Abstract
Intraepithelial lymphocytes (IEL) utilize the integrin αeβ7 on their surface to bind to E-cadherin on epithelial cells in the gut and breast. In oral mucosa and skin IEL express αeβ7 and the cutaneous lymphocyte-associated antigen (CLA) but the mechanisms of adhesion of these subsets to keratinocytes are unknown. Levels of αeβ7 and CLA were up-regulated on peripheral blood lymphocytes (PBL) by transforming growth factor-β (TGF-β) and interleukin-12 (IL-12), respectively, and both groups of lymphocytes adhered onto oral and skin keratinocytes. Adhesion of IL-12-activated PBL was totally abolished by anti-lymphocyte-associated function antigen type 1 (anti-LFA-1) antibodies but was unaffected by anti-αeβ7 antibodies indicating that adhesion of the CLA-positive subset is mediated via LFA-1 interaction with intercellular adhesion molecule-1 (ICAM-1). Adhesion of TGF-β-activated PBL to E-cadherin-positive oral and skin keratinocytes was partially inhibited by anti-αeβ7 antibodies but was unaffected by the blocking antibody E4.6 against E-cadherin which detects the binding site for αeβ7-positive lymphocytes in breast and gut epithelium. TGF-β-activated PBL also bound to an E-cadherin-negative oral keratinocyte cell line and adhesion was inhibited by anti-αeβ7 antibodies. These results strongly suggest that in oral epithelium and epidermis αeβ7-positive lymphocytes do not bind to E-cadherin and there may be a novel second ligand for the αeβ7 integrin.
INTRODUCTION
Lymphocytes are distributed widely throughout the body and are found in organized lymphoid masses as well as at extralymphoid sites, such as oral mucosa, skin and lung. In the gastrointestinal tract, intraepithelial lymphocytes (IEL) are a distinct cell population with a characteristic surface profile which differs from those found in the lamina propria and peripheral blood.1 In particular these lymphocytes are CD8+, CD45RO+, show a restricted T-cell receptor repertoire2 and express high levels of the integrin αeβ7.3 In culture the surface profile of IEL changes and expression resembles that of peripheral blood lymphocytes (PBL). However, transforming growth factor-β (TGF-β) restores the unique integrin profile of IEL by up-regulating αeβ7 and is able to do the same on PBL.4 It has been postulated that lymphocytes entering the gastrointestinal tract from the peripheral blood do so via an interaction of α4β7 on their surface with the addressin mucosal-associated cell adhesion molecule (MAdCam) on endothelial cells.5–7 Subsequent to migration, the α4 subunit is down-regulated and αe is up-regulated under the influence of TGF-β in the microenviroment of the intestine.4,8
The role of the αeβ7 integrin in gut epithelium has been the subject of recent research and there is evidence to suggest that it functions as an adhesion molecule and interacts with E-cadherin on the enterocyte surface. Adhesion to both breast and gut carcinoma cell lines can be inhibited by antibodies to αeβ74,9,10 and E-cadherin,11,12 and IEL adhere to cells transfected with E-cadherin.11 In mice, the E-cadherin epitope for αeβ7 binding lies on domain 1 and is distinct from that mediating homotypic E-cadherin binding.13
The oral mucosa forms part of the gastrointestinal tract but, like the skin, it is covered by stratified squamous epithelium and contains fewer lymphocytes than the intestine. In normal oral mucosa and skin between one-half and two-thirds of IEL are αeβ7-positive but numbers are greatly increased in disease and in oral lichen planus almost all IEL are αeβ7-positive.14 Although this increase is not seen in lichen planus-affected skin,14 epidermotropism in cutaneous T-cell lymphomas 15 and a variety of inflammatory dermatoses has been associated with expression of αeβ7 by IEL.16 These findings raise the possibility that in the oral mucosa and skin, as well as the intestine, αeβ7 functions as an adhesion molecule to retain lymphocytes within the epithelium.
A proportion of IEL in oral mucosa and skin also express the cutaneous lymphocyte-associated antigen (CLA).14,17,18 Expression of CLA defines a population of PBL that are thought to migrate selectively into skin from peripheral blood via an interaction with E-selectin on the surface of vascular endothelial cells.19–21 Whether these lymphocytes use the same mechanism of adhesion to bind to oral and skin keratinocytes is not known and although there have been some reports of E-selectin expression by oral keratinocytes22 there have been none to our knowledge of such expression by skin keratinocytes. Lymphocytes which express CLA have high surface levels of LFA-1 (lymphocyte function-associated antigen 1)23 and an interaction between LFA-1 and keratinocyte intercellular adhesion molecule-1 (ICAM-1) has been shown to be important in adhesion of activated PBL to epidermis.24 It is therefore possible that such an interaction plays a role in adhesion of CLA-positive lymphocytes.
The purpose of this study was to determine the mechanism of adhesion of CLA-positive and αeβ7-positive lymphocytes to oral and skin keratinocytes. We report that CLA-positive lymphocytes adhere to oral and skin keratinocytes via a LFA-1/ICAM-1 interaction and that, although αeβ7-positive lymphocytes do adhere to oral and skin keratinocytes via αeβ7, its ligand does not appear to be E-cadherin and there may be a novel second ligand in oral mucosa and skin.
