Abstract
The contribution of B7 molecules to the induction and maintenance of the T-cell response to the human pathogenic fungus Cryptococcus neoformans was investigated. T-cell activation by C. neoformans was regulated by B7 molecules. This costimulatory signal was necessary for initiation and maintenance of the T-cell response, through early and late requirements for B7–CD28 interaction. Blocking B7-2 inhibited the normal T-cell proliferative response. This inhibition was due, in part, to a reduced capability of T cells to produce interleukin-2 (IL-2). In contrast, the same T-cell population produced more interferon-γ. Suppression of the normal lymphoproliferation and IL-2 secretion responses to encapsulated C. neoformans by antibodies to B7 was largely reversed by addition of the monoclonal antibody 2H1, that is reactive with the major capsular polysaccharide, glucuronoxylomannan. Overall, our data indicate that B7 molecules play a critical role in T-cell activation by C. neoformans and suggest that appropriate manipulation could drive T helper type 1 cell development.
INTRODUCTION
Cryptococcus neoformans is an opportunistic pathogenic yeast. Disseminated cryptococcosis occurs rarely in healthy individuals, whereas individuals with compromised cellular immunity are at increased risk for cryptococcosis.1 Cryptococcus neoformans is surrounded by a capsule whose major constituent is the polysaccharide, glucuronoxylomannan (GXM), and at least two minor carbohydrate antigens, galactoxylomannan and mannoprotein.2 Capsular polysaccharide is a prominent virulence factor because it is antiphagocytic3 and interferes with antigen processing and presentation by non-professional antigen-presenting cells (APC). This interference is observed as inhibition of T-cell activation when monocytes exposed to C. neoformans are used as APC.4,5 Subsequent studies underlined the role of GXM encapsulation in suppression of the T-cell response.6 Opsonization of encapsulated cryptococci with anti-GXM monoclonal antibody (mAb) enhances the ability of monocytes to process C. neoformans yeast cells, leading to an enhanced T-cell proliferative response.7
Antigen presentation and immune recognition are two critical events in the generation of effective inflammatory responses to microbial pathogens. The generally accepted model of T-cell activation requires two signals.8 The first signal is the occupancy of the T-cell receptor (TCR) by a complex of the antigenic peptide and major histocompatibility complex (MHC) molecules on the APC surface. The second signal results from binding costimulatory (CS) factors or a ligand molecule on the APC surface to a receptor on the T-cell surface. Based on the two-signal model, T cells triggered by TCR in the absence of costimulation become anergic.9
The major CS signal appears to be provided by the B7 molecules B7-1 (CD80) and B7-2 (CD86) on the APC. Recent studies have reached different conclusions for the relative roles of B7-1 and B7-2 in mediating CS interactions with CD28/CTLA-4 and subsequent T-cell differentiation. Some reports suggest that B7-1 and B7-2 have overlapping functions in vitro,10,11 while other studies imply that interleukin-4 (IL-4) production by T cells is particularly dependent on B7-2 signalling.12,13In vitro differentiation of TCR transgenic T cells to the T helper type 1 (Th1) functional phenotype is inhibited by incubation with mAb to B7-1, whereas mAb to B7-2 impairs the development of Th2 clones.14 Administration of mAb to B7-1 and/or mAb to B7-2 during an in vivo immune response has provided different results depending on the system involved.14–16 In experimental autoimmune encephalomyelitis, treatment with mAb to B7-1 diminishes the severity of neurological disease, which is mediated by Th1 cells, whereas administration of mAb to B7-2 enhances disease manifestations.14,15 In contrast, in the non-obese diabetic mouse that develops autoimmune diabetes, blocking B7-2 reduces disease severity while blocking of B7-1 enhances disease severity.16 In both systems Th1 and Th2 components are present throughout the course of autoimmune disease. In contrast, infectious pathogens often elicit strong, highly polarized type 1 or type 2 immune responses. However, few studies have examined the role of B7-1 versus B7-2 in providing costimulation for T-cell effector functions in these systems.
In a previous study, we demonstrated that C. neoformans can induce B7-1 and B7-2 molecule expression in human monocytes, but the magnitude of the effect is dependent on yeast encapsulation and is influenced by the presence of capsule-specific antibody.17 The present study evaluated the contribution of CS molecule expression to regulation of both T-cell activation and phenotypic T-cell shifting (to a Th1 response) in response to encapsulated and acapsular cryptococci.
