Abstract
Chemokines and their receptors play an important role in the process of leucocyte recruitment at sites of inflammation. However, recent evidence suggests that these proteins can also regulate non-leucocyte cell functions such as angiogenesis, migration and proliferation. We have investigated the expression of the CXC chemokine receptor 4 (CXCR4) on primary cultures of type II alveolar epithelial cells, their transformed counterpart, the A549 cell line and also on other epithelial cell lines from various tissues. We found that all epithelial cell types tested express mRNA for CXCR4. Flow cytometric analysis and immunocytochemical staining shows that CXCR4 chemokine receptor is abundantly expressed on the surface of A549 epithelial cells. Furthermore, A549 cells responded to the CXCR4 ligand, stromal-derived factor-1α (SDF-1α) with a rapid and robust calcium mobilization and not to other CXC chemokines, suggesting that CXCR4 is functionally active and is able to couple to G-protein signalling mechanisms. A549 cells did not proliferate in response to either SDF-1α or interleukin-8 (IL-8) CXC chemokines. These findings may have important implications for epithelial physiology and pathology.
INTRODUCTION
Chemokines and their receptors play a important role in the process of leucocyte recruitment and activation at sites of inflammation.1 Chemokines are a family of small molecular weight proteins (8–13 000 MW) that are classified into four distinct groups depending on the positioning of the cysteine motif at the NH2 terminus. The family members include CXC, CC, C and CX3C chemokines of which CXC and CC are the largest and most well-characterized group.2–4 The specific effects of chemokines on their target cells are mediated by members of a family of seven transmembrane-spanning, G- protein-coupled receptors.5 To date a total of 17 chemokine receptors have been identified. Five receptors selectively bind certain CXC chemokines, these include chemokine receptors CXCR1–5,6 whilst the CC receptor (CCR) family currently consists of nine receptors, CCR1–8 and CCR10.7–11 Recently, receptors for fractalkine (CX3CR1)12 and lymphotactin (XCR1)13 have been identified. A further chemokine receptor known as the Duffy antigen receptor for chemokines (DARC) has been shown to bind promiscuously to both CXC and CC chemokines.14
The actions of chemokines and the expression of their receptors have mainly been described on different leucocyte subpopulations. However, new evidence has shown that non-haematopoietic cell types are capable of binding and responding to a number of chemokines. CXC chemokines, such as interleukin-8 (IL-8), growth-related oncogene (GRO-α), platelet factor-4 (PF-4) and interferon inducible protein-10 (γIP-10), have been implicated in the regulation of keratinocyte and endothelial cell functions including the stimulation and inhibition of proliferation, angiogenesis and cell migration.7,15,16 Recently, it has been shown that vascular endothelial cells express a multitude of chemokine receptors, with CXCR417–19 and duffy antigen receptor for chemokines (DARC)20 being particularly prominent. It has been speculated that these receptors may serve as facilitators of endothelial cell inflammatory responses or participate in neovascularization and wound healing.
Like the endothelium, epithelium can also be considered to play a major role in the maintenance of tissue homeostasis. Epithelial cells are particularly important as they comprise an interface between the body and the environment. Consequently, they must have the capacity to generate brisk inflammatory responses in order to provide prompt clearance of offending agents, leading to normal tissue repair, tissue remodelling and return to homeostasis. There are key differences between endothelial and epithelial inflammatory responses and in their adhesive interactions with polymorphonuclear leucocytes (PMNL) (reviewed in 21). It appears that β2-integrins are central to both PMNL transepithelial and transendothelial migration. However, in contrast to endothelial cells, PMNL adhesion to epithelial cells is mediated exclusively by CD11b/CD18 which binds either to intracellular adhesion molecule-1 (ICAM-1) or to other suitable counter ligands on the epithelial cell surface. Furthermore, PMNL transepithelial migration occurs preferentially in the basal to apical direction and does not require platelet endothelial cell adhesion molecule (PECAM; CD31)- or selectin-mediated rolling events, although other as yet undefined carbohydrate-mediated interactions are suspected. Recently, CD47 has been shown to be important in PMNL–epithelial transmigration.21 Given the differences between leucocyte recruitment at the endothelial and epithelial cell surfaces it is possible that these cells would exhibit differential chemokine receptor expression. However, there is no evidence as to whether epithelial cells express chemokine receptors.
