Abstract
Klebsiella pneumoniae has been isolated from liver abscesses in patients with leukaemia or diabetes. The resistance of Klebsiella infection in lipopolysaccharide (LPS)-hyporesponsive mice is unclear. Female C3H/HeJ and C3H/HeN mice, 6–8 weeks old, were intraperitoneally (i.p.) injected with K. pneumoniae. The results showed that C3H/HeJ mice were 24 times more susceptible [lethal dose 50% (LD50) 250 colony-forming units] than C3H/HeN mice to K. pneumoniae infection. C3H/HeJ mice, uninfected or infected with K. pneumoniae, had higher liver interleukin (IL)-10 levels and IL-10 mRNA levels than C3H/HeN mice. Previously, pretreatment with IL-1β and tumour necrosis factor-α (TNF-α) protected C3H/HeJ mice from lethal bacterial infection. Therefore the effects of pretreatment with IL-1β and TNF-α or antimurine IL-10 antibody i.p. 1 hr before this infection in both strains of C3H mice were examined. Pretreatment with TNF-α or anti-IL-10 antibody enhanced the survival of both strains of mice. TNF-α, in combination with IL-1β, enhanced the survival and bacterial clearance better than single pretreatment in C3H/HeJ mice. Anti-IL-10 antibody increased bacterial clearance and significantly reduced liver cytokine mRNA levels in C3H/HeJ mice more than it did in the controls during infection. These results indicate that exogenous cytokine modulation or neutralization of IL-10 enhance the resistance of LD50 infection in C3H/HeJ mice.
INTRODUCTION
Sepsis increases the length of hospital stays and medical costs, and when severe may cause multiple organ failure and death.1,2 Recently in Taiwan, sepsis due to pyogenic liver abscess has been associated with Klebsiella pneumoniae.3,4 However, the virulence of Klebsiella from clinical isolates is unclear. Gram-negative peritonitis in mice produces an intense local inflammation before becoming a systemic infection.5 Lipopolysaccharide (LPS) from the Gram-negative bacteria causes cytokine release and cytokines play important roles in the pathogenesis of sepsis.6,7 Cytokines also aid the recruiting and activating of neutrophils and macrophages8,9 that are critical to the infected host. For instance, cytokines protect C3H/HeJ mice from lethal Escherichia coli infection.10 However, excessive cytokine production may harm the host.11,12 Therefore, modulation of the cytokine response is necessary for maintaining homeostasis in the host. We hypothesized that LPS-resistant C3H/HeJ and LPS-sensitive C3H/HeN mice would respond differently to a virulent K. pneumoniae infection, similar to other virulent bacterial infections.10
Although, the molecular basis of LPS-hyporesponsiveness of C3H/HeJ mice is not completely understood, several defects are associated with LPS-mediated macrophage function.13–15 C3H/HeJ mice produce less cytokines than other mice, and are more susceptible to virulent bacterial infections,14,16,17 pretreatment with tumour necrosis factor-α (TNF-α) alone or in combination with interleukin-1 (IL-1) might protect C3H/HeJ mice from lethal Klebsiella infection as in Escherichia coli 018K+ infection.10 Recently, IL-10 has been shown to down-regulate the expression of TNF-α and chemokines from macrophages.18,19 Pretreatment with IL-10 protects mice, whereas neutralization of IL-10 increases lethality from endotoxaemia.20 However, neutralization of IL-10 enhances TNF-α or nitric oxide production and promotes the clearance of Mycobacterium avium, Candida albicans and K. pneumoniae in other infected mice.21–23 Our preliminary observation found that uninfected C3H/HeJ mice had a higher IL-10 basal level in the liver than C3H/HeN mice, thus we hypothesized that neutralization of IL-10 might protect C3H/HeJ mice from Klebsiella infection.