MATERIALS AND METHODS
Monoclonal antibodies
Mouse anti-human αeβ7 (CD103) unconjugated (Serotec, Oxford, UK); rat anti-mouse fluorescein isothiocyanate (FITC) -conjugated immunoglobulin G (IgG; Serotec, Oxford, UK); rat anti-human CLA IgM (HECA-452), and FITC-conjugated negative control rat IgM (Professor L. J. Picker, University of Texas, Dallas, TX); mouse IgG2a FITC-conjugated (Serotec, Oxford, UK); E4.6 mouse anti-E-cadherin specific for the αeβ7 binding epitope11 (Dr M. Brenner, Boston, MA); HECD-1 mouse anti-E-cadherin, non-blocking (R&D Systems, Abingdon, Oxon, UK); 3S3 mouse anti-β1 integrin (Serotec, Oxford UK); BerACT8 mouse anti-αeβ7 (Dako, Cambridge, UK) and BBIG-E4 mouse anti-E-selectin (R&D Systems, Oxon, UK). TS 1/18 (mouse anti-LFA-1) and 6.5B5 (mouse IgG anti ICAM-1) were both used as protein A-purified and concentrated hybridoma supernatants from cell lines TS1/18 [American Type Culture Collection (ATCC), Rockville, MD]25 and 6.5B5 (Professor D. O. Haskard, Imperial College of Medicine, London, UK),26 respectively. Secondary antibodies were biotinylated rabbit anti-mouse or horseradish peroxidase goat anti-mouse (Biorad, Hemel Hempstead, UK) and FITC-conjugated rabbit anti-mouse antibody (Dako, Cambridge, UK).
Cells
UP, a human skin keratinocyte cell line (gift from Dr F. M. Watt, International Cancer Research Fund, London, UK), H357 and H376 oral squamous cell carcinoma cell lines (gift of Prof. S. S. Prime, Bristol Dental School, UK), the breast epithelial cell line 16E6.A5 (gift of Dr V. Band, Tufts University, New England Medical Centre, Boston, MA) and the MCF7-breast adenocarcinoma cell line (ATCC, Manassas, VA), were cultured at 37°/5% CO2 in full keratinocyte growth medium (KGM) containing Dulbecco’s modified Eagle’s medium/HamF-12 medium (DMEM), adenine 1·8×10−4 m, hydrocortisone 0·4 μg/ml, insulin 5 μg/ml, cholera toxin 10−10 m, epidermal growth factor 10 μg/ml, transferrin 5 μg/ml (all Sigma, Poole, UK), fungizone 2·5 μg/ml (Life Technologies, Paisley, UK) and 10% fetal bovine serum (FBS; Globepharm, Guildford, UK).
Lymphocytes were separated from buffy coats (National Blood Transfusion Service, Brentwood, UK) as described previously.27 In brief, mononuclear cells were removed by density gradient centrifugation, washed and incubated in Petri dishes for 45 min at 37°/5°CO2 in RPMI-1640 (Life Technologies, Paisley, UK) and 10% FBS. Non-adherent cells were washed and rosetted with S-aminoethyl-isothiouronium hydrobromide (AET)-treated (Sigma) sheep red blood cells (TCS, Botelan, Claydon, UK). Rosettes were separated by density gradient centrifugation and the red cells were lysed. Isolated T cells were resuspended in RPMI-1640 plus 10% heat-inactivated FBS and were cultured at 0·5×106 cells/ml for 5–6 days at 37° RPMI-1640 and 10% FBS containing either 1 μg/ml phytohaemagglutinin (PHA-M; Sigma) and 20 U/ml IL-2 (R&D Systems, Oxon, UK) (PHA-activated); PHA-M, IL-2 and 5 ng/ml TGF-β (R&D Systems, Oxon, UK) to up-regulate αeβ74 (TGF-β-activated), or PHA-M, IL-2 and 0·2 ng/ml IL-12 (R&D Systems, Oxon, UK) to up-regulate CLA28 (IL-12-activated).
Flow cytometry
To confirm up-regulation of αeβ7 and CLA, T lymphocytes were washed in phosphate-buffered saline (PBS; Oxoid, Basingstoke, UK), resuspended at 1×106 cells/ml and incubated with either 10 μl of anti-αeβ7 antibody or 20 μl of anti-CLA antibody on ice for 30 min. The T-cell aliquots were washed with PBS and 10 μl of secondary antibody added and incubated for 30 min on ice. The cells were washed twice in PBS, resuspended and fixed with freshly prepared 0·5 ml of 1% paraformaldehyde (Sigma) in PBS. Cells were acquisitioned using a fluorescence-activated cell sorter and lysys ii software (FACSort Becton Dickinson San José, CA). Forward- and side-scatter gates were set to identify lymphocytes and 10 000 events were acquired.
In all experiments the proportion of CLA-positive lymphocytes was between 42 and 55% and the proportion of αeβ7-positive lymphocytes was between 35 and 51%.