We have examined the mRNA expression of chemokine receptor CXCR4 in various epithelial cell types and have analysed the protein expression and functional role of CXCR4 on the type II alveolar epithelial cell line, A549.
MATERIALS AND METHODS
Reagents
l-glutamine, penicillin, streptomycin, Hank’s balanced salt solution (HBSS), trypsin, Histopaque, fura-2/AM, recombinant human epidermal growth factor, bradykinin and propidium iodide were purchased from Sigma Chemical Co. (Poole, UK). Dulbecco’s modified minimum essential medium (DMEM), fetal calf serum (FCS), Superscript II and Taq polymerase were purchased from Life Technologies (Paisley, UK). Mouse anti-human CXCR4 (clone 12G5) and recombinant IL-8 were purchased from R & D Systems (Abingdon, UK). RNAzol B was purchased from Biogenesis (Poole, UK), Citifluor from Agar Aids, London, UK, and recombinant stromal-derived factor-1α (SDF-1α) was obtained from Peprotech (London, UK).
Cell lines
A549 type II alveolar epithelial cells, RT-4 bladder epithelial cells and MCF-7 mammary epithelial cells were purchased from the European Collection Animal Cell Cultures, Aylesbury, UK.
Cell culture
A549 cells were grown as monolayers in plastic tissue culture flasks at 37°, 5% CO2. DMEM supplemented with 5% FCS, 2 mm l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin was used to maintain the cells. The well-differentiated bladder epithelial cell line RT-4 and the mammary epithelial cell line MCF-7 were cultured as above, except that 10% FCS was used. All cells were subcultured following brief treatment with trypsin/ethylenediamine tetra-acetic acid (EDTA).
Mononuclear leucocyte isolation
Mononuclear leucocytes (MNL) were isolated from blood anticoagulated with 20 U/ml heparin collected by venepuncture from normal donors. MNL were separated from granulocytes by density gradient centrifugation using Histopaque (30 min, 400 g). The isolated MNL were washed once with HBSS without Ca2+ or Mg2+ before performing cell counts and viability determinations using propidium iodide. MNL were at least 98% viable before RNA extraction.
Reverse transcription–polymerase chain reaction (RT–PCR) analysis
Total RNA was extracted from cultured epithelial cells and MNL using RNAzol B according to the manufacturer’s instructions. RNA isolated from primary type II alveolar cell cultures was a gift from Colin Bingle (University of Sheffield). RNA was reverse transcribed using Superscript II and receptor specific oligonucleotide primers for CXCR4; sense primer ATGGAGGGGATCAGTATATACAC, antisense primer AGAAGTTCAAAAGTGAGGTCGAT. PCR was performed using 2·5 μl cDNA added to a reaction mixture containing 50 mm KCl, 10 mm Tris–HCl, 1·5 mm MgCl2, 200 μm deoxynucleotide triphosphates, 1 μm of each receptor specific primer and 2·5 units Taq polymerase in a reaction volume of 50 μl. The enzyme was added after heating the probe to 94° (hot start). Samples were incubated in a Techne PHC-3 thermal cycler (Cambridge, UK) for a total of 30 cycles. Each cycle consisted of 1 min at 95°, 1·5 min at 59° and 1·5 min at 72°. The amplification of a segment of the constitutively expressed β-2-microglobulin gene was used as an endogenous control and to also check for genomic DNA contamination. PCR products were visualized on a 1·5% tris-acetate-EDTA buffer (TAE) agarose gel containing ethidium bromide under UV illumination. DNA fragments whose sizes corresponded to that of CXCR4 were gel-purified and subjected to DNA sequence analysis.
