Abstract
The human high affinity receptor for immunoglobulin G, FcγRI, in dibutyryl cyclic AMP (dbcAMP)-differentiated U937 cells, is coupled to the activation of phospholipase C (PLC) and the conventional protein kinase C (PKC) isoforms, α, β, and γ. Here we demonstrate that aggregation of FcγRI activates the tyrosine-kinase regulated form of phosphatidylinositol-3-kinase (PI-3-kinase) and that an increase of phosphatidylinositol trisphosphate (PIP3) is essential for the activation and translocation of PLCγ1 in these cells. In addition, activation of the PKC isoforms was ablated by specific inhibitors of PI3-kinase or by overexpression of a dominant negative p85 subunit of PI3-kinase. The findings reported here demonstrate that PLCγ1 and PKC activation by FcγRI are downstream of PI3-kinase, and that in contrast to cytokine primed cells, only the tyrosine-kinase activated isoform of PI3-kinase is coupled to FcγRI in dbcAMP-differentiated cells.
INTRODUCTION
Receptors for the constant (Fc) region of immunoglobulin G (IgG) (FcγRs) are expressed on the surface of many different cell types of the immune system and play an important role in linking the cellular and humoral arms of the immune response.1 On myeloid cells aggregation of FcγRs leads to a number of cellular responses, including the internalisation of immune complexes, degranulation and the release of proteases, activation of the respiratory burst and the release of cytokines. These processes can lead to targeted cell killing through antibody-directed cellular cytotoxicity,2,3 which is critically important for clearing virus-infected cells and in cancer surveillance.4
FcγRs comprise a family of receptors for IgG (FcγRI, FcγRII, and FcγRIII) that are distinguished by the affinity for ligand.1 Of these the human high affinity receptor, FcγRI, is an integral type I membrane glycoprotein5 constitutively expressed on monocyte and macrophage cell types. The cytoplasmic tail of FcγRI contains no obvious signalling motif. However, FcγRI has been shown to associate with the immune-receptor tyrosine activation motif (ITAM)-containing molecules, γ chain6,7 and the low-affinity receptor FcγRIIa.7 Aggregation of FcγRI results in signal transduction events as evidenced by tyrosine phosphorylation of proteins,7–10 tyrosine-kinase dependent calcium transients,11,12 and the generation of lipid second messengers through the activation of phospholipases,7–9,12 and lipid kinases.8,13,14
The lipid kinases, phosphatidinositol-3-kinase (PI3-kinase), which catalyse the phosphorylation of inositol phospholipids at the 3-position of the inositol ring,15 have been increasingly implicated in regulating a number of cellular responses, including mitogenesis, enhanced cell motility, and vesicular trafficking, although the exact mechanism by which PI3-kinase mediates cell signalling during these events is still poorly understood.16 The products of PI3-kinases have been found to activate certain calcium-independent protein kinases C (PKC)17 and to bind to a subset of Src homology 2 (SH2) domains.18 Furthermore, phosphatidylinositol-3,4-biphosphate (PtdIns-3,4-P2) and/or phosphatidylinositol-3,4,5- trisphosphate (PtdIns-3,4,5-P3) have been found to bind and stimulate several pleckstrin homology (PH) domain-containing proteins, including the serine, threonine kinase, cellular homologue of the viral oncogene V-atk (Akt/PKB) protein kinase,19 the phosphoinositide-dependent kinase (PDK) protein kinase,20 and the general receptor for phosphoinositides-1 (Grp1) exchange factor for ADP ribosilation factor-1 (Arf1).21 More recently, it was shown that the PH domain of phospholipase Cγ (PLCγ) will bind to PtdIns-3,4,5-P3,14 resulting in translocation to membranes. By this translocation, PtdIns-3,4,5-P3 enhances PLCγ1-mediated hydrolysis of PtdIns-4,5-P2 thereby increasing intracellular Ins-1,4,5-P3 levels. In support of this, overexpression of a constitutively active form of the p110 catalytic subunit of PI3-kinase increases intracellular InsP3 levels,22 raising the possibility that phosphatidylinositol-trisphosphate (PIP3) may regulate cytosolic calcium transients. Moreover, inhibitors of PI3-kinase diminish the intracellular calcium transient seen in adrenal glomerulosa cells, neutrophils, and rat leukaemia cells.23 Furthermore, it has recently been shown that, in HepG2 cells expressing platelet-derived growth factor receptor (PDGFR), inhibition of PI3-kinase markedly reduced the release of intracellular calcium.24
We have previously shown that aggregation of FcγRI in U937 cells results in distinct signalling patterns and calcium transients, depending on the differentiation state of the cell.7 Thus, in cells differentiated to a macrophage phenotype with dibutyryl cyclic AMP (dbcAMP), phospholipase C is activated whereas in cytokine (interferon-γ; IFN-γ) primed cells, FcγRI activates phospholipase D.7,12 A role for PI3-kinases in signal transduction has been shown in cytokine-primed cells as aggregation of FcγRI results in prolonged elevation of PIP3 as a result of sequential activation of both Class I PI3-kinases.13 The role of PI3-kinases in dbcAMP-differentiated cells has not been studied. Here we show that in contrast to the cytokine primed cells only the tyrosine-kinase dependent form of PI3-kinase is activated by FcγRI aggregation in dbcAMP-differentiated cells and that this activation is necessary for the activation and translocation of PLCγ1 and PKCs.