Expression of E-cadherin on the surface of H376 and H357 epithelial cell lines was determined by flow cytometry. Subconfluent cells were washed twice in PBS containing 1 mm CaC12 (before harvesting with 0·1% trypsin also containing 0·1 mm CaC12 Sigma). Cells were resuspended in 200 μl of wash buffer (PBS) with 0·1% heat-inactivated bovine serum albumin fraction V (BSA) (Sigma) and 0·1% azide (Sigma). Primary antibody (HECD1, 4:1000) was added for 1 hr on ice, the cells were washed twice and again resuspended in 200 μl wash buffer. The secondary, FITC-conjugated rabbit anti-mouse was added on ice for 1 hr at a dilution of 1 in 50. Cells were washed three times and resuspended in 0·5 ml wash buffer. Labelled cells were scanned on a FACS calibre cytometer (Becton-Dickinson, Oxford, UK) connected to an Apple Macintosh computer fitted with cellquest software, acquiring 1×104 events. The same settings were used for each experiment.
Cell-based immunosorbent assay (ELISA)
Expression of ICAM-1, E-cadherin and E-selectin on the epithelial cell lines was determined by cell-based enzyme-linked immunosorbent assay (ELISA). Epithelial cells were seeded at 4×104 cells/well into 96-well flat bottom plates in 200 μl KGM and grown to confluence overnight. They were washed in DMEM, incubated with 50 μl/well of primary monoclonal antibody at 37° for 30 min, washed three times in DMEM and fixed at 4° with 2% paraformaldehyde. The monolayers were washed and incubated at 37° for 1 hr with blocking solution containing 200 μl/ml of 1% BSA in Hanks’ buffered saline solution (Life Technologies, Paisley, UK). Epithelial cells were washed three times with 1% BSA in PBS and incubated for 30 min with 50 μl/well of biotinylated rabbit anti-mouse immunoglobulins or biotinylated goat anti-rat immunoglobulins (Dako, Cambridge, UK). They were washed and incubated for 30 min with 200 μl of avidin–biotin–horseradish peroxidase complex (Dako, Cambridge, UK), washed three times in PBS, and 200 μl of substrate solution containing O-phenylenediamine for 30 min at room temperature and read immediately at 450 nm on a microtitre plate reader.
Lysate preparation and Western Blotting
Cultures of H357, H376, UP, 16E6.A5 and MCF7 (control breast cell line for HECD-1) were established by seeding 4×105 cells into 35-mm diameter tissue culture plates (Corning, Stone, UK). After 24 hr the cultures were washed twice with ice-cold PBS and harvested on ice into 20 mm Tris–HC1 (pH 7·4) containing 1 mm ethylenediamine tetraacetic acid (EDTA), 1% Nonidet P-40, 1% aprotinin and 1 mm phenylmethylsulphonyl fluoride (all Sigma). Twenty-five micrograms were resolved by electrophoresis on 7·5% polyacrylamide minigels along with 10 μl of molecular weight markers (Kaleidoscope-Biorad, Hemel Hempstead, UK). Gel was blotted at 30 V overnight onto nitrocellulose membranes and transfer was confirmed using Ponceau S red 0·5% (Sigma) (w/v in 5% trichloroacetic acid). For immunoblotting, the nitrocellulose membranes were incubated for 1 hr with PBS containing soya milk (2%) and Tween-20 (0·02%) (Sigma). E-cadherin primary antibody was HECD-1 (R&D) and it was used at 1:200 dilution. The secondary antibody was horseradish peroxidase-conjugated goat anti-mouse (Biorad) at a 1:3000 dilution. Both antibodies were diluted in PBS/Tween and incubated for 2 and 1 hr, respectively, at room temperature. Immunoreactive bands were visualized using an enzyme-linked chemiluminescence (ECL) detection kit (Amersham, Little Chalfont, UK)
Adhesion assays
PHA-M- or TGF-β1- or IL-12-activated T lymphocytes (200 μl/well of 6×105/ml) were cocultured for 1 hr at 37° in duplicate wells in 96-well plates which contained either H357, H376, UP or 16E6.A5 cell lines. Prior to coculture aliquots of T cells were incubated with BerACT8, TS 1/18 or 3S3 antibodies and epithelial cell monolayers with E4.6 and HECD-1 antibodies for 15 min at 37°. The T lymphocytes and monolayers were washed with DMEM. Following coculture. non-adherent T lymphocytes were removed by washing four times with fresh RPMI-1640 and 10% FBS and cocultures were fixed with 70% alcohol for 30 min at room temperature (200 μl/well). They were stained with methylene blue (Sigma) for 5 min, the stain removed by aspiration and 200 μl of PBS added to each well. The number of adherent T lymphocytes was then quantified at ×320 magnification using phase contrast microscopy and a 10×10 ocular grid. Randomly selected and non-overlapping fields were counted and cumulative mean plots were calculated for each well. Percentage adhesion was calculated from the number of lymphocytes added to each well prior to washing.
RESULTS
Adhesion molecule profile of epithelial cell lines
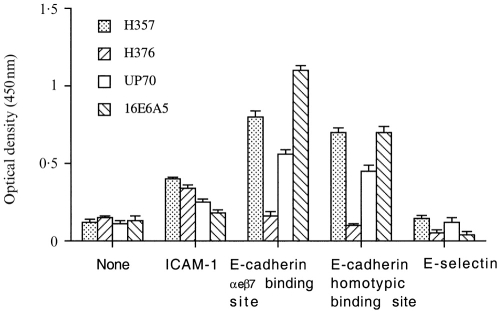
Both oral keratinocyte cell lines and the skin cell line expressed surface ICAM constitutively (Fig. 1) and although levels could be reduced by excluding serum from the culture medium (data not shown) they were never abolished. Levels of ICAM-1 were higher on the oral than the skin cell lines and low levels were expressed on the breast 16E6.A5 cell line.