Flow cytometry
A549 cells were removed from flasks non-enzymatically and resuspended at 2×105 cells in ice-cold wash buffer (0·2% bovine serum albumin–phosphate-buffered saline (BSA–PBS)+0·1% sodium azide). Cells were incubated with 10 μg/ml mouse anti-human CXCR4 (clone 12G5) antibody for 40 min at 4°. Cells were kept on ice to minimize down-modulation of CXCR4 from receptor internalization. After washing twice with cold wash buffer cells were incubated with fluoroscein isothiocyanate (FITC)-conjugated rabbit anti-mouse immunoglobulin G (IgG) in the dark for 30 min at 4°. Finally, cells were washed twice more and resuspended in wash buffer. Fluorescence-activated cell sorting (FACS) analysis was done with a FACScan flow cytometer (Becton Dickinson). Controls received isotype-matched irrelevant IgG or mouse anti-human human leucocyte antigen (HLA) class I antibody at the same concentration.
Immunocytochemistry
Cultured A549 epithelial cells were washed with PBS, fixed with 2% paraformaldehyde and stained with 8 μg/ml monoclonal anti-CXCR4 (clone 12G5) for 1 hr at room temperature. Cells were washed, stained with FITC-conjugated rabbit anti-mouse IgG for 30 min at room temperature, washed again and mounted with Citifluor. Photographs were taken using a Nikon SLR camera attached to a Leica DM IRB inverted fluorescence microscope.
Calcium mobilization assay
Microfluorimetry using fura-2/AM was employed for measurements of intracellular calcium [Ca2+]i. A549 cells were grown on poly-d-lysine-coated glass coverslips and loaded with 2 μm fura-2/AM which was added to the culture medium for 45 min at 37°. Coverslips were transferred to assay buffer (in mm: NaCl 137, KCl 5·36, MgSO4 0·81, Na2PO4 0·34, KH2PO4 0·44, NaHCO3 4·17, HEPES 10, CaCl2 1·26 and glucose 2·02) and fluorescence of fura-2 in individual cells was measured using a Nikon diaphot microscope with an epifluorescence ×40 objective. Chemokines were added from concentrated stocks in water. Emission fluorescence at 510 nm was measured upon excitation at 340 and 380 nm. Data are presented by the ratio of fluorescence intensity at 340 nm divided by 380 nm. To establish the integrity of epithelial cells, [Ca2+]i was also measured using bradykinin as a control stimulant.
Proliferation assay
Increases in A549 cell number were detected by labelling cellular DNA using the fluorescent dye propidium iodide. A549 cells were plated at a density of 2×104 cells/well in a 96-well plate and incubated in culture medium for 24 hr to allow adherence. After washing, DMEM supplemented with 0·5% FCS with or without chemokine was added and the cells incubated for 24, 48 and 72 hr at 37°, 5% CO2. At the end of the culture period A549 cells were fixed with 3·7% formaldehyde, stained with 5 μg/ml propidium iodide for 5 min at room temperature in the dark and then air dried. Fluorescence was measured at 530 nm excitation and 620 nm emission using an automated fluorescent microplate reader (Denly Wellfluor, Watertown, MA). The amount of fluorescence detected was assumed to be directionally proportional to the number of cells within a well.22 Results are expressed as mean fluorescence values from triplicate wells±SEM.
RESULTS
RT–PCR analysis of CXCR4
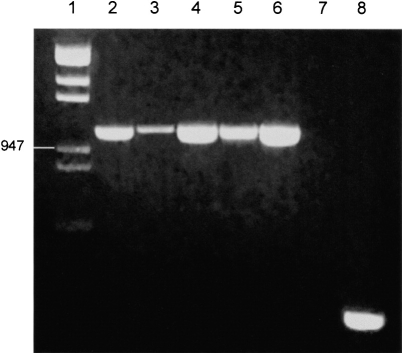
In order to assess the gene expression of CXCR4 in epithelial cells RT–PCR was conducted on RNA extracted from primary cultures of type II alveolar epithelial cells and from epithelial cell lines derived from various tissues. RT–PCR analysis and subsequent gel electrophoresis showed bands of equivalent size to MNL CXCR4 for all epithelial cell types tested (Fig. 1). DNA sequencing of these bands confirmed that mRNA for CXCR4 is present in both primary cultures of human type II alveolar epithelial cells and their transformed counterpart, the A549 cell line. Moreover, CXCR4 expression was also detected in the well-differentiated bladder epithelial cell line RT-4, and the mammary epithelial cell line MCF-7, suggesting that mRNA for CXCR4 may be widely expressed in epithelium from various tissues. The expression of CXCR4 in these epithelial cells appears to be comparable to that of the constitutively expressed β-2-microglobulin gene, suggesting that mRNA for CXCR4 may be relatively abundant in epithelial cells.