MATERIALS AND METHODS
Reagents and media
RPMI-1640 with glutamine and fetal bovine serum (FBS) were obtained from Gibco BRL (Paisley, UK). Human serum (HS) from healthy blood type AB donors was obtained from Sigma (St Louis, MO). The mAb 2H1 is a murine immunoglobulin G1 (IgG1) that binds to GXM of serotypes A, B, C and D. It was purified from ascites fluid by protein-G affinity chromatography.18 GXM was isolated from culture supernatant fluid of a serotype A strain [ATCC 24064; American Type Culture Collection (ATCC), Rockville, MD] that was grown on a liquid synthetic medium19 in a gyrator shaker for 4 days at 30°. GXM was isolated using differential precipitation with ethanol and hexadecyl trimethyl ammonium bromide (CTAB, Sigma).20
RPMI-1640, FBS, HS, C. neoformans yeast cells and mAb 2H1 were tested for endotoxin contamination by a Limulus amoebocyte lysate assay (Sigma) which had a sensitivity of ≈0·05–0·1 ng of Escherichia coli lipopolysaccharide (LPS) per ml. All reagents tested negative for LPS by this assay. Mouse isotype control IgG1,k mouse isotype control IgM and fluorescein isothiocyanate (FITC)-conjugated monoclonal anti-rabbit immunoglobulins were provided by Sigma. FITC-conjugated mouse mAb to human CD86 (B7-2; Cat. no. 217632), mouse mAb to human CD80 (B7-1; Cat. no. 217625) and mouse mAb to human CD86 (B7-2; Cat. no. 217630) were purchased from Calbiochem-Novabiochem Corporation (San Diego, CA). FITC-conjugated mouse mAb to human MHC class II (Cat. no. 131-020) and phycoerythrin (PE)-conjugated mouse mAb to human CD14R (Cat. no. 163-050) were purchased from Ancell Corporation (Bayport, MN).
Micro-organisms
The two strains of C. neoformans used in this study were obtained from Dr J. Orendi (Central Bureau Schimmel (CBS) Cultures, Delft, the Netherlands). Strain 6995 (CBS 6995=NIH 37; National Institutes of Health, Bethesda, MD) is a thinly encapsulated isolate of serotype A. Cryptococcus neoformans strain 7698 is an acapsular mutant (CBS 7698=NIH B-4131). The cultures were maintained by serial passage on Sabouraud agar (BioMerieux, Lyon, France) and harvested by suspending a single colony in RPMI-1640, washed twice, counted on a haemocytometer and adjusted to the desired concentration. The C. neoformans yeast cells were killed before use by autoclaving.
Preparation of peripheral blood monocytes
Heparinized venous blood, obtained from healthy donors, was diluted with RPMI-1640. Peripheral blood mononuclear cells (PBMC) were separated by density gradient centrifugation on Ficoll–Hypaque.21 The cells at the interface were washed twice in RPMI-1640, placed into cell culture Petri dishes (Nunc Inter Med, Roskilde, Denmark) at a concentration of 2×106–3×106/ml in RPMI-1640 supplemented with 5% FBS and 100 U penicillin/ml and 100 μg of streptomycin/ml, and incubated at 37° in 5% CO2 for 1 hr. The non-adherent cells were removed by washing the dishes three to five times with warm RPMI-1640. Adherent cells were carefully recovered with a rubber policeman. The harvested cells were 95–98% esterase positive and >98% viable as evaluated by trypan blue dye exclusion. Non-adherent cells were E rosetted as previously described.4 The cells recovered were T lymphocytes [(E+), >98% CD3+], as evaluated by flow cytometry analysis.
Lymphocyte proliferation assay
Monolayers of PBMC (2×104/cells) adherent to flat-bottomed 96-well plates were incubated with or without heat-inactivated C. neoformans (2×105) for 24 hr at 37° in 5% CO2 in RPMI-1640 plus 10% HS, and used throughout as APC. Then the PBMC monolayers were washed to remove unbound micro-organisms. Subsequently, autologous T(E+) cells (1×105) in RPMI-1640 plus 10% HS were added to the cultures. At various days, the cultures were pulsed for 18 hr with 0·5 μCi [3H]thymidine (Amersham International, Amersham, UK); thereafter the cells were collected onto filter paper using a cell harvester (Flow Laboratories, McLean, VA). The dried filters were counted directly in a β-scintillation counter (Packard Instruments, Downers Grove, IL). Proliferation was expressed as mean values of indicated replicates±standard error (SE).
Flow cytometry analysis
Human monocytes (1×106) in RPMI-1640 containing 10% HS were incubated for 18 hr with cells of encapsulated strain 6995 [effector-to-target ratio (E:T) = 1:3] or acapsular strain 7698 (E:T = 1:3) in polycarbonate tubes. After treatment, the monocytes were recovered by washing (1500 g for 10 min) twice in phosphate-buffered saline (PBS) containing 0·5% bovine serum albumin and 0·4% sodium azide. The monocytes were mixed with 80 μl of FITC-conjugated mAb to CD86 (1:100) or the irrelevant FITC-conjugated, isotype-matched anti-rabbit IgG1. After 30 min of incubation on ice, the cells were washed twice and then stained for 30 min with PE-conjugated antibody to CD14. For each sample, B7-2 expression was measured on the surface of CD14-positive cells using a fluorescence-activated cell sorter (FACS; Becton Dickinson, San Jose, CA). Human monocytes were also analysed for MHC class II expression. Monocytes (1×106) were challenged with encapsulated strain 6995 or acapsular C. neoformans (7698) with or without GXM (250 μg/ml) at an E:T ratio of 1:2 in the presence or absence of mAb 2H1 (10 μg/ml). After 18 hr of incubation, monocytes were recovered, washed in PBS containing 0·5 mm ethylenediamine tetraacetic acid (EDTA), and fixed with 3% paraformaldehyde for 10 min at room temperature. To stain the intracellular pool of MHC class II molecules, the cells were permeabilized for 10 min at room temperature with HEPES-buffered PBS containing 0·1% saponin (Sigma) and stained with FITC-labelled antibody to human MHC class II (Ancell Corp., Bayport, MN) in HEPES-buffered PBS containing 0·1% saponin and 5% FCS as previously described.22
Cytokine determination
Cytokine levels in the culture supernatants were measured with a human enzyme-linked immunosorbent assay (ELISA) kit for human interferon-γ (IFN-γ) and IL-2 (Biosource International, Camarillo, CA) and for human IL-10 (Poiesys S.r.l., Padova, Italy).