MATERIALS AND METHODS
Cell culture
U937 cells were cultured in RPMI-1640 (Gibco, Paisley, UK) supplemented with 10% fetal calf serum, 2 mm glutamine, 10 IU/ml penicillin and 10 mg/ml streptomycin at 37°, 6·8% carbon dioxide in a water saturated atmosphere. U937:Δp85 cells (a generous gift from Dr L. Stephens, Barbraham Institute, Cambridge, UK) were similarly cultured, but in addition were cultured in the presence of 0·6 mg/ml G418 and 0·1 mg/ml hygromycin B (Calbiochem, Nottingham, UK). Expression of Δp85 was induced with 15 mm isopropyl B-D-thiogalactoside (IPTG), 5 nm phorbol 12-myristate 13-acetate (PMA) and 100 μm zinc chloride for a period of 16 hr. All cells were differentiated with 1 mm dbcAMP (Sigma, Poole, UK.) 48 hr prior to experimentation.
FcγRI cross-linking
Cells were harvested by centrifugation and then incubated at 4° with 1 μm human monomeric IgG (Serotec, Oxford, UK) to occupy surface FcγRI. Excess unbound ligand was removed by dilution and centrifugation of the cells. Cells were resuspended in ice cold RPMI-1640/10 mm HEPES/0·1% bovine serum albumin (BSA) and cross-linking antibody (sheep antihuman IgG; 1:50 dilution) was added.12 Cells were then warmed to 37° for the times specified in the assays.
Measurement of PI3-kinase activity
PI3-kinase activity was measured as described previously.13 Briefly, cells were labelled with 500 μCi/ml [32P]PO4 for 90 min at 37°. Following labelling, cells were stimulated by cross-linking FcγRI, and the reactions stopped at specified times with ice-cold PBS. Generated PIP3 is resolved from lipid extracts by thin-layer chromatography (TLC), by reference to standards, and counted by liquid scintillation.
Cell fractionation
Subcellular fractions were prepared as described previously.25 Briefly, cells were lysed in nuclear preparation buffer and freeze–thawed three times, then the intact nucleus was isolated, and the cytosol was separated from nuclear-free membranes by high speed centrifugation.
Immunoprecipitation of translocated p85 and PLCγ1
The p85 subunit of PI3-kinase, and PLCγ1 were immunoprecipitated from the membrane fraction. Rabbit polyclonal anti-p85α antibody (Santa Cruz Biotechnology, Santa Cruz, CA), or mouse monoclonal anti-PLCγ1, D4 (raised against a C-terminal peptide26), were incubated with protein-A-agarose (50% slurry, Pharmacia Biotech, Uppsala, Sweden) at 4° rocking for 2 hr in order to form precipitating complexes. Samples from the membrane fraction were either incubated with either anti-p85-precipitating complexes, or anti-PLCγ1-precipitating complexes, placed in a tumbler at 4° for 4 hr, to immunoprecipitate specific proteins.