Figure 1.
Cell surface expression of ICAM-1 (6.5β5 mAb), E-selectin (BBIG-E4 mAb) and E-cadherin (E4.6 mAb: αeβ7 binding site and HECD-1: homotypic binding site) on oral (H357 and H376) and skin (UP) keratinocyte cell lines and breast cell line (16E6.A5) quantified by cell-based ELISA; n = 6.
The oral keratinocyte cell line H357 expressed E-cadherin as assessed by surface ELISA (Fig. 1) and staining was positive with both the HECD-1 antibody which detects the homotypic binding site of E-cadherin as well as E4.6 which detects the binding site for αeβ7-positive lymphocytes. In contrast E-cadherin was not detectable on the oral H376 cell line using either the HECD-1 or the E4.6 antibody.
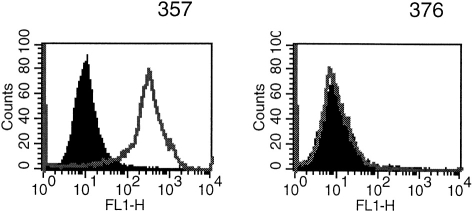
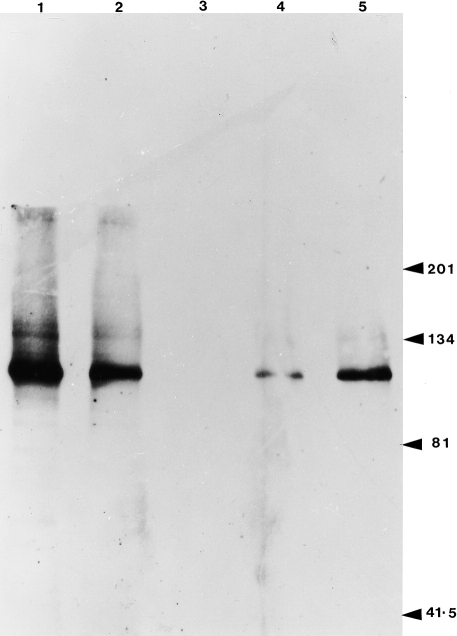
This lack of surface expression of E-cadherin by the H376 cell line was confirmed by flow cytometry (Fig. 2) and by Western blotting which also showed the oral H357, the skin UP and the breast 16E6.A5 cell lines were positive (Fig. 3). The oral H376 cell line had a phenotype which also suggested it was E-cadherin-negative: the cells were spindle shaped and formed only loose intercellular attachments which were easily disrupted. It is interesting to note that there appeared to be a greater amount of the E-cadherin epitope detected by E4.6 on the breast compared with the oral and skin cell lines (Fig. 1).
Figure 2.
Flow cytometric analysis of E-cadherin expression (as detected by HECD-1) by H357 and H376 oral keratinocyte cell lines. Negative control (black filled histogram) had secondary antibody only. H357 cells are positive and H376 are negative for E-cadherin.
Figure 3.
Immunoblot analysis of the cell surface expression of E-cadherin using the HECD1 mAb, which detects the homotypic binding site. Lane 1, breast cell lines, 16.E6.A5; lane 2, MCF7 breast cell line; lane 3, oral keratinocyte cell line H376; lane 4, oral keratinocyte cell line H357; lane 5, skin cell line UP.
No evidence of E-selectin expression was detected on any of the oral or skin cell lines using surface ELISA (Fig. 1).
Adhesion of TGF-β-stimulated PBL to oral and skin keratinocytes is inhibited by antibodies to αeβ7 but not E-cadherin
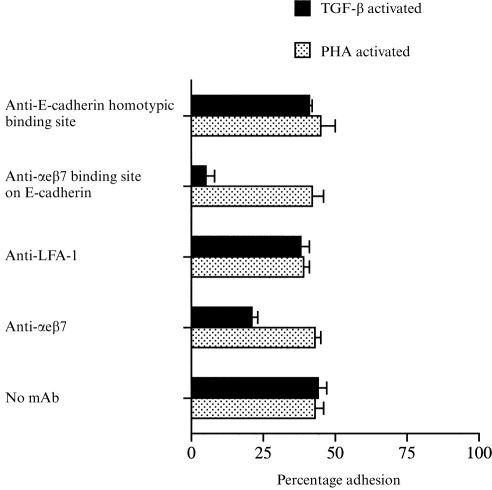
PHA-activated, IL-12-activated and TGF-β-activated PBL bound to oral and skin keratinocytes (Fig. 4) but antibodies against αeβ7 (BerACT8) only inhibited adhesion in the TGF-β-stimulated group, indicating that the inhibition was specific. In contrast, antibodies against LFA-1 almost totally abolished binding of PHA-activated and IL-12 activated PBL but only partially reduced binding of the TGF-β-activated group. A combination of antibodies against αeβ7 and LFA-1 totally abolished binding of TGF-β-activated PBL indicating that all adhesion is mediated by either LFA-1 or αeβ7. The control antibody against the β1 integrin did not affect binding in any group.
Figure 4.