RNA isolation
Total RNA was isolated by acid guanidine isothiocyanate–phenol–chloroform extraction as described.23 In brief, peripheral blood leucocytes (PBL, 5×106) were lysed with denaturing solution containing 4 mm guanidine isothiocyanate, 25 mm sodium citrate, 0·5% sarcosyl, 0·1 m 2-mercaptoethanol. Sequentially, 0·1 vol. 2 m sodium acetate (pH 4), 1 vol. of water-saturated phenol and 0·2 vol. of chloroform–isoamylic alcohol mixture (49:1) was added to the lysate and mixed thoroughly after the addition of each reagent. The final mixture was cooled for 15 min on ice and centrifuged at 10 000 g for 20 min at 4°. The aqueous phase was mixed with an equal volume of isopropanol and the RNA was sedimented by centrifugation (10 000 g for 30 min at 4°), washed in 75% ethanol, vacuum dried (10 min) and dissolved in diethyl pyrocarbonate (DEPC)-treated water. The amount of RNA was determined by spectrophotometry.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of cytokine mRNA and GAPDH mRNA
For each sample, 1 μg of total RNA was reverse transcribed in a volume of 20 ml RT buffer (Tris–HCl 1 m pH = 8·3, MgCl2 1 m, KCl 1 m, dithiothreitol 150 mm, dNTPs 25 mm) containing 20 U RNAsin, 1 μg oligo-dT primer and 200 U murine Moloney leukaemia virus (MMLV; Gibco BRL). The RT reaction was performed for 1 hr at 37° using a HYBAID OMN-E Thermocycler, and the samples were stored at −20° until PCR analysis was performed. The cDNA was amplified in the presence of 0·05 mm 5′ and 3′ primers (Stratagene, La Jolla, CA), 2 mm deoxynucleotides (Promega, Milan, Italy) and 0·5 U of Ampli Tac Polymerase (Pharmacia, Uppsala, Sweden) and 10× PCR buffer. The PCR was for 35 cycles as follows: 5 min denaturation at 94° and 5 min annealing at 60° and 1·5 min at 72°. Cytokine-specific primer pairs amplified GAPDH (600 bp), IFN-γ (501 bp) and IL-2 (457 bp) were provided by Stratagene. The primer pair sequences were: GAPDH sense, CCACCCATGGCAAATTCCATGGC; GAPDH antisense, TCTAGACGGCAGGTCAGGTCCACC; IFN-γ sense, ATGAAATATACAAGTTATATCTTGGCTTT; IFN-γ antisense, GATGCTCTTCGACCTCGAAACAGCAT; IL-2 sense ATGTACAGGATGCAACTCCTGTCTT; IL-2 antisense GCTAGTGTTGAGATGATGCTTTGAC.
PCR products were analysed by electrophoresis on 2% agarose gels stained with ethidium bromide to quantify the size of the banding pattern, 0·5 μg of 100 bp DNA ladder (Gibco BRL) was run in parallel. Gels were scanned on a densitometer and analysed using Molecular Analyst.
RESULTS
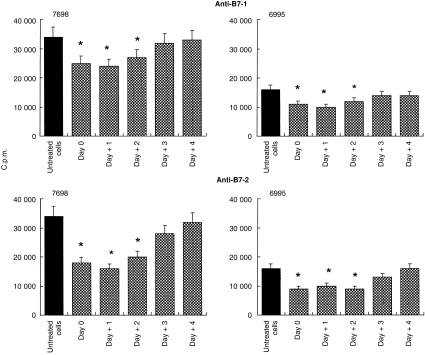
In a first series of experiments, we determined whether the B7 signal was involved in the initiation of T-cell activation or whether B7 ligation also regulated later phases of the TCR to cryptococcal cells. To this end, Cryptococcus-laden monocytes were cocultured with autologous T cells. Under these experimental conditions, 18–25% of monocytes phagocytosed encapsulated C. neoformans and 40–60% phagocytosed acapsular C. neoformans. Anti-B7-1 or anti-B7-2 mAbs were added at the time of culture preparation (day 0) or on days +1, +2, +3, and +4 after initiation of the culture, and lymphoproliferation was measured. The results (Fig. 1) show that the mAb to B7-2 significantly reduced the T-lymphocyte proliferative response to both encapsulated and acapsular cryptococci if the antibody was added at any time during the first 2 days after culture preparation. The time–course of the inhibitory effect mediated by the mAb to B7-1 was similar to that observed using mAb to B7-2. However, mAb to B7-1 inhibited T-cell activation to a lesser extent than mAb to B7-2 (Fig. 1). Since the inhibitory effect of antibody to B7-2 was greater than the effect of antibody to B7-1, most subsequent experiments focused on the action of the mAb to B7-2.