Figure 1.
Expression of chemokine receptor CXCR4 mRNA in epithelial cells by RT–PCR: Lane 1 Hin dIII & Eco RI λ DNA digest, lane 2 MNL, lane 3 primary type II alveolar epithelial cells, lane 4 A549, lane 5 RT4, lane 6 MCF-7, lane 7 negative control, lane 8 β-2-microglobulin fragment (A549). mRNA for CXCR4 is present in all types of epithelial cells tested. PCR data represents a typical one of four independent experiments.
Cell-surface expression of CXCR4
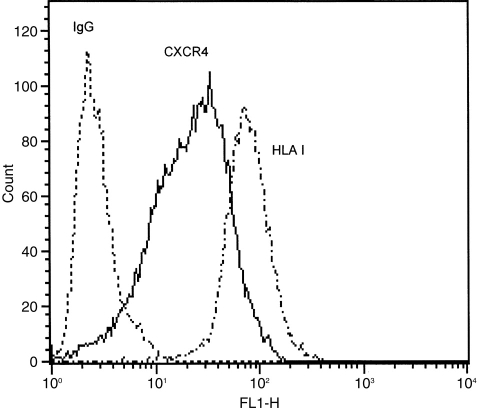
The cell-surface expression of CXCR4 on A549 cells was evaluated by both flow cytometry and immunocytochemistry using a specific monoclonal anti-CXCR4 antibody (12G5) as described in Materials and Methods. A significant shift is seen in the fluorescence histogram of anti-CXCR4 stained cells when compared to the isotype-matched IgG control (Fig. 2) indicating the presence of cell surface CXCR4 protein on A549 epithelial cells. Furthermore, the levels of immunofluorescence staining in anti-CXCR4 and anti-HLA class I stained cells are almost comparable, suggesting that CXCR4 is apparently as abundantly expressed on the epithelial cell surface as HLA class I. The localization of CXCR4 to the cell surface of A549 cells was confirmed using immunocytochemical staining (Fig. 3). No observable staining was seen with an isotype-matched IgG control.
Figure 2.
Flow cytometry histograms of CXC4 on A549 epithelial cells. Cells were stained with either anti-CXCR4 or anti-HLA class I antibody followed by FITC-conjugated anti-IgG. Controls received equivalent concentrations of isotype-matched IgG. For all panels, data are shown as cell number vs. the relative fluorescence. Each histogram shows data from a single representative experiment although each analysis was repeated at least three times. A549 cells abundantly express CXCR4 at the cell surface with almost comparable levels to HLA class I antigen.
Figure 3.
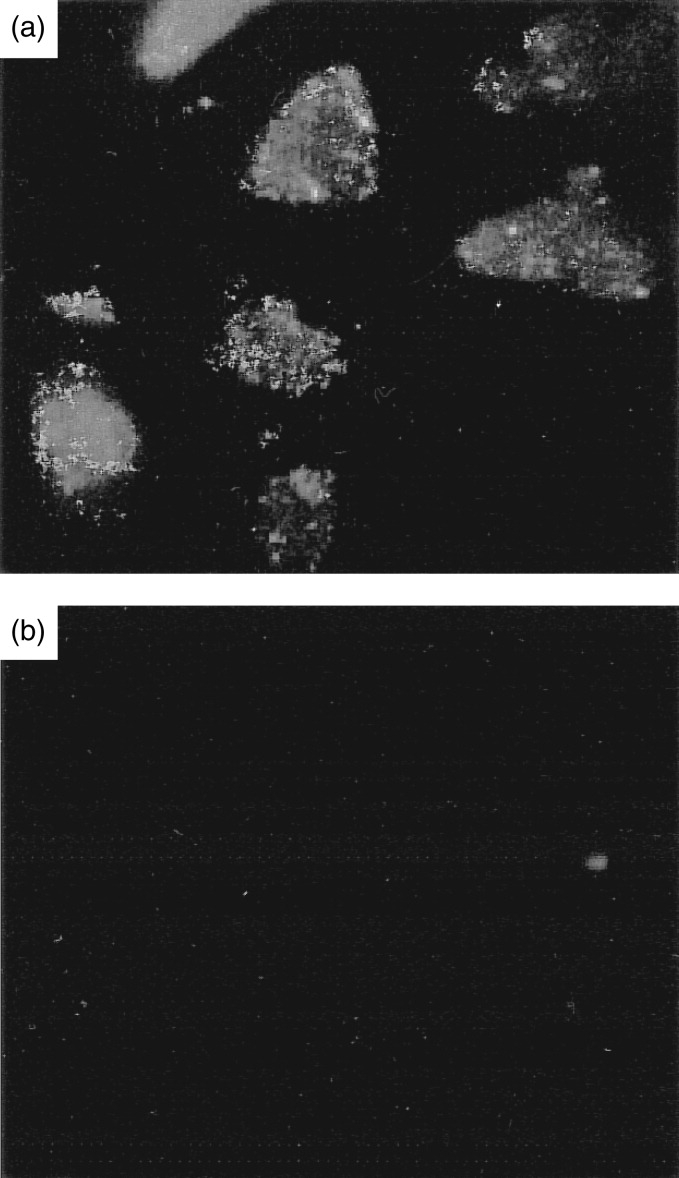