The percentage adhesion of PHA-activated, IL-12-activated and TGF-β-activated PBL to the E-cadherin-positive H357 oral keratinocyte cell line. Lymphocytes were incubated with or without antibodies against αeβ7 (BerACT8), LFA-1 (TS1/18), β1 integrin (3S3), αeβ7 binding site on E-cadherin (E4.6) and the homotypic binding site on E-cadherin (HECD-1). Essentially similar results were found with the skin cell line UP – results not shown. For each experiment n = 6 or above.
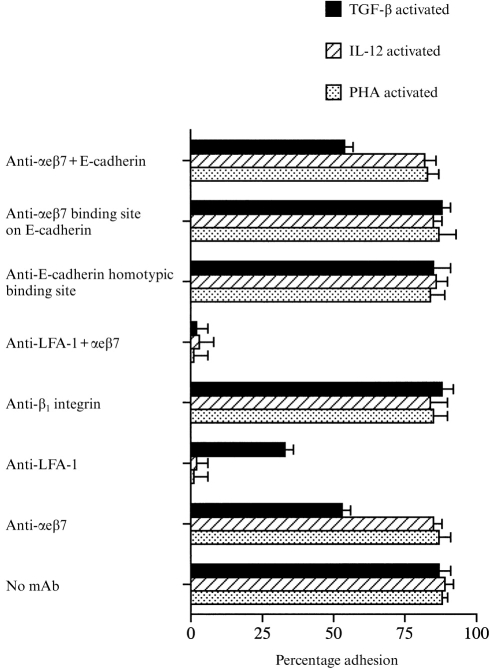
Surprisingly, although binding of TGF-β-activated PBL to oral and skin keratinocytes was partially mediated by αeβ7, adhesion was not inhibited at all by the E4.6 antibody against E-cadherin (Fig. 4) which did prevent binding of TGF-β-activated cells to the 16E6.A5 breast cell line (Fig. 5). Similarly a combination of the E4.6 and anti-αeβ7 antibody did not increase the inhibition of binding to oral or skin keratinocytes above that seen with antibodies to αeβ7 alone. Non-blocking antibodies against E-cadherin were without affect on any of the cell lines (Fig. 4).
Figure 5.
The percentage adhesion of PHA-activated and TGF-β-activated PBL to the breast cell line 16E6.A5. Lymphocytes were incubated either with or without antibodies against the homotypic binding site on E-cadherin (HECD-1), the αeβ7 binding site on E-cadherin (E4.6), LFA-1 (TS1/18) and αeβ7 (BerACT8). For each experiment n = 6 or above.
These results suggest that αeβ7-positive PBL bind to oral and skin keratinocytes either through a second epitope on E-cadherin that is unaffected by the E4.6 antibody or that there is a novel ligand on skin and oral keratinocytes distinct from E-cadherin. In order to help distinguish between these two possibilities adhesion of TGF-β-activated lymphocytes to the E-cadherin-negative oral keratinocyte cell line H376 was examined.
TGF-β-stimulated PBL adhere to an E-cadherin-negative oral keratinocyte cell line
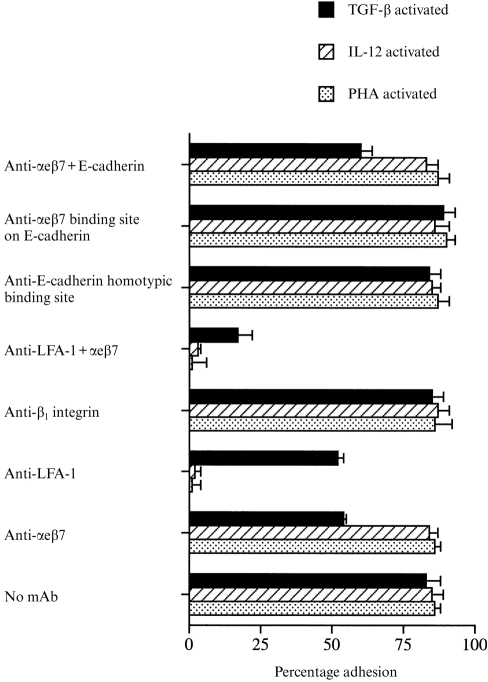
TGF-β-activated PBL adhered to the same extent to the E-cadherin-negative oral keratinocyte cell line H376 (Fig. 6) as to the E-cadherin-positive cell line H357 (Fig. 4) and binding was inhibited by antibodies against αeβ7, indicating that the cells were adhering via αeβ7 itself (Fig. 6). Binding of PHA-activated and IL-12-activated PBL was unaffected. As with the E-cadherin-positive oral cell line, adhesion in the TGF-β-activated group was unaffected by the E4.6 and HECD-1 antibodies against E-cadherin and inhibition of binding was not significantly greater with a combination of the E4.6 and anti-αeβ7 antibody. Binding of PHA-activated and IL-12-activated PBL was almost totally abolished by antibodies against LFA-1, which also partially reduced binding in the TGF-β-activated group. However, binding of TGF-β-activated lymphocytes could not be totally abolished by a combination of antibodies against αeβ7 and LFA-1.
Figure 6.
