins MK. In vivo effects of GK1.5 (anti-L3T4a) monoclonal antibody on induction and expression of delayed-type hypersensitivity. Cell Immunol. 1985;92:414. doi: 10.1016/0008-8749(85)90022-x. [DOI] [PubMed] [Google Scholar]
- 5.Issekutz TB, Stolz JM, Van Der Meide P. Lymphocyte recruitment in delayed-type hypersensitivity: the role of IFN-γ. J Immunol. 1988;140:2989. [PubMed] [Google Scholar]
- 6.Skoglund CA, Scheynius A, Holmdahl R, Van Der Meide P. Enhancement of DTH reaction and inhibition of the expression of class II transplantation antigens by in vivo treatment with antibodies against γ-interferon. Clin Exp Immunol. 1986;71:428. [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson JA, Troutt AB, Kelso A. Contact sensitivity to oxazolone: involvement of both interferon-γ and interleukin-4 in oxazolone-specific Ig and T-cell response. Immunology. 1993;78:185. [PMC free article] [PubMed] [Google Scholar]
- 8.Gocinski BL, Tigelaar RE. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990;144:121. [PubMed] [Google Scholar]
- 9.Ptak W, Szczpanik M, Rabamabhadran R, Askenase PW. Immune or normal γδ T cells that assist αβ T cells in elicitation of contact sensitivity preferentially use Vγ5 and Vδ4 variable region gene segments. J Immunol. 1996;156:976. [PubMed] [Google Scholar]
- 10.Dieli F, Asherson GL, Sireci G, et al. γδ cells involved in contact sensitivity preferentially rearrange the Vγ3 region and require interleukin-7. Eur J Immunol. 1997;26:206. doi: 10.1002/eji.1830270131. [DOI] [PubMed] [Google Scholar]
- 11.Dieli F, Ptak W, Sireci G, et al. Cross-talk between Vβ8+ and γδ+ T lymphocytes in contact sensitivity. Immunology. 1998;93:256. doi: 10.1046/j.1365-2567.1998.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H, Diiulio NA, Fairchild RL. T cell populations primed by haptene sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon-γ-producing (Tc1) effector CD8+ T cells and interleukin (IL) -4/IL-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinbrink K, Sorg C, Mavher E. Low zone tolerance to contact allergens in mice: a functional role for CD8+ T helper type 2 cells. J Exp Med. 1996;183:759. doi: 10.1084/jem.183.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gautam SC, Chikkala NF, Hamilton TA. Anti-inflammatory action of IL-4. J Immunol. 1992;148:1411. [PubMed] [Google Scholar]
- 15.Berg DJ, Leach MW, Kuhn R, et al. Interleukin 10 but not interleukin 4 is a natural suppressant of cutaneous inflammatory response. J Exp Med. 1995;182:99. doi: 10.1084/jem.182.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weigmann B, Schwing J, Huber H, et al. Diminished contact hypersensitivity response in IL-4 deficient mice at a late phase of the elicitation reaction. Scand J Immunol. 1997;45:308. doi: 10.1046/j.1365-3083.1997.d01-402.x. [DOI] [PubMed] [Google Scholar]
- 17.Dieli F, Asherson GL, Colonna Romano G, Sireci G, Gervasi F, Salerno A. IL-4 is essential for the systemic transfer of delayed hypersensitivity by T cells. Role of γ/δ cells. J Immunol. 1994;152:2698. [PubMed] [Google Scholar]
- 18.Salerno A, Dieli F, Sireci G, Bellavia A, Asherson GL. Interleukin-4 is a critical cytokine in contact sensitivity. Immunology. 1995;84:404. [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science (Wash DC) 1991;254:707. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 20.Kopf M, Le Gros G, Bachmann M, Lamers M, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 21.Asherson GL, Dieli F. Immune deviation in the mouse: transfer of selective depression of the contact sensitivity and interleukin-2 response, with retention of interferon gamma production requires CD8+ T cells. Immunology. 1992;76:427. [PMC free article] [PubMed] [Google Scholar]
- 22.Dieli F, Asherson GL, Bonanno CT, Sireci G, Salerno A. Major histocompatibility complex control of the class of the immune response to the haptene trinitrophenyl. Immunology. 1995;84:355. [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald HR, Cerottini JC, Ryser CG, et al. Quantitation and cloning of cytolytic T lymphocytes and their precursors. Immunol Rev. 1980;51:93. doi: 10.1111/j.1600-065x.1980.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 24.Vadas MA, Miller JF.A.P, Gamble J, Whitelaw A. A radioisotopic method to measure delayed type hypersensitivity in the mouse. Int Arch Allergy Appl Immunol. 1975;49:670. doi: 10.1159/000231449. [DOI] [PubMed] [Google Scholar]
- 25.Subramaniam M, Saffaripour S, Watson SR, Mayadas TN, Hynes RD, Wagner DD. Reduced recruitment of inflammatory cells in a contact hypersensitivity response in P-Selectin-deficient mice. J Exp Med. 1995;181:2277. doi: 10.1084/jem.181.6.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dieli F, Taniguchi M, Asherson GL, et al. Development of haptene-induced IL-4-producing CD4+ T lymphocytes requires early IL-4 production by αβ T lymphocytes carrying invariant Vα14 TCR α chains. Int Immunol. 1998;10:244. doi: 10.1093/intimm/10.4.413. [DOI] [PubMed] [Google Scholar]
- 27.Shornick LP, De Togni P, Mariathasan S, et al. Mice deficient in IL-1β manifest impaired contact hypersensitivity to trinitrochlorobenzene. J Exp Med. 1996;183:1427. doi: 10.1084/jem.183.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuang I, Chiang L, Chou C, Hsieh H. Different kinds of antigen-presenting cells exert different effects on T-helper cells development. Int Arch Allergy Immunol. 1996;111:366. doi: 10.1159/000237394. [DOI] [PubMed] [Google Scholar]
- 29.Muller K, Jaunin F, Masouye I, Saurat J, Hauser C. Th2 cells mediate IL-4 dependent local tissue inflammation. J Immunol. 1990;150:5576. [PubMed] [Google Scholar]
- 30.Werfel T, Hentschel M, Kapp A, Renz H. Dichotomy of blood- and skin-derived IL-4-producing allergen-specific T cells and restricted V beta repertoire in nickel-mediated contact dermatitis. J Immunol. 1997;158:2500. [PubMed] [Google Scholar]
- 31.Salerno A, Dieli F. Role of γδ cells in immune response in humans and mice. Crit Rev Immunol. 1998;18:327. doi: 10.1615/critrevimmunol.v18.i4.30. [DOI] [PubMed] [Google Scholar]
- 32.Havran WL, Allison JP. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature. 1990;344:68. doi: 10.1038/344068a0. [DOI] [PubMed] [Google Scholar]
- 33.Barks JL, McQuillan JJ, Iademarco MF. TNF-α and IL-4 synergistically increase vascular cell adhesion molecule-1 expression in cultured vascular smooth muscle cells. J Immunol. 1997;159:4532. [PubMed] [Google Scholar]
- 34.Bochner BS, Klunk DA, Sterbinsky SA, Coffman RL, Chleimer RP. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J Immunol. 1995;154:799. [PubMed] [Google Scholar]
- 35.de Ruco LP, Laat PA, Matteucci C, et al. Expression of ICAM-1 and VCAM-1 in human malignant mesothelioma. J Pathol. 1996;179:266. doi: 10.1002/(SICI)1096-9896(199607)179:3<266::AID-PATH592>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]