In the present study, we pretreated both strains of C3H mice with IL-1 and TNF-α or antimurine IL-10 antibody, 1 hr before intraperitoneal (i.p.) injection of K. pneumoniae. The results showed that C3H/HeJ mice were more susceptible than C3H/HeN mice to a virulent K. pneumoniae infection. Furthermore, exogenous cytokine modulation or neutralization of IL-10 enhanced the survival in infected C3H/HeJ mice.
MATERIALS AND METHODS
Animals
Mice of C3H/HeN and C3H/HeJ strains were obtained from the animal facility of National Cheng Kung University Medical College. These mice were originally obtained from the Jackson Laboratory (Bar Harbor, ME). They were maintained on standard laboratory chow and water ad libitum. Six- to eight-week-old females were used in all experiments.
Reagents
Murine recombinant TNF-α [1·33×108 U/mg specific activity (SA)], IL-1β (1·33×108 U/mg SA) and antimurine IL-10 antibody were obtained commercially (R&D, Minneapolis, MN).
Treatment and bacterial infection of mice
Klebsiella pneumoniae (TVGH5395) was isolated clinically at Taichung Veterans General Hospital. K. pneumoniae (ATCC9997, capsular type 2) was obtained from the American Type Culture Collection (ATCC; Rockville, MD). The K. pneumoniae isolate reacted with anticapsular type 1 but not type 2 serum. The micro-organisms were cultured in tryptic soy broth (TSB) overnight at 37° with shaking to reach ≈109 bacteria/ml. Mice were injected i.p. with various doses of K. pneumoniae in 0·2 ml of sterile normal saline. C3H mice were tested with their lethal dose 50% (LD50) doses. The effect of cytokine modulation or neutralization of IL-10 was studied in C3H mice treated with various doses of IL-1β and TNF-α alone or combined or antimurine IL-10 antibody, i.p. 1 hr before the infection.
Bacterial counts in serum and liver
Blood samples of mice were obtained by open heart collection into tubes. The liver of each animal was removed, weighed (50 mg) and homogenized in 2 ml heparinized saline using a glass tissue grinder. Serum and liver homogenates were diluted with normal saline and aliquots of the suspension were plated onto tryptic soy agar (TSA) plates to determine bacterial colony counts.
Cytokine assay
Animals were killed at the indicated times postinfection. About 200 mg of the liver of each animal were removed and homogenized separately as previously described.21 Samples were stored at −20° until assay. Determination of TNF-α, IL-1β and IL-10 production in each sample was performed by commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D, Minneapolis, MN). Cytokines in the serum or liver homogenates were measured according to the manufacturer’s instructions. The detection limits of TNF-α, IL-1β and IL-10 were 50, 15 and 5 pg/ml, respectively.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total cellular RNA was extracted from livers and spleens (50 mg) using Ultraspec RNA extraction solution (Biotecx Lab. Inc., Houston, TX). Equal amounts of total RNA from each sample were subjected to first-strand cDNA synthesis using Moloney murine leukaemia virus reverse transcriptase, the oligo-dT15 primer (except that the reverse transcription of TNF-α mRNA was performed using a gene-specific primer to replace the oligo-dT15 primer), and dNTP in the presence of RNase inhibitor in a 50-μl reaction at 42° for 60 min. PCR was performed with 10% of the product of cDNA synthesis, Taq polymerase, 0·4 μm of each pair of cytokine gene-specific primers (CLONTECH, Palo Alto, CA) and 0·2 mm dNTP in a reaction buffer. As a control for the RNA isolation and reverse-transcription β-actin gene expression was used. The cDNA samples were amplified using a three-temperature PCR system, usually consisting of denaturation at 94° for 45 seconds, primer annealing at 60° for 45 seconds, and extension at 72° for 2 min. Quantification of cytokine mRNA levels was performed in the exponential phase of amplification. The reaction product was visualized by electrophoresis in a 3% agarose gel (consisting of 2% Nusieve GTG agarose and 1% agarose), and by staining with 0·5 μg/ml ethidium bromide.
Statistical methods
The Mann–Whitney rank test was used for the analysis of data; P-values less than 0·05 were considered significant. All anayses were performed with statview version 3.0 for Macintosh (Abacus Concepts, Inc., Berkeley, CA).