Adhesion of PHA-activated, IL-12-activated and TGF-β-activated PBL to the E-cadherin-negative oral keratinocytes cell line H376. Lymphocytes were incubated either with or without antibodies to αeβ7 (BerACT8), LFA-1 (TS1/18), β1 integrin (3S3), αeβ7 binding site on E-cadherin (E4.6) and the homotypic binding site on E-cadherin (HECD-1). For each experiment n = 6 or above.
DISCUSSION
There is convincing evidence that αeβ7-positive lymphocytes adhere to gut and breast epithelium via an interaction with E-cadherin11,12 and we have confirmed these findings using a breast cell line. However, the results of this study also suggest there may be a novel second ligand for αeβ7 in oral mucosa and skin: TGF-β-activated PBL bind to oral and skin keratinocytes via αeβ7 but adhesion is not inhibited by antibodies against E-cadherin and moreover, the lymphocytes adhere via αeβ7 to an E-cadherin-negative oral keratinocyte cell line. This strongly suggests that binding of TGF-β-activated PBL is independent of E-cadherin.
It could be argued our results do not conclusively exclude the possibility that αeβ7-positive lymphocytes bind to E-cadherin on the H357 oral cell line. However, if this is the case the epitope is distinct from the one in breast and gut epithelium and because TGF-β-activated PBL bind to the E-cadherin-negative oral cell line, there must be an additional third ligand for αeβ7-positive lymphocytes on that cell line. Whilst it is possible that αeβ7-positive lymphocytes use two different ligands to adhere to oral keratinocytes it seems more likely that there is a common ligand on both cell lines that is independent of E-cadherin.
The biological significance of the interaction between αeβ7 on IEL and E-cadherin on gut and breast epithelium is unknown but may result in signals to both lymphocytes and epithelial cells which are important in immunological surveillance.13 It is likely that the interaction of αeβ7 with the alternative ligand on oral and skin keratinocytes plays a similar role but the presence of this second ligand may reflect functional as well as structural differences between skin and oral mucosa and the rest of the gastrointestinal tract. Gut epithelium is composed of only one cell layer and is separated from the intestinal lumen by tight junctions at the apical portion of the cell which are important in controlling permeability. Below this are the well-defined intermediate junctions or zonula adherens and at the base are the desmosomes. E-cadherin is present within the intermediate junctions of the zonula adherens and in small intercellular junctional structures which are distinct from desmosomes.29 In contrast, stratified squamous epithelium is many cell layers thick and does not contain tight junctions. The permeability barrier resides in the lipids located in the extracellular space at the top of the prickle cell layer. Intercellular connections are mediated by desmosomes throughout the epithelial layers and it is only recently that zonula adherens have been identified. These are not well-defined ultrastructurally but are characterized by E-cadherin in association with β-catenin and actin and are closely associated with desmosomes.30 It is possible that the binding site for αeβ7-positive lymphocytes on E-cadherin is not accessible in stratified squamous epithelium and one of the other members of the cadherin family, such as desmoglein or desmocollins which form part of the desmosome, may be the ligand. It seems unlikely that P-cadherin plays a role since it has been shown in the mouse not to support adhesion of αeβ7-positive lymphocytes.13
In this study, all binding of CLA-positive cells appeared to be mediated via LFA-1/ICAM-1 but we did not test directly whether the lymphocytes adhered via CLA/E-selectin. This seems unlikely given the assay conditions: levels of LFA-1 remain high on PBL activated with IL-12 to up-regulate CLA23,28 and the keratinocytes were ICAM-1-positive with no evidence of E-selectin expression. However, there have been reports that under certain conditions oral keratinocytes express a variant of E-selectin22 and we have shown that in oral lichen planus up to one-quarter of IEL coexpress CLA and αeβ7.14 Since LFA-1 is down-regulated when αeβ7 is up-regulated by TGF-β4 it seems likely that LFA-1 levels may be low on these coexpressing cells. Under these conditions it is possible that CLA may interact with E-selectin and play a role in lymphocyte adhesion to oral keratinocytes.
An interaction of LFA-1 on the surface of lymphocytes with ICAM-1 on keratinocytes has been assumed to play a major role in IEL adhesion in skin and oral mucosa and hence be important in immunologically mediated dermatological disease. This assumption has been based partly on in vitro studies which have shown that activated PBL adhere to ICAM-1-positive skin and oral keratinocytes via LFA-124 and partly on in vivo observations which show that, in diseased skin and oral mucosa, keratinocyte expression of ICAM-1 is often associated with intraepithelial accumulation of LFA-1-positive lymphocytes.31,32 However, one of the problems with this hypothesis is that it is not able to explain all binding of IEL in vivo. ICAM-1 is not usually expressed in normal oral epithelium and epidermis and in disease IEL are not always related to keratinocyte ICAM-1 expression.33 In addition, several studies using a modified Stamper Woodruff assay on diseased and inflamed skin have shown that binding of lymphocytes to epidermis can only be partly inhibited by antibodies to LFA-1.34,35 The present study has shown that subpopulations of lymphocytes use different mechanisms to adhere to oral and skin keratinocytes in vitro and that interaction of αeβ7, albeit with an unknown ligand, may be a second important adhesion mechanism in vivo. The relative importance of these adhesion mechanisms is difficult to assess and may vary between normal and diseased tissue and also between different immunologically mediated diseases. IEL which express αeβ7 have low levels of LFA-123 and it seems likely the two mechanisms are mutually exclusive for any given lymphocyte. Recent evidence from immunocytochemical studies of diseased skin suggests expression of αeβ7 by IEL correlates with epidermotropism16 and large numbers of αeβ7-positive IEL are found in inflamed gingiva and in the buccal mucosa in oral lichen planus.14,36 It may be that interaction of αeβ7 with the unknown ligand on keratinocytes is a more important adhesion mechanism than LFA-1/ICAM-1 interaction.