Figure 1.
Effect of mAb to B7-1 or B7-2 on the T-cell proliferative response to monocytes treated with acapsular (7698) or encapsulated (6995) C. neoformans (E:T ratio 1:2). The mAb to B7-1 or B7-2 (2 μg/ml) was added at the time of coculture preparation (monocytes plus autologous T lymphocytes, day 0) or at various days (+1, +2, +3, +4) after culture initiation. The determination of lymphoproliferation was performed on day 7 of culture. Values represent the mean c.p.m.±SE of six separate experiments from six different donors. In all determinations c.p.m. values of unstimulated cells were always < 2000. *P < 0·05 (mAb to B7-1 or to B7-2 treated versus respective untreated cells).
The IgG1 mAb to GXM known as 2H1 has been shown to augment host resistance to C. neoformans in several experimental models24–26 and to promote granulomatous inflammatory responses. In vitro, addition of mAb 2H1 to suspensions of monocytes, T lymphocytes and C. neoformans enhanced lymphoproliferation7 and promoted B7-1 expression on monocytes.17 Since mAb 2H1 is a potent opsonin for C. neoformans and doubled the phagocytic capacity of monocytes towards encapsulated C. neoformans in our experimental system, we considered the possibility that antibody-mediated opsonization could reverse the suppression of lymphoproliferation which results from the treatment of cells with mAb to B7-2. Consequently, we examined the ability of mAb 2H1 to restore mAb to B7-1- or B7-2-mediated inhibition of T-cell proliferation.
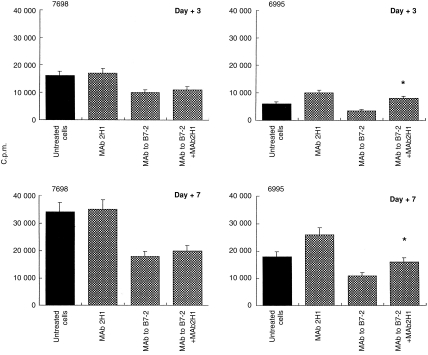
The addition of mAb to B7-2 produced the expected decrease of lymphoproliferation in response to encapsulated or acapsular cryptococci alone (Fig. 2). The presence of mAb 2H1 reversed the inhibitory effect for encapsulated cryptococci, but had no effect on the mAb to B7-2-mediated suppression of the proliferative response to acapsular cryptococci. In a similar manner, mAb 2H1 restored the lymphoproliferative capacity of cells treated with mAb to B7-1 (data not shown).
Figure 2.
Effect of anticapsular antibody on the proliferative response of T lymphocytes to C. neoformans. Monocytes were treated with acapsular (7698) or encapsulated (6995) C. neoformans (E:T ratio 1:2) in the presence or absence of anti-GXM mAb 2H1 (10 μg/ml), mAb to B7-2 (2 μg/ml), or mAb to B7-2 (2 μg/ml) plus mAb 2H1 (10 μg/ml). Proliferation was measured on days 3 and 7 of culture. The c.p.m. represents the mean±SE of four separate experiments from four different subjects. In all determinations c.p.m. values of unstimulated cells were always < 2000. *P < 0·05 (mAb to B7-2 plus mAb 2H1-treated versus mAb to B7-2-treated cells).
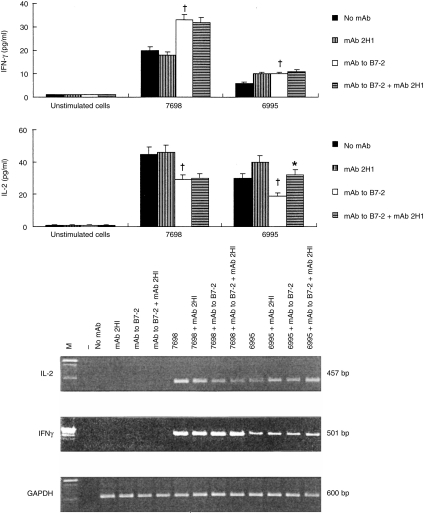
It has been reported that over-expression of B7-1 and/or B7-2 is able to regulate IFN-γ production by human peripheral blood T cells cultured in the presence of P-815 cells transfected with human CD80 or CD86.8 However, reports differ on the role of B7-1 or B7-2 CS signals to help in the generation of a Th1- or Th2-type response, respectively.14–16 We addressed the contribution of B7-1 or B7-2 in driving the T-cell phenotype by blocking either molecule with specific mAb. An increase in IFN-γ secretion was observed when mAb to B7-2 was used (Fig. 3). In particular, production of IFN-γ was consistently enhanced when the acapsular (7698) strain was used (P < 0·01). In the presence of the encapsulated strain 6995, IFN-γ secretion was also augmented but to a lesser extent (P < 0·05, Fig. 3). As previously observed, mAb 2H1 was able to augment IFN-γ secretion by T cells responding to encapsulated C. neoformans27,28 but no synergistic effect was observed with mAb to B7-2 in induction of IFN-γ.