Gel electrophoresis and Western blots
Electrophoresis and Western blots were done as previously described.12 Briefly, proteins were resolved on 8% polyacrylamide gels (sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE)). The resolved proteins were transferred to 0·45 μm nitrocellulose membranes. Following blocking, the membranes were incubated with a rabbit polyclonal anti-p85α antibody (Santa Cruz Biotechnology); or mouse monoclonal anti-PLCγ1 (clone D4), or antiphosphotyrosine 4G10 (Santa Cruz Biotechnology), for 4 hr at room temperature. The membranes were then washed and incubated with suitable horseradish peroxidase (HRP)-conjugated secondary antibodies (Amersham, Little Chalfont, UK) for 4 hr at room temperature. Proteins were visualized using an ECL detection system (Amersham).
Measurement of inositol-1,4,5,-trisphosphate
Ins-1,4,5-P3 was measured using the BIOTRAK TRK 1000 kit (Amersham). Briefly, this is a competition binding assay in which cellular generated (unlabelled) InsP3 competes with a fixed, known amount of [3H]InsP3 for binding to the InsP3 receptor present in homogenates from bovine adrenal glands, which has a high affinity and specificity for InsP3.
Measurement of total inositol phosphates
Total inositol phosphates were assayed as previously described.27 Briefly, PtdInsP2 pools are labelled by preincubating cells with [3H]inositol. Prior to assay the cells are pretreated with 10 mm LiCl. Inositol phosphates are extracted and resolved by anion-exchange chromatography and quantified by liquid scintillation.
Measurement of DAG generation
Mass DAG was measured as previously described.12 Briefly, cellular lipids extracts are reconstituted into mock membrane micelles and incubated with DAG kinase and [32P-γ]ATP. Any DAG present in the cell sample will be phosphorylated to form PtdOH. PtdOH is resolved by TLC relative to standards and quantified by liquid scintillation.
PKC enzyme activity assay
PKC enzyme activity was assayed as previously described.25 Briefly, the assay is based upon the PKC catalysed transfer of the γ-phosphate group of ATP to a peptide substrate specific for PKC. The samples were assayed in the presence of 1·5 mm calcium, or substituting calcium with 1·5 mm egtazic acid (EGTA)-containing buffer.