RESULTS
Mortality from infection
The virulence of our K. pneumoniae isolate (TVGH5359) was similar to that of a virulent strain of K. pneumoniae (ATCC9997). C3H/HeJ females were more susceptible (LD50 2·5×102 CFU) than C3H/HeN females (LD50 6×103 CFU) to K. pneumoniae i.p. infection at 2 days postinfection (data not shown).
IL-10 mRNA and protein in infected mice
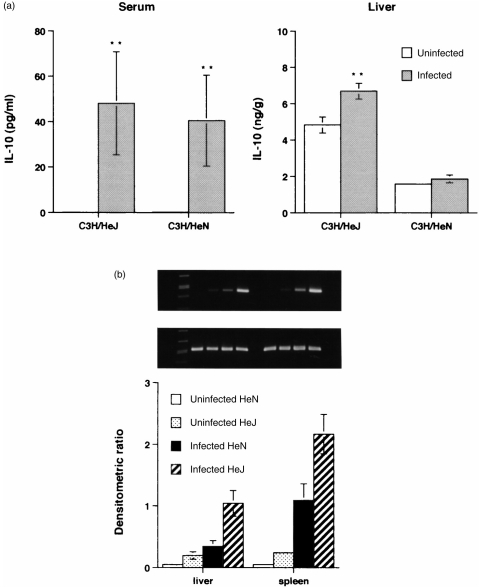
Preliminary experiments were performed to determine whether IL-10 levels were different between these two strains of mice under each LD50 CFU of Klebsiella infection. The result showed that the basal and infected liver IL-10 levels in C3H/HeJ mice were much higher (P < 0·01) than in C3H/HeN mice (Fig. 1a). Serum IL-10 levels (Fig. 1a) increased in both strains of mice at 32 hr postinfection, but were not significantly different from each LD50 dose. Therefore, we examined their IL-10 mRNA levels under the same CFU of Klebsiella infection. IL-10 mRNA levels in liver and spleen homogenates of C3H/HeJ mice were much higher than those in C3H/HeN mice, with 3×103 CFU of K. pneumoniae 1 day postinfection (Fig. 1b).
Figure 1.
IL-10 production (a) in serum and liver homogenates from C3H/HeJ and C3H/HeN mice, after i.p. injection with LD50 2·5×102 and 6×103 CFU K. pneumoniae, repectively, at 32 hr postinfection. Each value is the mean±SEM from five animals. **P < 0·01 as compared with uninfected controls. (b) IL-10 (top panel) and β-actin (middle panel) mRNA expression in the liver and spleen of C3H/HeJ and C3H/HeN mice 24 hr after i.p. injection with 3×103 CFU K. pneumoniae. Molecular weight markers are in the left lane. Each value is the mean±SEM densitometric ratio of IL-10/β-actin from five animals. All mice survived until time of killing.
Effect of cytokine modulation or neutralization of IL-10 on animal survival
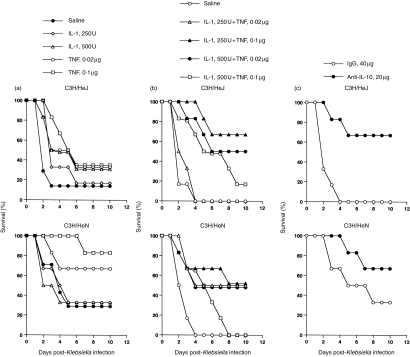
The effect of cytokine or anti-IL-10 antibody on the survival of both infected C3H mouse strains is shown in Fig. 2. Pretreatment with TNF-α alone (0·1 μg/mouse), or in combination with IL-1β (250 U/mouse), increased the survival of the LD50 250 CFU Klebsiella-infected C3H/HeJ mice (Fig. 2a,b). However, pretreatment with TNF-α alone protected C3H/HeJ mice from the infection much less than when combined with IL-1β (33% versus 68% of survival at 10 days postinfection, respectively). Pretreatment with TNF-α alone also protected C3H/HeN mice from the LD50 6000 CFU infection. Furthermore, C3H/HeN mice pretreated with IL-1β (500 U) had a significantly increased mortality. By contrast, pretreatment with TNF-α combined with IL-1β did not increase the survival of C3H/HeN mice as compared with that in mice treated with TNF-α alone. On the other hand, an increase in the concentration of combined cytokines decreased the survival in both infected mice. Pretreatment of C3H/HeJ and C3H/HeN mice with 20 μg of anti-IL-10 antibody enhanced their survival from 0% (control serum) to 70% and 30% (control serum) to 70%, respectively, 10 days postinfection (Fig. 2c). The protection of these infected mice by the pretreatment with 20 μg of anti-IL-10 antibody was better (70% of survival) than that of the lower (5–10 μg) or higher (40 μg) doses of anti-IL-10 antibody (20–30% and 50% of survival in C3H/HeJ and C3H/HeN mice, respectively).