Acknowledgments
Dean Brown was in receipt of an MRC (UK) Studentship. The authors would like to thank Dr Peter Kilshaw for valuable discussions and Mrs Christine Hall for help in preparation and typing of the manuscript.
References
- 1.Jarry A, Cerf-Bensussan N, Brousse N, Selz F, Guy-Grand D. Subsets of CD3+ (T cell receptor α/β or γ/δ) and CD3-lymphocytes isolated from normal human gut epithelium display phenotypical features different from their counterparts in peripheral blood. Eur J Immunol. 1990;20:1097. doi: 10.1002/eji.1830200523. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg RS, Yockey CE, Gross GG, Ebert EC, Balk SP. Human intestinal intraepithelial lymphocytes are derived from a limited number of T cell clones that utilise multiple v. Tcell receptor genes. J Immunol. 1993;150:5144. [PubMed] [Google Scholar]
- 3.Cerf-Bensussan N, Jarry A, Brousse N, Lisowska-Grospierre B, Guy-Grand D, Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987;17:1279. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- 4.Cepek KL, Parker CM, Madara JL, Brenner MB. Integrin alpha E beta 7 mediates adhesion of T lymphocytes to epithelial cells. J Immunol. 1993;150:3459. [PubMed] [Google Scholar]
- 5.Berlin C, Berg EL, Briskin MJ, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 6.Berlin C, Bargatze RF, Campbell JJ, et al. Alpha 4 integrins mediate lymphocyte attachment and rolling under physiological flow. Cell. 1995;80:413. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 7.Bargatze RF, Jutila MA, Butcher EC. Distinct roles for l-selectin and integrins αeβ7 and LFA-1 in lymphocyte homing to Peyers patch high endothelium in situ: the multistep model confirmed and refined. Immunity. 1995;3:99. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 8.Kilshaw PJ, Murant SJ. Expression and regulation of β7 (βp) integrins on mouse lymphocytes: relevance to the mucosal immune system. Eur J Immunol. 1991;21:1591. doi: 10.1002/eji.1830211041. [DOI] [PubMed] [Google Scholar]
- 9.Robert AI, O’connell SM, Ebert EC. Intestinal intraepithelial lymphocytes bind to colon cancer cells by HML-1 and CD11a. Cancer Res. 1993;53:1068. [PubMed] [Google Scholar]
- 10.Benmerah A, Badrichani A, Ngohou K, Megarbane B, Begue B, Cerf-Benussan N. Homotypic aggregation of CD103 (alpha E beta 7) and lymphocytes by an anti-CD103 antibody, HML-4. Eur J Immunol. 1994;24:2243. doi: 10.1002/eji.1830240946. [DOI] [PubMed] [Google Scholar]
- 11.Cepek KL, Shaw SK, Parker CM, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha e beta 7 integrin. Nature. 1994;372:190. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 12.Karecla PI, Bowden SJ, Green SJ, Kilshaw PJ. Recognition of E-cadherin on epithelial cells by the mucosal T cell integrin αM290β7 (αβ7) Eur J Immunol. 1995;25:852. doi: 10.1002/eji.1830250333. [DOI] [PubMed] [Google Scholar]
- 13.Karecla PI, Green SJ, Bowden SJ, Coadwell J, Kilshaw PJ. Identification of a binding site for integrin αeβ7 in the N terminal domain of E-cadherin. K = J Bio Chem. 1996;271:30909. doi: 10.1074/jbc.271.48.30909. [DOI] [PubMed] [Google Scholar]
- 14.Walton LJ, Thornhill MH, Macy MG, Farthing PM. Cutaneous lymphocyte associated antigen (CLA) and αeβ7 integrins are expressed by mononuclear cells in skin and oral lichen planus. J Oral Pathol Med. 1997;26:402. doi: 10.1111/j.1600-0714.1997.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 15.Sperling M, Kaudewitz P, Braun-Falco O, Stein H. Reactivity of T cells in mycosis fungoides exhibiting marked epidermotropism with the monoclonal antibody HML-1 that defines a membrane molecule on human mucosal lymphocytes. Am J Pathol. 1989;134:955. [PMC free article] [PubMed] [Google Scholar]
- 16.Dietz SB, Whitaker-Menezes D, Lessin SR. The role of alpha E beta 7 integrin (CD103) and E-cadherin in epidermotropism in cutaneous T-cell lymphoma. J Cutan Pathol. 1996;23:312. doi: 10.1111/j.1600-0560.1996.tb01303.x. [DOI] [PubMed] [Google Scholar]
- 17.Picker LJ, Michie SA, Rott LS, Butcher EC. A unique phenotype of skin-associated lymphocytes in humans. Preferential expression of the HECA-452 epitope by benign and malignant T cells at cutaneous sites. Am J Pathol. 1990;135:1053. [PMC free article] [PubMed] [Google Scholar]
- 18.Bos JD, de Boer OJ, Tibosch E, Das PK, Pals ST. Skin-homing T-lymphocytes: detection of cutaneous lymphocyte-associated antigen (CLA) by HECA-452 in normal human skin. Arch Dermatol Res. 1993;285:179. doi: 10.1007/BF00372006. [DOI] [PubMed] [Google Scholar]