Figure 3.
IFN-γ and IL-2 production (upper panel) by PBMC (2×106/ml) unstimulated or stimulated with acapsular (7698) or encapsulated (6995) C. neoformans (E:T ratio 1:2) in the presence or absence of mAb 2H1 (10 μg/ml), mAb to B7-2 (2 μg/μl), or mAb to B7-2 (2 μg/ml) plus mAb 2H1 (10 μg/ml). The mAbs were added at the time of culture preparation. IFN-γ and IL-2 were determined in supernatant fluids after 7 days of culture. The results are the means±SE of four separate experiments from four different donors. *P < 0·05 (mAb to B7-2 versus no mAb) †P < 0·05 (mAb to B7-2 plus mAb 2H1 versus mAb to B7-2-treated cells). Analysis of IL-2 and IFN-γ gene expression (lower panel) by PBMC cultured for 7 days unstimulated (no mAb) or stimulated as indicated. –, No DNA was added to amplification mixture during PCR. M, DNA markers. The results are from one representative experiment of three performed.
Consistent with inhibition of lymphoproliferation, IL-2 production decreased when mAb to B7-2 was added to culture (P < 0·01). The addition of mAb 2H1 to encapsulated cryptococci partially restored the down-regulation of IL-2 induced by mAb to B7-2 (P < 0·01, Fig. 3). Indeed, mAb to B7-2 inhibited T-cell proliferation in response to encapsulated and acapsular cryptococci and this inhibition was completely reversed by addition of 20 U/ml of exogenous IL-2 (data not shown). In agreement with our previous observations, addition of mAb 2H1 induced a significant increase of IL-2 from T lymphocytes when the encapsulated strain was used.7 The molecular events responsible for IFN-γ and IL-2 modulation caused by addition of mAb to B7-2 were further assessed by RT-PCR. As illustrated in Fig. 3, analysis of mRNA from cells cultured with Cryptococcus-laden monocytes in the presence of mAb to B7-2 showed that IL-2 inhibition occurs at the transcriptional level. In contrast, enhancement of IFN-γ mRNA expression was observed in the same experimental conditions.
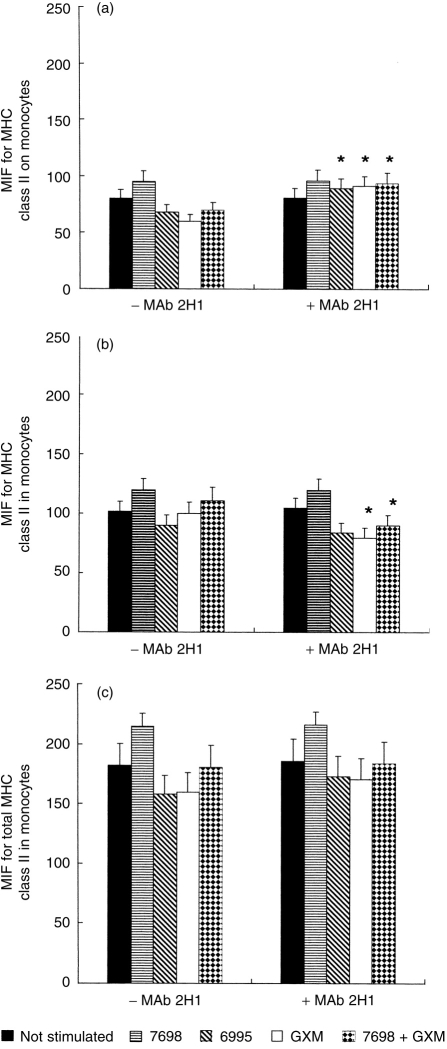
To further investigate the mechanism by which mAb 2H1 restored the effects produced by inhibiting B7-2, we evaluated the effect of mAb 2H1 on MHC expression. Since C. neoformans has been shown to down-regulate MHC class II molecule expression on monocytes through a polysaccharide-related mechanism, we hypothesize that mAb 2H1 may reverse this effect by binding to the capsule and serving as an opsonin. To evaluate MHC class II expression on monocytes, mAb was added to cocultures of encapsulated (strain 6995) and acapsular (7698) C. neoformans and monocytes, and MHC class II expression was measured as both surface and total expression. In the absence of mAb 2H1, MHC class II expression is reduced when monocytes are cultured with the encapsulated strain, GXM, or the acapsular strain plus GXM (Fig. 4a). However, addition of mAb 2H1 reversed this phenomenon for all three conditions (Fig. 4a). To establish whether the mAb 2H1 effect was due to enhanced synthesis of MHC class II or to a redistribution of the intracellular pool of these molecules, we measured both surface and total expression and obtained a measure of the intracellular pool by subtracting surface mean fluorescence intensity from total mean fluorescence (Fig. 4c) in the presence and absence of mAb 2H1 (Fig. 4b). In the presence of mAb 2H1 there was a consistent up-regulation of MHC class II on monocytes with simultaneous down-regulation of these molecules in the intracytoplasmic pool. Although small, the effect is consistent with increased translocation of MHC class II to the cellular periphery.