(a) Immunocytochemical staining of an A549 type II alveolar epithelial cell showing localization of CXCR4 chemokine receptor at the cell surface. (b) IgG isotype-matched control (magnification ×400).
Intracellular Ca2+ mobilization
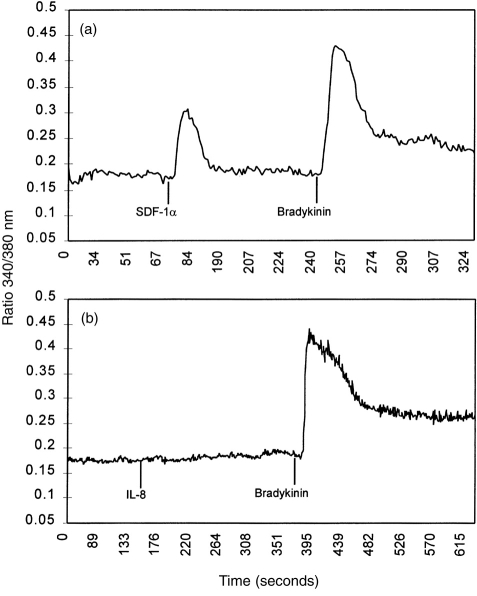
Calcium mobilization studies were carried out to determine whether CXCR4 expressed by epithelial cells is a functional receptor. Adherent fura-2/AM-loaded A549 cells were challenged with the CXCR4 ligand, SDF-1α. Stimulation of epithelial cells with 2 μg/ml SDF-1α caused a rapid and robust increase in intracellular calcium (Fig. 4), which was concentration dependent. The magnitude of the calcium response was lower than that evoked by stimulation with 100 nm bradykinin (Fig. 4). A549 cells challenged with either IL-8, GRO-α or buffer alone (data not presented) failed to produce a calcium response (Fig. 4), suggesting the absence of other functional CXC chemokine receptors.
Figure 4.
Calcium mobilization in A549 cells following chemokine stimulation. 2 μg/ml SDF-1α (a) produced a rapid and robust calcium mobilization whilst 2 μg/ml IL-8 (b) did not. In each experiment bradykinin 100 nm was used as a positive control to check epithelial cell integrity. These results indicate that epithelial expressed CXCR4 can couple to G-protein-linked signal transduction mechanisms.
A549 cell proliferation
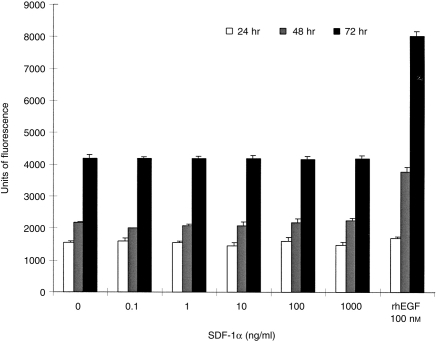
A fluorescence-based assay was used to assess the effect of SDF-1α on A549 epithelial cell proliferation. A549 cells stimulated with increasing concentrations (0·1 ng/ml to 1 μg/ml) of SDF-1α over 24, 48 and 72 hr produced equivalent fluorescence signals to unstimulated cells (Fig. 5), suggesting that SDF-1α does not have any detectable effects on proliferation of A549 cells under the conditions tested. However, A549 epithelial cells treated with 100 nm recombinant human epidermal growth factor showed considerable increases in fluorescence and therefore cell number over the same incubation period.