- 19.Picker LJ, Kishimoto TK, Smith CW, Warnock RA, Butcher EC. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991;349:796. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- 20.Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothlelial cell – leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picker LJ, Treer JR, Ferguson DB, Collins PA, Bergstresser PR, Terstappen LW. Control of lymphocyte recirculation in man. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissue selective homing receptor for skin-homing T cells. J Immunol. 1993;150:1122. [PubMed] [Google Scholar]
- 22.Pietrzak ER, Savage NW, Aldred MJ, Walsh LJ. Expression of the E-selectin gene in human gingival epithelial tissue. J Oral Pathol Med. 1996;25:320. doi: 10.1111/j.1600-0714.1996.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 23.Picker L. Control of lymphocyte homing. Curr Opin Immunol. 1994;6:394. doi: 10.1016/0952-7915(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 24.Nicholoff BJ, Lewinsohn DM, Butcher EC, Krensky AM, Clayberger C. Recombinant gamma interferon increases the binding of peripheral blood mononuclear leukocytes and a Leu3+ T lymphocyte clone to cultured keratinocytes and to a malignant cutaneous squamous cell carcinoma cell line that is blocked by antibody against the LFA-1 molecule. J Invest Dermatol. 1988;90:17. doi: 10.1111/1523-1747.ep12462420. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Madrid F, Krensky AM, Ware CF, et al. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, CFA-3. Proc Natl Acad Sci USA. 1982;79:7489. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wellicome SM, Thornhill MH, Thomas DS, et al. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumour necrosis factor, IL-1 or lipopolysaccharide. J Immunol. 1990;144:1558. [PubMed] [Google Scholar]
- 27.Thornhill MH, Kyan-Aung U, Lee TH, Haskard DO. T cells and neutrophils exhibit differential adhesion to cytokine-stimulated endothelial cells. Immunology. 1990;69:287. [PMC free article] [PubMed] [Google Scholar]
- 28.Leung DY, Gately M, Trumble A, Ferguson-Darnell B, Schlievert PM, Picker LJ. Bacterial superantigens induce T-cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen, via stimulation of interleukin 12 production. J Exp Med. 1995;181:747. doi: 10.1084/jem.181.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leiser J, Molitoris BA. Disease processes in epithelia: the role of the actin cytoskeleton and altered surface membrane polarity. Biochem Biophys Acta. 1993;1225:1. doi: 10.1016/0925-4439(93)90115-h. [DOI] [PubMed] [Google Scholar]
- 30.Haftek M, Hansen MU, Kaiser HW, Kreysel HW, Schmitt D. Interkeratinocyte adherens junctions: immunocytochemical visualisation of cell-cell junctional structures distinct from desmosomes in human epidermis. J Invest Dermatol. 1996;106:498. doi: 10.1111/1523-1747.ep12343791. [DOI] [PubMed] [Google Scholar]
- 31.Griffiths CE, Nickoloff BJ. Keratinocyte adhesion molecule-1 (ICAM-1) expression precedes dermal T lymphocyte infiltration in allergic contact dermatitis. (Rhus dermatitis) Am J Pathol. 1989;135:1045. [PMC free article] [PubMed] [Google Scholar]
- 32.Walton LJ, Macy MG, Thornhill MH, Farthing PM. Intraepithelial subpopulations of T-lymphocytes and Langerhans cells in oral lichen planus. J Oral Pathol Med. 1998;27:116. doi: 10.1111/j.1600-0714.1998.tb01926.x. [DOI] [PubMed] [Google Scholar]
- 33.Verdickt GM, Savage NW, Dodd NM, Walsh LJ. Expression of CD54 (ICAM-1) and CD11a (LFA-1) adhesion molecules in oral mucosal inflammation. J Oral Pathol. 1992;21:65. doi: 10.1111/j.1600-0714.1992.tb00981.x. [DOI] [PubMed] [Google Scholar]
- 34.Nicholoff BJ, Griffiths CE. T-lymphocytes and monocytes bind to keratinocytes in frozen sections of biopsy specimens of normal skin treated with gamma interferon. J Am Acad Dermatol. 1989;20:736. doi: 10.1016/s0190-9622(89)70083-9. [DOI] [PubMed] [Google Scholar]
- 35.Bruynzeel I, van der Raaij EMH, de Haan P, Willemze R. Increased adherence to keratinocytes of peripheral blood mononuclear leukocytes of a patient with drug induced erythema multiforme. Br J Dermatol. 1993;129:45. doi: 10.1111/j.1365-2133.1993.tb03310.x. [DOI] [PubMed] [Google Scholar]
- 36.Tonetti M, Straub AM, Lang NP. Expression of the cutaneous lymphocyte antigen and the αeβ7 integrin by intraepithelial lymphocytes in healthy and diseased gingiva. Archs Oral Biol. 1995;40:1125. doi: 10.1016/0003-9969(95)00084-4. [DOI] [PubMed] [Google Scholar]