Figure 4.
MHC class II expression on the surface (a), inside (b) and in whole (c) monocytes exposed or not to acapsular (7698) or encapsulated (6995) C. neoformans (E:T ratio 1:2), to GXM (250 μg/ml) or to the acapsular strain (7698) plus GXM (250 μg/ml) in the presence or absence of mAb 2H1 (10 μg/ml) for 18 hr. The intracellular pool of MHC class II expression was determined by subtracting the surface mean fluorescence intensity from the total mean fluorescence in the presence and absence of mAb 2H1. The results are the mean±SE of three separate experiments from three different donors. *P < 0·05 (mAb 2H1-treated versus untreated cells).
DISCUSSION
This study was designed to determine the potential role of CS molecules, B7-1 and B7-2, in the induction and maintenance of T-cell activation and in promoting Th1 cell development to C. neoformans. Expression of B7-1 and B7-2 is necessary for T-cell activation and the paucity of these CS molecules has been implicated in immune evasion of tumour cells.29 The manipulation of B7 molecules and their ligands on T lymphocytes has been proposed as an efficient tool for therapeutic intervention in parasitic30,31 as well as autoimmune disease.14–16,32
The results reported here show that optimal C. neoformans-induced T-cell activation is dependent on availability on the APC of CS molecules, B7-1 and B7-2. Blockade of B7 molecules by antibodies reactive with B7-1 and B7-2 reduced the T-cell proliferative response to both encapsulated and acapsular cryptococci. The contribution of B7 molecules to the T-cell lymphoproliferative response appears to be more important for B7-2 than B7-1 molecules. Despite the fact that B7–CD28 is important in T-cell proliferation, as shown by the significant reduction in proliferation produced by blocking B7 molecules, T-cell proliferation was never completely abrogated. This residual response suggests that a subpopulation of T cells may not require the B7 signal to respond to C. neoformans. Alternatively, CS molecules other than B7 could be involved.
Our data provide evidence for the involvement of B7 both at the beginning of T-cell activation and also at later stages. This conclusion is based on our observation that T-cell proliferation is significantly decreased if the B7 blocking mAb is added up to 48 hr after coculture of yeast-stimulated monocytes and T cells. As a consequence, the prolonged interaction of B7–CD28 plays a pivotal role in triggering and maintaining the T-cell response.
Both B7-1 and B7-2 molecules have been shown to modify the T-cell cytokine profile promoting Th1 and Th2 generation, respectively.33,34 Our results show that by blocking B7-2 there is a consistent increase of IFN-γ confirming that blocking B7-2 favours Th1 development14 and strongly suggest that measures which influence B7 molecule expression could help in the development of protective Th1 responses against C. neoformans.35
A dichotomous effect in IL-2 and IFN-γ production was observed when B7-2 molecules were blocked by specific mAb; IL-2 was inhibited and IFN-γ was enhanced. The molecular analysis showed that regulation of IL-2 and IFN-γ secretion occurred at the transcriptional level for both cytokines. A GXM-binding mAb selectively bypassed the mAb to B7-2-mediated inhibitory effect on both T-cell proliferation and IL-2 production implying stimulation of an alternative activation pathway.
Having previously established that GXM-binding mAb has the capacity to enhance IL-2 and facilitate T-cell proliferation,7 we considered the possibility that addition of mAb 2H1 to the system might reverse the T-cell suppression mediated by mAb specific for B7-2. Such a reversal did, in fact, occur. The mAb 2H1 partially reversed the suppressive effect of mAb to B7-2 on IL-2 and T-cell activation. This correlated with the augmented internalization of encapsulated C. neoformans by monocytes suggesting a potential involvement of the phagocytic process in the observed phenomenon. However, mAb 2H1 had no effect on IFN-γ production, suggesting that in this system the increased proliferation of T cells and IL-2 secretion did not necessarily correlate with their capacity to secrete IFN-γ.
The mechanism by which antibody reactive with the cryptococcal capsule could partially reverse the suppressive effect of a mAb reactive with B7-2 could be due, at least in part, to the capability of mAb 2H1 to up-regulate B7-117 and MHC class II expression. This is consistent with the ability of mAb to B7-117 as well as mAb to MHC class II molecules5 to reduce lymphoproliferation. Our results suggest that the increase in MHC class II expression mediated by mAb 2H1 is attributable, in part, to increased translocation of the intracellular pool of MHC class II molecules to the cellular periphery. The observation that the addition of mAb 2H1 and GXM was sufficient to increase expression of MHC class II molecules on surface monocytes further suggests that this effect can be caused by immune complexes alone. Complexes may be able to trigger increased MHC expression directly by stimulating Fc receptors.36 Alternatively, mAb 2H1 has been shown to promote IFN-γ release from suspensions of lymphocytes, monocytes and C. neoformans; IFN-γ is known to enhance expression of MHC class II molecules.37
The demonstration that a pathogen-specific antibody can affect the regulation of MHC class II molecules is a novel function for humoral immunity and suggests another potential mechanism for synergy between humoral and cellular immunity. In this regard, it is noteworthy that administration of anti-GXM mAbs to mice with C. neoformans infection has been associated with a more intense granuloma formation;38 the enhanced expression of MHC class II molecules observed in this study may contribute to this effect.