RESULTS
FcγRI aggregation stimulates PI3-kinase activity
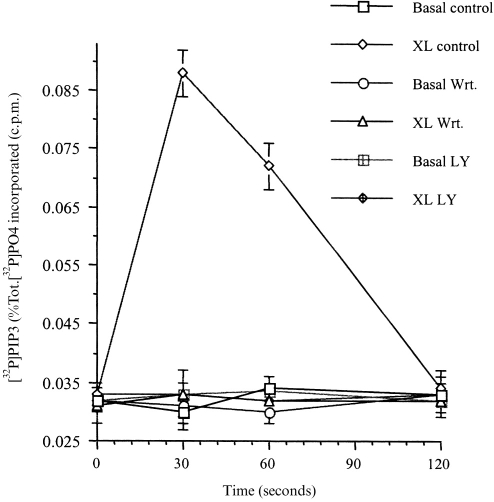
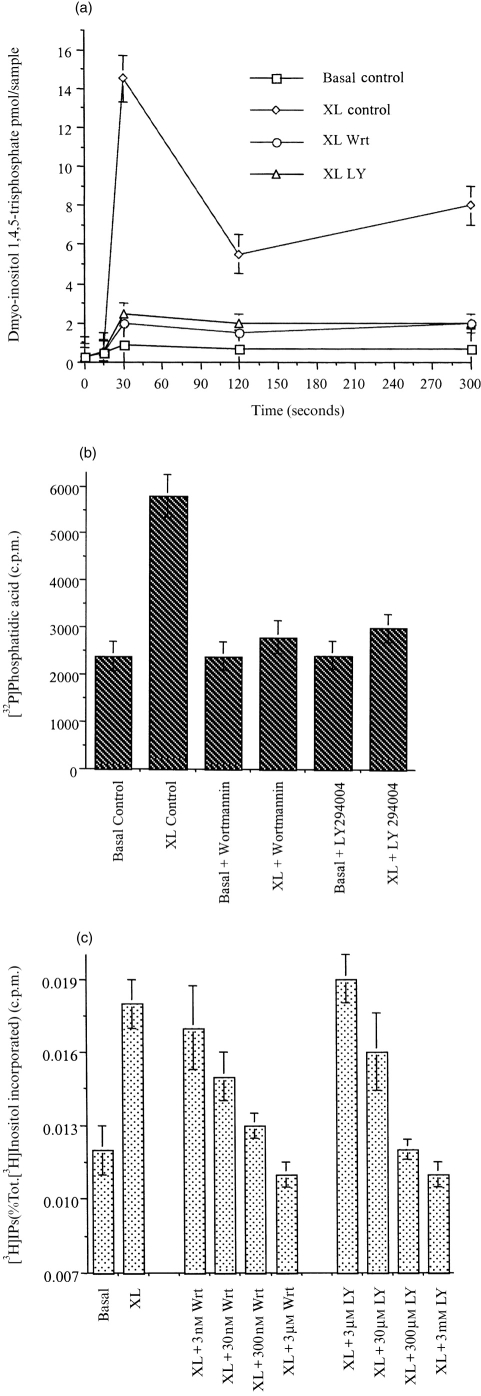
Following the aggregation of FcγRI, in dbcAMP-differentiated U937 cells, the cellular levels of PIP3 rose rapidly, reaching a peak 30 s after receptor aggregation (Fig. 1). After this peak, PIP3 levels fell and returned to basal levels 2 min after receptor stimulation (Fig. 1). All of this stimulated increase in PIP3 levels was abolished by pretreating the cells (for 30 min) with the PI3-kinase inhibitors wortmannin (50 nm) or LY294002 (250 μm) (Fig. 1).
Figure 1.
PI3-kinase is activated by FcγRI aggregation in dbcAMP-differentiated U937 cells.PIP3 production was measured following aggregation of FcγRI in untreated cells (XL), compared to cells pretreated for 30 min with 50 nm wortmannin (XL Wrt.), and LY294002 250 μm (XL LY); or in untreated cells were no crosslinking antibody was added (Basal); and in pretreated cells were no crosslinking antibody was added (Basal Wrt.); (Basal LY). Data is the mean±SD of triplicate measurements for each time point and are derived from three separate experiments.
PI3-kinase activation by FcγRI aggregation is tyrosine kinase dependent
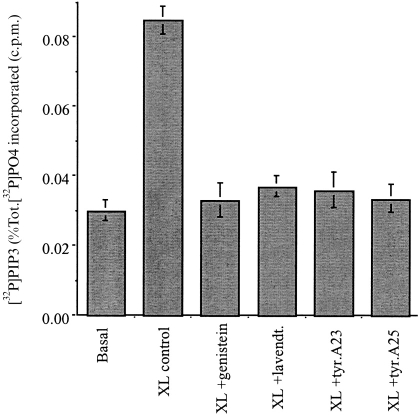
As signal transduction mediated by FcγRI requires the recruitment of non-receptor tyrosine kinases,7–10 the relationship of PI3-kinase activity to tyrosine kinase activation was investigated. Preincubation of cells for 30 min with tyrosine kinase inhibitors abolished the increase in PIP3 following FcγRI aggregation. Thus, genistein (0·37 mm); lavendustin A (2 μm); tyrphostin A23 (160 μm) or tyrphostin A25 (28 μm) all inhibited peak (30 s) PI3-kinase activity (Fig. 2).
Figure 2.
Inhibition of tyrosine-kinase activity blocks FcγRI-stimulated PI3-kinase activity. Peak 30 s) PIP3 production was measured in cells following FcγRI aggregation (XL), compared to cells pretreated with for 30 min with genistein 0·37 mm (XL+Gen); lavendustin A 2-μm (XL+lavendt.); tyrphostin A23 160 μm (XL+tyr.A23); or tyrphostin A25 28 μm (XL+tyr.A25); or in cells in which not crosslinking antibody was added (Basal). Data is the mean±SD of triplicate measurements for each time point and are derived from five separate experiments.