Figure 5.
The effects of SDF-1α on A549 proliferation. A549 epithelial cells were plated into 96-well plates and stimulated for 24, 48 and 72 hr with increasing concentrations of SDF-1α. Fluorescence from three replicate wells were measured and data are represented as mean units of fluorescence±SEM (one of three similar experiments). SDF-1α had no effect on A549 proliferation, whilst cells treated with recombinant human epidermal growth factor showed marked increases in fluorescence, and therefore cell number.
DISCUSSION
Epithelial cells represent an important cellular interface between tissues and the external environment. They are situated at sites that are prone to infection from foreign pathogens and by necessity are key regulators of the immune response. Recently the expression of several chemokine receptors has been shown on endothelial cells17–19 indicating that these receptors are not restricted to haemopoietic cells. Furthermore, the expression of ‘classical’ G-protein-linked seven-transmembrane chemoattractant receptors by epithelial cells has been reported. The complement C5a receptor (C5aR) has been detected by RT–PCR and immunostaining in both bronchial and alveolar epithelial cells,23 whilst the platelet-activating factor receptor (PAFR) has recently been shown to be expressed on both lung epithelial and endothelial cells, where it is believed to act as the major attachment site for virulent Streptococcus pneumoniae.24 The promiscuous chemokine receptor DARC has been reported to be expressed on both renal and spleen capillary endothelial and epithelial cells25 as well as on type I alveolar epithelial cells.20 This evidence suggests that epithelial cells may express other chemokine receptors than DARC.
Using a RT–PCR based strategy utilizing CXCR4 specific primers, we clearly demonstrate that mRNA for the chemokine receptor CXCR4 is expressed not only in both primary cultures and transformed type II alveolar epithelial cells (pneumocytes) but also in a number of epithelial cell lines derived from various other tissues. However, we could not detect the expression of mRNA for DARC in either A549 or RT-4 epithelial cells by this highly sensitive method. We also found that A549 cells do not express mRNA for other chemokine receptors (CXCR1–3 or CCR1–5; data not shown) indicating that, unlike endothelial cells CXCR4 is the only chemokine receptor expressed on epithelial cells. Flow cytometric analysis was consistent with the PCR data and reveals that CXCR4 is abundantly expressed at the epithelial cell surface with similar expression levels to the HLA class I antigen. Calcium mobilization studies show that SDF-1α, the sole ligand for CXCR4, was able to elicit a rapid and robust calcium mobilization in A549 cells. Therefore, CXCR4 expressed by epithelium can couple to signal transduction mechanisms upon ligand binding showing that epithelial CXCR4 is functionally active. Other CXC chemokines (IL-8 and GRO-α) tested did not produce a calcium mobilization, suggesting the absence of other chemokine receptors on epithelial cells, in agreement with the PCR data.
Recent studies have shown that CXCR4,26 CCR2b, CCR3, CCR527 and CCR828 serve as cofactors in association with CD4 to permit infection of either T-cell tropic or macrophage-tropic human immunodeficiency virus-1 (HIV-1) strains into permissive cells. Kozak et al. suggest that CXCR4 is utilized only when CD4 is present on the cell surface in relatively high concentrations,29 which suggests that epithelial cells would be non-permissive for HIV infection. However, CD4-independent HIV infection has been shown to occur. Some strains of HIV-2 and simian immunodeficiency virus (SIV) can replicate efficiently in CD4-negative human cells in vitro because of an ability of these stains to use CXCR4 for entry in the absence of CD4.30,31 These findings suggest that some HIV isolates, particularly HIV-2, could enter epithelial cells by utilizing CXCR4 alone. Consequently, HIV infection may have more widespread cellular targets than first envisaged.