In conclusion, our results provide direct and unequivocal evidence for the involvement of B7 molecules in the induction of T-cell responses to C. neoformans antigens. The findings that mAbs to GXM and B7-2 could affect T-cell proliferation and IL-2 secretion suggest that some combinations of these antibodies could be used for optimal T-cell activation. For example, it may be possible to direct a T-cell response towards Th1 by modulating the activation of B7 molecules. In this regard, blocking of B7-2 in T cells produced more IFN-γ but not more IL-2, whereas addition of mAb 2H1 partially reversed these effects. The results emphasize the critical role of B7 molecules in the triggering, establishment, and maintenance of the T-cell response to C. neoformans and provide encouragement for the plans to use antibody therapy in the treatment of cryptococcal infections.
Acknowledgments
The authors are grateful to Eileen Mahoney Zannetti for excellent and editorial assistance. This study was supported by the National Research Program on AIDS ‘Opportunistic Infections and Tuberculosis’ contract 50A.0.35, Italy and by United States Public Health Service grant AI 14209 from the National Institute of Allergy and Infectious Diseases (T. R. Kozel).
References
- 1.Kwong-Chung KJ, Bennett JE. Medical mycology. Malvern, PA: Lea and Febinger; 1992. p. 397. [Google Scholar]
- 2.Cherniak R, Sundstrom JB. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun. 1994;62:1507. doi: 10.1128/iai.62.5.1507-1512.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozel TR, Gotschlich E. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast macrophages. J Immunol. 1982;129:1675. [PubMed] [Google Scholar]
- 4.Vecchiarelli A, Dottorini M, Pietrella D, et al. Role of alveolar macrophages as antigen presenting cells in Cryptococcus neoformans infection. Am J Respir Cell Mol Biol. 1994;11:130. doi: 10.1165/ajrcmb.11.2.8049074. [DOI] [PubMed] [Google Scholar]
- 5.Vecchiarelli A, Pietrella D, Dottorini M, et al. Encapsulation of Cryptococcus neoformans regulates fungicidal activity and the antigen presentation process in human alveolar macrophages. Clin Exp Immunol. 1994;98:217. doi: 10.1111/j.1365-2249.1994.tb06128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Retini C, Vecchiarelli A, Monari C, Bistoni F, Kozel TR. Encapsulation of Cryptococcus neoformans with glucuronoxylomannan inhibits the antigen-presenting capacity of monocytes. Infect Immun. 1998;66:664. doi: 10.1128/iai.66.2.664-669.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vecchiarelli A, Retini C, Monari C, Casadevall A. Specific antibody to Cryptococcus neoformans alters human leukocyte cytokine synthesis and promotes T-cell proliferation. Infect Immun. 1998;66:1244. doi: 10.1128/iai.66.3.1244-1247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Gool SW, Vandenberghe P, de Boer M, Ceuppens JL. CD80, CD86 and CD40 provide accessory signals in a multiple-step T-cell activation model. Immunol Rev. 1996;153:47. doi: 10.1111/j.1600-065x.1996.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 10.Natesan M, Razi-Wolf Z, Reiser H. Costimulation of IL-4 production by murine B7-1 and B7-2 molecules. J Immunol. 1996;156:2783. [PubMed] [Google Scholar]
- 11.Levine B, Ueda L, Craighead N, Huang ML, June CH. CD28 ligands CD80 (B7-1) and CD86 (B7-2): induce long-term autocrine growth of CD4+ T cells induce similar patterns of cytokine secretion in vitro. Int Immunol. 1995;7:891. doi: 10.1093/intimm/7.6.891. [DOI] [PubMed] [Google Scholar]
- 12.Freeman GJ, Boussiotis VA, Anumanthan A, et al. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 13.Ranger AM, Das MP, Kuchroo VK, Glimcher LH. B7-2 (CD86) is essential for the development of interleukin-4 producing T cells. Int Immunol. 1996;8(Suppl. 10):1549. doi: 10.1093/intimm/8.10.1549. [DOI] [PubMed] [Google Scholar]
- 14.Kuchroo VK, Das MP, Brown JA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 development pathways: application to autoimmune disease therapy. Cell. 1995;80:707. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 15.Racke MK, Scott DE, Quigley L, et al. Distinct roles for B7-1 (CD80) and B7-2 (CD86): in the initiation of experimental allergic encephalomyelitis. J Clin Invest. 1995;96:2195. doi: 10.1172/JCI118274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenschow DJ, Ho SC, Sattar H, et al. Differential effect of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vecchiarelli A, Monari C, Retini C, et al. Cryptococcus neoformans differently regulates B7-1 (CD80) and B7-2 (CD86): expression on human monocytes. Eur J Immunol. 1998;28:114. doi: 10.1002/(SICI)1521-4141(199801)28:01<114::AID-IMMU114>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Casadevall A, Mukherjee J, Devi SJN, Schneerson R, Robbins JB, Scharff MD. Antibodies elicited by a Cryptococcus neoformans glucuronoxylomannan-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis. 1992;65:1086. doi: 10.1093/infdis/165.6.1086. [DOI] [PubMed] [Google Scholar]