FcγRI aggregation stimulates tyrosine-kinase dependent (p85) isoform of PI3-kinase
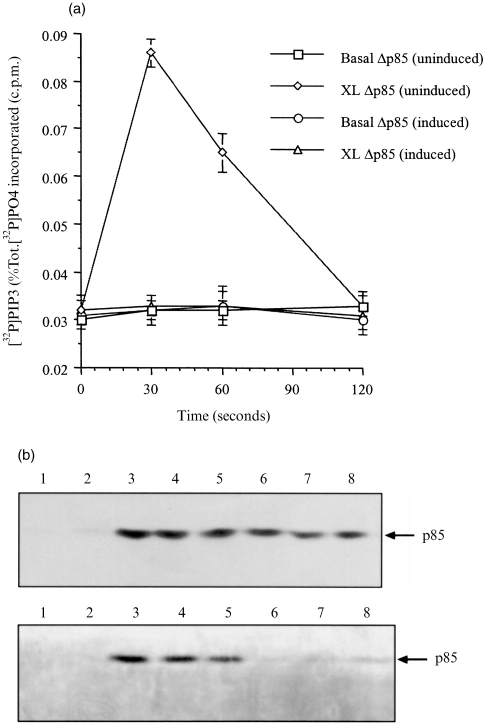
To examine whether the tyrosine-kinase regulated form of PI3-kinase accounted for all the increase in PIP3 after FcγRI aggregation, U937 cells expressing a dominant negative form of p85 were used (U937:Δp85); this dominant negative protein is regulated by an IPTG-inducible promoter. Over expression of this protein (Δp85), ablates p85-mediated PI3-kinase activation.13,28 Protein studies have shown that under IPTG induction the truncated p85 is expressed eight- to tenfold above the wild-type protein.13
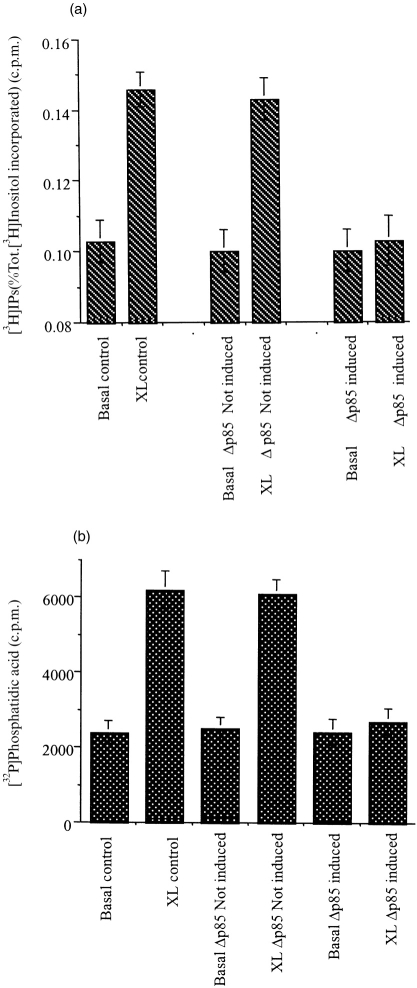
Initial studies were performed in non-induced U937:Δp85 to ensure that the kinetics were identical to the wild-type U937 cells. This comparison showed that in both cell types the temporal pattern of PIP3 levels in the cells was identical (Fig. 3b). After over expression of Δp85, the pattern of PIP3 was altered (Fig. 3a), and no increase in PIP3 levels was observed following FcγRI aggregation (Fig. 3a).
Figure 3.
(a) PI3-kinase coupled to FcγRI aggregation was inhibited in cells expressing the dominant negative, Δp85.Production of PIP3 following FcγRI aggregation in cells in which the Δp85 gene has been induced (XL Δp85 (induced)); or not induced (XL Δp85 (uninduced)); and non-crosslinked basal levels, induced (Basal Δp85 (induced)), not induced (Basal Δp85 (uninduced)). Data are the means±SD of triplicate measurements for each time point and are derived from three separate experiments. (b) The p85 subunit of PI3-kinase translocates to the nuclear-free membrane fraction and gets phosphorylated on tyrosine residues.Western blot analysis of immunoprecipitated p85 subunit of PI3-kinase translocated to the nuclear-free membrane fraction of dbcAMP differentiated U937 cells following FcγRI aggregation time course (upper panel) lanes: 1 unstimulated; 2 time 0; 3 15 s; 4 30 s; 5 1 min; 6 2 min; 7 5 min; 8 10 min. Tyrosine phosphorylation of translocated p85 (lower panel) lanes: 1 unstimulated; 2 time 0; 3 15 s; 4 30 s; 5 1 min; 6 2 min; 7 5 min; 8 10 min.