IL-8 and other CXC chemokines are secreted by a variety of cells, including epithelial cells, upon activation with pro-inflammatory agents.1 However, SDF-1α and SDF-1β (which are derived from SDF-1 by alternative splicing) are unlike other chemokines because their expression is not regulated by pro-inflammatory stimulants. It appears that SDF-1α is constitutively expressed in a broad range of tissues, including bone marrow, thymus, spleen and liver.32 It is therefore possible that SDF-1α may have other important cellular functions as well as being a leucocyte chemoattractant. Koch et al. (1991) reported a dose-dependent growth-enhancing effect of the CXC chemokine, IL-8 on endothelial cells.7 However, our results show that neither SDF-1α or IL-8 (data not shown) have any significant proliferative effects on the A549 epithelial cell line. Transgenic mice lacking the CXCR4 gene have defective formation of the large vessels supplying the gastrointestinal tract and are defective in vascular development, haematopoiesis and cardiogenesis. Therefore, it appears that CXCR4 and SDF-1 are important in organ vascularization and embryogenesis33 as well as in the immune response. Further experimental evidence is necessary to elucidate the precise functional role of CXCR4 on epithelial cells.
The demonstration that chemokine receptor CXCR4 is constitutively expressed in primary type II alveolar epithelial cells and epithelial cell lines from various tissues indicates that this receptor may have a functional role on epithelial physiology and pathology. Whether CXCR4 participates in inflammatory responses is unclear. As both leucocyte and epithelial CXCR4 bind to SDF-1α in the same way, one might speculate that CXCR4 expressed on the epithelium is unlikely to participate in chemokine presentation to passing leucocyte populations. However, it is possible that epithelial CXCR4 may facilitate the recruitment of phagocytic cells to sites of inflammation by direct effects on epithelial cells. CXCR4 may also have other functional roles within the immune response or participate in wound healing or neovascularization. If this is so, it is also possible that CXCR4 may be involved in the pathophysiology of several acute or chronic inflammatory disease states associated with the epithelium.
Acknowledgments
We would like to thank Molly Hashmi and Ruth Shepherd for technical assistance with the calcium mobilization assay, and Arthur Moir and Paul Brown for synthesis of oligonucleotide primers and DNA sequencing. The funding of this work included financial support from the Arthritis and Rheumatism Council (P. N. Monk, grant MO543) and the Children’s Appeal, Sheffield Children’s Hospital.
Abbreviations
- CXCR4
CXC chemokine receptor 4
- CCR
CC chemokine receptor
- SDF-1α
stromal derived factor-1α
- PMNL
polymorphonuclear leucocytes
- MNL
mononuclear leucocytes.
References
- 1.Taub DD. Chemokine–leukocyte interactions. The voodoo that they do so well. Cytokine Growth Factor Rev. 1996;7:355. doi: 10.1016/s1359-6101(97)89237-4. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 3.Kelner GS, Kennedy J, Bacon KB, et al. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 4.Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 5.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 6.Legler DF, Loetscher M, Roos RS, Clark-Lewis IB, M., Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998;187:655. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 8.Baba M, Imai T, Nishimura M, et al. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J Biol Chem. 1997;272:14893. doi: 10.1074/jbc.272.23.14893. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida R, Imai T, Hieshima K, et al. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem. 1997;272:13803. doi: 10.1074/jbc.272.21.13803. [DOI] [PubMed] [Google Scholar]
- 10.Tiffany HL, Lautens LL, Gao JL, et al. Identification of CCR8: a human monocyte and thymus receptor for the CC chemokine I-309. J Exp Med. 1997;186:165. doi: 10.1084/jem.186.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonini JA, Martin SK, Dralyuk F, Roe MW, Philipson LHS, Steiner DF. Cloning, expression, and chromosomal mapping of a novel human CC-chemokine receptor (CCR10) that displays high-affinity binding for MCP-1 and MCP-3. DNA Cell Biol. 1997;16:1249. doi: 10.1089/dna.1997.16.1249. [DOI] [PubMed] [Google Scholar]