Figure 2.
Effect of cytokine or anti-IL-10 antibody on survival rates of C3H mice after i.p. injection with each LD50 CFU K. pneumoniae as in Fig. 1. Mice received 0·2 ml of TNF-α (0·02, 0·1 μg) or IL-1β (250, 500 U) (a), the two cytokines combined (b), or anti-IL-10 antibody (5, 10, 20, 40 μg) (c) i.p. 1 hr before challenge with K. pneumoniae. Each group consisted of six animals. Experiments were repeated twice. The best results of anti-IL-10 antibody pretreatment from both strains of mice are selected to show in the figures.
Effect of cytokine modulation or neutralization of IL-10 on the bacterial clearance
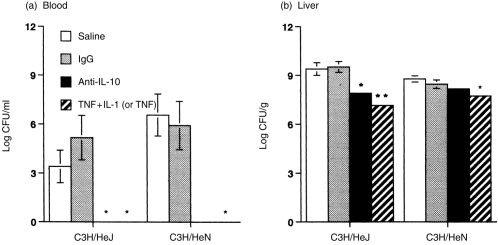
Having observed the protective effect of cytokine or anti-IL-10 antibody in Klebsiella-infected mice, we then studied their relationships with bacterial clearance. Pretreatment with anti-IL-10 antibody (20 μg) or TNF-α (0·1 μg) combined with IL-1β (250 U), significantly (P < 0·01) reduced bacterial CFU in blood and liver samples in C3H/HeJ mice with LD50Klebsiella infection (Fig. 3). In infected C3H/HeN mice, pretreatment with anti-IL-10 antibody or TNF-α alone had the same efficiency as that in C3H/HeJ mice regarding bacterial clearance in the blood. However, the bacterial CFU loading in the liver of C3H/HeN mice was only reduced twofold and 10-fold, respectively, by pretreatment with anti-IL-10 antibody or TNF-α, as compared to the controls. In comparison, anti-IL-10 antibody reduced bacterial CFU loading in the liver of C3H/HeJ mice about 40-fold, but in C3H/HeN mice the reduction was only twofold.
Figure 3.
Effect of cytokine or anti-IL-10 antibody on bacterial counts in blood (a) and liver homogenates (b) of C3H mice 32 hr after i.p. injection with each LD50 CFU K. pneumoniae. Mice received 0·2 ml of saline, goat immunoglobulin G (IgG; 20 μg), TNF-α (0·1 μg) and IL-1β (250 U), or anti-IL-10 Ab (20 μg) i.p. 1 hr before challenge with K. pneumoniae. Each value is the mean±SEM from five animals. *, P < 0·05, **, P < 0·01 as compared with the infected saline or IgG controls.