The kinetics of the translocation of p85-subunit of PI3-kinase to the membrane was assessed together with its tyrosine phosphorylation. Following FcγRI aggregation, p85 was rapidly translocated to the membrane fraction. Thus, no p85 could be detected in the membrane preparation in non-stimulated cells. However, 15 s after receptor aggregation p85 was present in the membrane fraction and remained associated with the membrane during subsequent time points (30 s, 1 min, 2 min, 5 min, and 10 min). At the later time points, the amount of p85 in the membrane preparation appeared to decrease (Fig. 3b upper panel). The p85 subunit in the membrane preparation was tyrosine phosphorylated (Fig. 3b lower panel). Thus, even at the earliest time point of translocation (15 s), p85 was tyrosine phosphorylated. Tyrosine phosphorylation of p85 could also be detected at 1 min and 2 min of the time course but not at later time points.
Aggregation of FcγRI activates phosphatidylinositol-specific phospholipase Cγ1
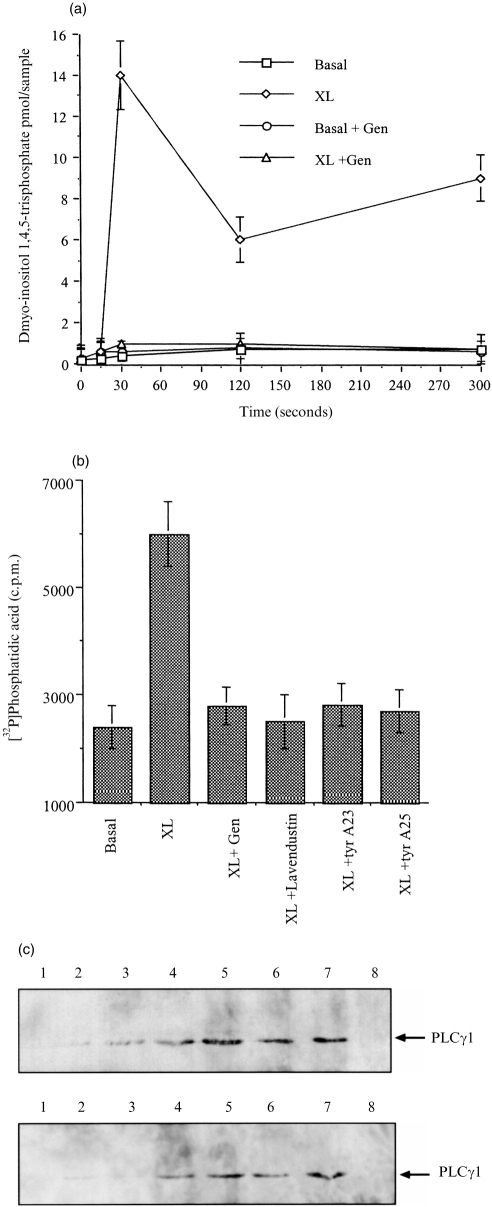
In dbcAMP-differentiated U937 cells, aggregation of FcγRI results in the generation of inositol-1,4,5-trisphosphate (InsP3) (Fig. 4a), and diacylglycerol (DAG) (Fig. 4b), which is dependent on tyrosine kinase activity as it is blocked by genistein (0·37 mm), lavendustin A (2 μm), tyrphostin A23 (160 μm), tyrphostin A25 (28 μm), inhibitors of tyrosine kinases (Fig. 4a,b).
Figure 4.