- 12.Imai T, Hieshima K, Haskell C, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida T, Imai T, Kakizaki M, Nishimura M, Takagi S, Yoshie O. Identification of single C motif-1/lymphotactin receptor XCR1. J Biol Chem. 1998;273:16551. doi: 10.1074/jbc.273.26.16551. [DOI] [PubMed] [Google Scholar]
- 14.Neote K, Darbonne W, Ogez J, Horuk R, Schall TJ. Identification of a promiscuous inflammatory peptide receptor on the surface of red blood cells. J Biol Chem. 1993;268:12247. [PubMed] [Google Scholar]
- 15.Tuschil A, Lam C, Haslberger A, Lindley I. Interleukin-8 stimulates calcium transients and promotes epidermal cell proliferation. J Invest Dermatol. 1992;99:294. doi: 10.1111/1523-1747.ep12616634. [DOI] [PubMed] [Google Scholar]
- 16.Maione TE, Gray GS, Petro J, et al. Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science. 1990;247:77. doi: 10.1126/science.1688470. [DOI] [PubMed] [Google Scholar]
- 17.Murdoch C, Monk PN, Finn A. CXC chemokine receptor expression on human endothelial cells. Cytokine. 1999;11 doi: 10.1006/cyto.1998.0465. in press. [DOI] [PubMed] [Google Scholar]
- 18.Gupta SK, Lysko PG, Pillarisetti KOE, Stadel JM. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem. 1998;273:4282. doi: 10.1074/jbc.273.7.4282. [DOI] [PubMed] [Google Scholar]
- 19.Volin MV, Joseph L, Shockley MS, Davies PF. Chemokine receptor CXCR4 expression in endothelium. Biochem Biophys Res Commun. 1998;242:46. doi: 10.1006/bbrc.1997.7890. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhuri A, Nielsen S, Elkjaer ML, Zbrzezna V, Fang FP, Pogo AO. Detection of Duffy antigen in the plasma membranes and caveolae of vascular endothelial and epithelial cells of nonerythroid organs. Blood. 1997;89:701. [PubMed] [Google Scholar]
- 21.Parkos CA. Cell adhesion and migration. I. Neutrophil adhesive interactions with intestinal epithelium. Am J Physiol. 1997;273:G763. doi: 10.1152/ajpgi.1997.273.4.G763. [DOI] [PubMed] [Google Scholar]
- 22.McCaffrey TA, Agarwal LA, Weksler BB. A rapid fluorometric DNA assay for the measurement of cell density and proliferation in vitro. In Vitro Cell Dev Biol. 1988;24:247. doi: 10.1007/BF02623555. [DOI] [PubMed] [Google Scholar]
- 23.Haviland DL, McCoy RL, Whitehead WT, et al. Cellular expression of the C5a anaphylatoxin receptor (C5aR): demonstration of C5aR on nonmyeloid cells of the liver and lung. J Immunol. 1995;154:1861. [PubMed] [Google Scholar]
- 24.Cundell DR, Gerard NP, Gerard C, Idanpaan Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 25.Hadley TJ, Lu ZH, Wasniowska K, Martin AW, Peiper SC.H.J, Horuk R. Postcapillary venule endothelial cells in kidney express a multispecific chemokine receptor that is structurally and functionally identical to the erythroid isoform, which is the Duffy blood group antigen. J Clin Invest. 1994;94:985. doi: 10.1172/JCI117465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 27.Doranz BJ, Rucker J, Yi Y, et al. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 28.Horuk R, Hesselgesser J, Zhou Y, et al. The CC chemokine I-309 inhibits CCR8-dependent infection by diverse HIV-1 strains. J Biol Chem. 1998;273:386. doi: 10.1074/jbc.273.1.386. [DOI] [PubMed] [Google Scholar]
- 29.Kozak SL, Platt EJ, Madani N, Ferro FE, Jr, Peden KK, Kabat D. CD4, CXCR-4, and CCR-5 dependencies for infections by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J Virol. 1997;71:873. doi: 10.1128/jvi.71.2.873-882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Endres MJ, Clapham PR, Marsh M, et al. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 31.Clapham PR, McKnight A, Weiss RA. Human immunodeficiency virus type 2 infection and fusion of CD4-negative human cell lines: induction and enhancement by soluble CD4. J Virol. 1992;66:3531. doi: 10.1128/jvi.66.6.3531-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada HST, Honjo T. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 33.Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]