Effect of cytokine modulation or neutralization of IL-10 on cytokine mRNA and protein levels
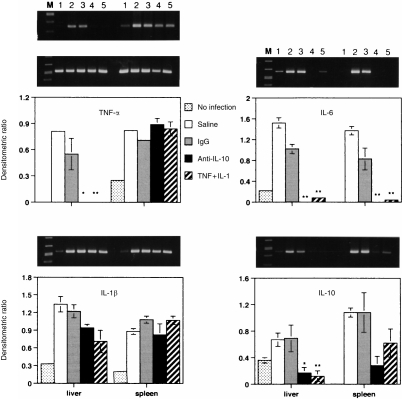
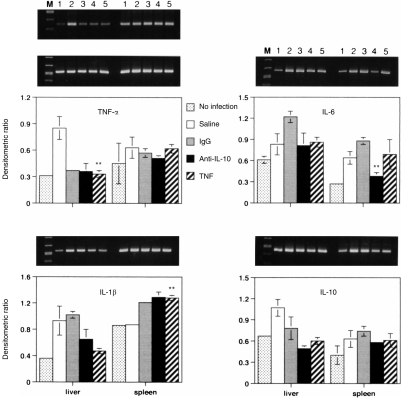
Pretreatment with either anti-IL-10 antibody (20 μg) or TNF-α (0·1 μg) and IL-1β (250 U) or TNF-α alone (0·1 μg) of C3H/HeJ and C3H/HeN mice infected with each LD50 of K. pneumoniae significantly (P < 0·01) reduced serum IL-10 level at 32 hr postinfection (from 30 to 50 pg/ml to 0 and 40–55 pg/ml to 4 pg/ml, respectively, for each strain of mouse). Serum TNF-α and IL-1β, were either not detected or were minimal in all groups of mice with LD50 of Klebsiella infection at 32 hr postinfection (data not shown). There was no significant difference in liver TNF-α, IL-1β and IL-10 levels between controls and animals pretreated with either anti-IL-10 antibody or TNF-α and IL-1β (data not shown); except that liver IL-10 levels in C3H/HeJ mice (4·2–7 ng/g) were much higher than in C3H/HeN mice (1·8–2 ng/g) in all treated groups. The effects of cytokine modulation on cytokine mRNA levels in liver and spleen were then measured by RT-PCR. Since IL-10 mRNA levels in liver and spleen homogenates of C3H/HeJ mice were much higher than those in C3H/HeN mice with infection (Fig. 1b), lower cycles for PCR were used to detect the difference of IL-10 mRNA in samples of C3H/HeJ mice. The transcription of TNF-α, IL-6 and IL-10 genes in the liver of pretreated C3H/HeJ mice was reduced significantly from the control levels during the infection (Fig. 4). The cytokine mRNA levels in the spleen were similarly reduced by the treatments as in the liver of C3H/HeJ mice, except for TNF-α and IL-1β. By contrast, in infected C3H/HeN mice pretreated with TNF-α alone or anti-IL-10 antibody, the reduction of mRNA of IL-6, and IL-10 in the liver or spleen was minimal (Fig. 5).
Figure 4.
Effect of cytokine or anti-IL-10 antibody on cytokine gene expression in liver and spleen from C3H/HeJ mice 32 hr after i.p. injection with LD50 CFU K. pneumoniae. Treatment conditions were identical to Fig. 3. The agrose gel bands show the RT-PCR amplified cytokine (top panel) and β-actin (middle panel) mRNA from the liver and spleen. Lane M, molecular marker; lane 1, uninfected control; lanes 2 and 3, saline and immunoglobulin G (IgG) controls; lane 4, anti-IL-10 (20 μg); lane 5, TNF-α (0·1 μg) and IL-1 (250 U). Each value is the mean±SEM densitometric ratio of cytokine/β-actin from five animals. *, P < 0·05, **, P < 0·01 as compared with the infected saline or IgG controls.
Figure 5.
Effect of cytokine or anti-IL-10 antibody on cytokine gene expression in liver and spleen from C3H/HeN mice 32 hr after i.p. injection with LD50 CFU K. pneumoniae. Treatment conditions were identical to those described in Fig. 4. The description of lanes is identical to Fig. 4 except lane 5 is TNF-α alone (0·1 μg).