- 19.Cherniak R, Reiss E, Turner SH. A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr Res. 1982;103:239. [Google Scholar]
- 20.Cherniak R, Reiss E, Slodki ME, Plattner RD, Blumer SO. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans. Mol Immunol. 1980;17:1025. doi: 10.1016/0161-5890(80)90096-6. [DOI] [PubMed] [Google Scholar]
- 21.Monari C, Retini C, Palazzetti B, Bistoni F, Vecchiarelli A. Regulatory role of exogenous IL-10 in the development of immune response versus Cryptococcus neoformans. Clin Exp Immunol. 1997;109:242. doi: 10.1046/j.1365-2249.1997.4021303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 23.Netea MG, Drenth JPH, De Bont N, et al. A semi-quantitative reverse transcriptase polymerase chain reaction method for measurement of mRNA for TNF-α and IL-1β in whole blood cultures: its application in typhoid fever and exentric exercise. Cytokine. 1996;8:739. doi: 10.1006/cyto.1996.0098. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee J, Scharff MD, Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun. 1992;60:4534. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:4211. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldmesser M, Kress Y, Casadevall A. Effect of antibody to capsular polysaccharide on eosinophilic pneumonia in murine infection with Cryptococcus neoformans. J Infect Dis. 1998;177:1639. doi: 10.1086/515314. [DOI] [PubMed] [Google Scholar]
- 27.Retini C, Casadevall A, Pietrella D, Monari C, Palazzetti B, Vecchiarelli A. Specific activated T cells regulate interleukin-12 production by human monocytes stimulated with Cryptococcus neoformans. J Immunol. 1999;162:1618. [PubMed] [Google Scholar]
- 28.Larsen CP, Ritchie SC, Hendrix R, et al. Regulation of immunostimulatory function and costimulatory molecule (B7-1 and B7-2) expression on murine dendritic cells. J Immunol. 1994;152:5208. [PubMed] [Google Scholar]
- 29.Ohtani H, Naito Y, Saito K, Nagura H. Expression of costimulatory molecules B7-1 and B7-2 by macrophages along invasive margin of colon cancer: a possible antitumor immunity? Lab Invest. 1997;77:231. [PubMed] [Google Scholar]
- 30.McCoy K, Camberis M, Le Gros G. Protective immunity to nematode infection is induced by CTLA-4 blockade. J Exp Med. 1997;186:183. doi: 10.1084/jem.186.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frosch S, Kuntzlin D, Fleischer B. Infection with Trypanosoma cruzi selectively upregulates B7-2 molecules on macrophages and enhances their costimulatory activity. Infect Immun. 1997;65:971. doi: 10.1128/iai.65.3.971-977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daikh D, Wosfy D, Imboden JB. The CD28–B7 costimulatory pathway and its role in autoimmune disease. J Leukoc Biol. 1997;62:156. doi: 10.1002/jlb.62.2.156. [DOI] [PubMed] [Google Scholar]
- 33.Creery WD, Diaz-Mitoma F, Filion L, Kumar A. Differential modulation of B7-1 and B7-2 isoform expression on human monocytes by cytokines which influence the development of T helper cell phenotype. Eur J Immunol. 1996;26:1273. doi: 10.1002/eji.1830260614. [DOI] [PubMed] [Google Scholar]
- 34.Nakajima A, Watanabe N, Yoshino S, Yagita H, Okumura K, Azuma M. Requirement of CD28–CD86 co-stimulation in the interaction between antigen-primed T helper type 2 and B cells. Int Immunol. 1997;9(Suppl. 5):637. doi: 10.1093/intimm/9.5.637. [DOI] [PubMed] [Google Scholar]
- 35.Hoag KD, Lipscomb MF, Izzo AA, Street NE. IL-12 and IFN-γ are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Biol. 1997;17:733. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 36.De La Salle H, Galon J, Bausinger H, et al. Soluble CD16/Fc gamma RIII induces maturation of dendritic cells and production of several cytokines including IL-12. Adv Exp Med Biol. 1997;417:345. doi: 10.1007/978-1-4757-9966-8_56. [DOI] [PubMed] [Google Scholar]
- 37.Woodroofe MN, Hayes GM, Cuzner ML. Fc receptor density, MHC antigen expression and superoxide production are increased in interferon-gamma-treated microglia isolated from adult rat brain. Immunology. 1989;68:421. [PMC free article] [PubMed] [Google Scholar]
- 38.Feldmesser M, Casadevall A. Effect of serum IgG1 against murine pulmonary infection with Cryptococcus neoformans. J Immunol. 1997;158:790. [PubMed] [Google Scholar]