DISCUSSION
The virulence of the K. pneumoniae isolate (TVGH5395) in C3H/HeJ mice was similar to the virulence of a capsule type 2 strain of K. pneumoniae. However, the K. pneumoniae isolate was more virulent than other Gram-negative bacterial isolates (LD50 102, compared to LD50 109 CFU) in the i.p. challenged mice.16 C3H/HeJ mice were 24 times more susceptible to this infection than were C3H/HeN mice. The expression of IL-10 mRNA in C3H/HeJ mice was higher than that in C3H/HeN mice when both were infected with 3×103 CFU of K. pneumoniae (Fig. 1b). We also found that C3H/HeJ mice had a basal liver IL-10 level much higher (P < 0·01) than C3H/HeN mice, and both strains of mice had high serum IL-10 levels 32 hr postinfection with each LD50 CFU of K. pneumoniae (Fig. 1a). Since IL-10 is an important mediator of immune responses,24,25 the IL-10-mediated suppression of cytokine may be detrimental to the host when an active immune response is required for effective clearance of microbial pathogens.23 This suggests that IL-10 might be responsible in part for the susceptibility of C3H/HeJ mice to a virulent Klebsiella infection.
TNF-α plays a role in host defence against bacterial pneumonia in mice,26,27 and the preteatment of neutrophils with TNF-α enhances their bactericidal activity in the presence of complement.28 IL-1 released by macrophages and monocytes may act on monocytes as an autostimulating factor and may enhance bacterial clearance.10 C3H/HeJ mice have much lower cytokine production than C3H/HeN mice with an infection or LPS administration, therefore when provided with appropriate concentrations of cytokine, C3H/HeJ mice are able to overcome this defect to combat virulent pathogens.10 Accordingly, pretreatment with TNF-α alone resulted in an increase in the survival of C3H/HeJ mice from this Klebsiella infection which was better than that of mice pretreated with IL-1β alone. This different effect may be because IL-1β is not directly involved in activating phagocytosis of macrophages against microbial agents.29 C3H/HeJ mice pretreated with a combination of TNF-α and IL-1β had a significantly enhanced survival compared with those pretreated with cytokine alone at 10 days of infection. These results are similar to the result of cytokine treatment of C3H/HeJ mice with a virulent E. coli infection.10 Although C3H/HeN mice pretreated with TNF-α combined with IL-1β also had an increased survival rate of LD50 of K. pneumoniae infection, the percentage of survival was less than that of those mice pretreated with TNF-α alone. These results are compatible with other studies that demonstrated that TNF-α plays a protective role in mice with pneumonia.26,27 However, we observed that pretreatment with IL-1β had no protective effect during the infection in infected C3H/HeN mice. Furthermore, pretreatment with TNF-α combined with IL-1β seemed to have a greater benefit to C3H/HeJ mice, but less to C3H/HeN mice during infection. This agrees with the concept that cytokine plays roles in the pathogenesis of sepsis, as well as in resistance to infection.6–9
Because C3H/HeJ mice had a high basal IL-10 level, anti-IL-10 antibody might protect mice from the lethal infection. Since the host defence against microbial infection depends on the rapid clearance of the organisms from the site of infection, a high IL-10 basal level of C3H/HeJ mice might suppress the production of TNF-α and chemokines, and thus reduce the resistance of C3H/HeJ mice to Klebsiella infection. If neutralization of IL-10 could correct this immunological aberration, it might improve the initial clearance of K. pneumoniae and survival. The present results clearly show that anti-IL-10 antibody could provide significant protection in LD50 250 CFU-infected C3H/HeJ mice.
To investigate the mechanisms whereby neutralization of IL-10 improves survival rate, the transcription of TNF-α, IL-6 and IL-10 genes in the liver and spleen were compared with control animals. We found no enhancement of TNF-α in the livers of both strains of mice during the infection. This is different from the results of pretreatment with anti-IL-10 serum in CD-1 mice infected intratracheally with K. pneumoniae, which showed enhanced lung TNF-α production,23 and in C57BL/6 mice infected with Streptococcus pneumoniae which increased lung levels of TNF-α and interferon-γ.30 The discrepancy may be due to the differences in infection routes, animal strains, or bacterial strains in these studies. Although enhancement of TNF-α production by anti-IL-10 antibody was not observed in this study, the improved survival rate indicates that an enhanced immune response was associated with this treatment. Furthermore, most of cytokine mRNA levels in anti-IL-10 antibody-pretreated mice were much lower than those of the controls. This may be due to the decrease of bacterial CFU loading in the livers or blood (2–5 log of CFU) over the controls. Therefore, our results might not be inconsistent with these findings. Further study is needed to investigate the mechanism of anti-IL-10 antibody on the protection of infected C3H/HeJ mice. Taken together, these results suggest that cytokine modulation in vivo may provide potential therapeutic benefits in patients with immune deficiencies against virulent bacterial infections.
Acknowledgments
We thank Drs Dah-Chuan Yeh and Peter Yuk-Fong Liu for valuable discussion, Chang-Tel Fung (Taipei), Division of Infectious Diseases, Veterans General Hospitals, for typing of K. pneumoniae, and Jon-Son Kuo, Chairman of the Department of Education and Research, for the support. This work was supported in parts by the National Science Council Grant NSC862314 B075 A009, and Taichung Veterans General Hospital Grant TCVGH 877311C, Taiwan.
Abbreviations
- CFU
colony-forming units
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IL-10
interleukin-10
- LPS
lipopolysaccharide
- TNF-α
tumor necrosis factor-α.
References
- 1.Haley RW, Schaberg DR, Crossley KB, Von-Allmen SD, Magowan J.E. Jr. Extra charges and prolongation of stay attributable to nosocomial infections: a prospective interhospital comparison. Am J Med. 1981;70:51. doi: 10.1016/0002-9343(81)90411-3. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control. Increase in National hospital discharge survey rates for septicemia-United States, 1979. MMWR. 1990;39:31. [PubMed] [Google Scholar]
- 3.Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146:1913. [PubMed] [Google Scholar]
- 4.Wang LS, Lee FY, Cheng DL, Liu CY, Hinthorn DR, Jost PM. Klebsiella pneumoniae bacteremia: analysis of 100 episodes. J Formosan Med Asso. 1990;89:756. [PubMed] [Google Scholar]
- 5.Zanetti G, Heumann D, Gerain J, et al. Cytokine production after intravenous or peritoneal Gram-negative bacterial challenge in mice. J Immunol. 1992;148:1890. [PubMed] [Google Scholar]
- 6.Tracey KJ, Fong Y, Hesse DG, et al. Anti-cachetein/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 7.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 8.Shalaby MR, Aggarwal BB, Rinderknecht E, Sverdersky LP, Finkle BS, Palladino MA. Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985;135:2069. [PubMed] [Google Scholar]
- 9.Bermudez LEM, Young LS. Tumor necrosis factor alone or in combination with IL-2, but not IFN-γ, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988;140:3006. [PubMed] [Google Scholar]
- 10.Cross AS, Sadoff JC, Kelly N, Bernton E, Gemski P. Preteatment with recombinant murine tumor necrosis factor-α/cachectin and murines interleukin 1 α protects mice from lethal bacterial infection. J Exp Med. 1989;169:2021. doi: 10.1084/jem.169.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva AT, Bayston KF, Cohen J. Prophylactic and therapeutic effects of a monoclonal antibody to tumor necrosis factor α in experimental Gram-negative shock. J Inf Dis. 1990;162:421. doi: 10.1093/infdis/162.2.421. [DOI] [PubMed] [Google Scholar]
- 12.Ohlsson K, Bjork P, Bergenfeldt M, Hageman R, Thompson RC. Interleukin 1 receptor antagonist reduced mortality from endotoxin shock. Nature. 1990;348:550. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- 13.Vogel SN. The LPS gene. Insights into the genetic and molecular basis of LPS responsiveness and macrophage differentiation. In: Beutler B, editor. Tumor Necrosis Factor: the Molecules and Their Emerging Role in Medicine. New York: Raven Press; 1992. p. 485. [Google Scholar]
- 14.Weinstein DL, Lissner CR, Swanson RN, O’brien AD. Macrophage defect and inflammatory cell recruitment dysfunction in Salmonella susceptible C3H/HeJ mice. Cell Immunol. 1986;102:68. doi: 10.1016/0008-8749(86)90326-6. [DOI] [PubMed] [Google Scholar]
- 15.Shinji H, Akagawa KS, Yoshida T. LPS induces selective translocation of protein kinase C-β in LPS-responsive mouse macrophages, but not in LPS-nonresponsive mouse macrophages. J Immunol. 1994;153:5760. [PubMed] [Google Scholar]
- 16.Hagberg L, Briles DE, Eden CS. Evidence for separate genetic defects in C3H/HeJ and C3HeB/FeJ mice, that affect susceptibility to gram-negative infections. J Immunol. 1985;134:4118. [PubMed] [Google Scholar]
- 17.Evans T, Strivens JE, Carpenter A, Cohen J. Differences in cytokine response and induction of nitric oxide synthase in endotoxin-resistant and endotoxin-sensitive mice after intravenous Gram-negative infection. J Immunol. 1993;150:5033. [PubMed] [Google Scholar]
- 18.de Waal MR, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin-10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815. [PubMed] [Google Scholar]
- 20.Standiford TJ, Strieter RM, Lukacs NW, Kunkel ST. Neutralization of IL-10 increases lethality in endotoxemia. Cooperative effects of macrophage inflammatory protein-2 and tumor necrosis factor. J Immunol. 1995;155:2222. [PubMed] [Google Scholar]
- 21.Bermudez LE, Champsi J. Infection with Mycobacterium avium induced production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993;61:3093. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romani L, Puccetti P, Mencacci A, et al. Neutralization of IL-10 up-regulates nitric oxide production and protects susceptible mice from challenge with Candida albicans. J Immunol. 1994;152:3514. [PubMed] [Google Scholar]
- 23.Greenberger MJ, Strieter RM, Kunkel ST, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995;155:722. [PubMed] [Google Scholar]
- 24.Howard M, O’garra A, Ishida H, Malefyt RD, De Vries J. Biological properties of interleukin 10. J Clin Immunol. 1992;12:239. doi: 10.1007/BF00918147. [DOI] [PubMed] [Google Scholar]
- 25.Romagnani S. Lymphokine production by human T cells in disease state. In: Paul WE, Fathman CG, Metzger H, editors. Annual Review of Immunology. Vol. 12. Palo Alto, CA: Annual Review Inc.; 1994. p. 227. [DOI] [PubMed] [Google Scholar]
- 26.Laichalk LL, Kunkel SL, Strieter RMM, Danforth JM, Bailie MB, Standiford TJ. Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumonia. Infect Immun. 1996;64:5211. doi: 10.1128/iai.64.12.5211-5218.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takashima K, Tateda K, Matsumoto T, Iizawa Y, Nakao M, Yamaguchi K. Role of tumor necrosis factor in pathogeneis of pneumococcal pneumonia in mice. Infect Immun. 1997;65:257. doi: 10.1128/iai.65.1.257-260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrante A, Martin AJ, Bates EJ, et al. Killing of Staphylococcus aureus by tumor necrosis factor-α activated neutrophils. J Immunol. 1993;151:4821. [PubMed] [Google Scholar]
- 29.Czuprynski CJ, Brown JF. Recombinant murine interleukin-1α enhancement of nonspecific antibacterial resistance. Infect Immun. 1987;55:2061. doi: 10.1128/iai.55.9.2061-2065.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van der Poll T, Marchant A, Keogh CV, Goldman M, Lowry S. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J Infect Dis. 1996;174:994. doi: 10.1093/infdis/174.5.994. [DOI] [PubMed] [Google Scholar]