FcγRI aggregation activates PLCγ1, which is dependent on tyrosine kinases and translocates to the membrane. (a) Inositol-1,4,5-trisphosphate (InsP3) is generated in cells following FcγRI aggregation (XL), compared to cells pretreated with for 30 min with genistein 0·37 mm (XL+Gen); or in cells in which not crosslinking antibody was added (Basal). (b) Diacylglycerol (DAG) is produced in cells following FcγRI aggregation (XL), compared to cells pretreated with for 30 min with genistein 0·37 mm (XL+Gen); lavendustin A 2-μm (XL+lavendt.); tyrphostin A23 160 μm (XL+tyr.A23); and tyrphostin A25 28 μm (XL+tyr.A25); or in cells in which not crosslinking antibody was added (Basal). (c) Western blot analysis of immunoprecipitated PLCγ1 translocated to the nuclear-free membrane fraction of dbcAMP-differentiated U937 cells following FcγRI aggregation time course (upper panel) lanes: 1 unstimulated; 2 time 0; 3 15 s; 4 30 s; 5 1 min; 6 2 min; 7 5 min; 8 10 min. Tyrosine phosphorylation of translocated PLCγ1 (lower panel) lanes: 1 unstimulated; 2 time 0; 3 15 s; 4 30 s; 5 1 min; 6 2 min; 7 5 min; 8 10 min.
The PLC isoform activated following FcγRI aggregation was characterized by Western blot analysis. Following FcγRI aggregation, PLCγ1 rapidly translocates to the nuclear-free membrane fraction. Thus, no PLCγ1 could be detected in the membrane fraction prepared from resting cells. However, 15 s after receptor aggregation, a band corresponding to the correct molecular weight for PLCγ1 was detected in the membrane fraction by the anti-PLCγ1, monoclonal antibody Δ4 (Fig. 4c upper panel) and remained associated with the membrane for subsequent time points up to 5 min (15 s, 30 s, 1 min, 2 min and 5 min). The band corresponding to PLCγ1 was also positive when probed with the monoclonal antibody 4G10 indicating that this protein is tyrosine phosphorylated. Interestingly, the tyrosine phosphorylation was apparent only after 30 s (Fig. 4c lower panel) but remained tyrosine phosphorylated up to 5 min
FcγRI stimulation of PLCγ1 activity is downstream of PI3-kinase stimulation
Studies were undertaken to explore comparative kinetics of activation of PI3-kinase and PLCγ1 after FcγRI aggregation. As seen in Figs 1(a) and 4(a), the generation of lipid second messengers produced by PI3-kinase and PLCγ1, respectively, was very rapid in both cases reaching maximal activity 30 s after receptor stimulation. However, from the tyrosine phosphorylation data (Fig. 3a, lower panel and Fig. 4b, lower panel), it can be seen that the p85-subunit of PI3-kinase is tyrosine phosphorylated more rapidly than PLCγ1. To determine whether PI3-kinase activation precedes PLCγ1 activation, cells pretreated with PI3-kinase inhibitors were compared with control cells. Pretreating cells with wortmannin (50 nm) or LY294002 (250 μm) for 30 min, completely abolished the increase in InsP3 and DAG previously observed in response to FcγRI aggregation (Fig. 5a, b). To ensure that a transient or small peak in InsP3 was not missed an inositol phosphate (InsPs) accumulation assay was performed. Accumulation in total InsPs observed after receptor aggregation, was systematically reduced by pretreating cells with increasing amounts of either wortmannin or LY294002 (Fig. 5c).
Figure 5.
FcγRI stimulation of PLCγ1 is inhibited by PI3-kinase inhibitors. (a) InsP3 generation following aggregation of FcγRI in untreated cells (XL), compared to cells pretreated for 30 min with 50 nm wortmannin (XL Wrt.), or LY294002 250 μm (XL LY), and in cells where no crosslinking antibody was added (Basal). (b) Peak DAG generation following aggregation of FcγRI in untreated cells (XL), compared to cells pretreated for 30 min with 50 nm wortmannin (XL Wrt.), or LY294002 250 μm (XL LY), and in cells where no crosslinking antibody was added (Basal). (c) Total inositol phosphates generation following FcγRI for 30 min in untreated cells (XL), and in cells pretreated for 30 min with wortmannin 3 nm (XL+3 nm Wrt); wortmannin 30 nm (XL+30 nm Wrt); wortmannin 300 nm (XL+300 nm Wrt); wortmannin 3 μm (XL+3 μm Wrt); or LY294002 3 μm (XL+3 μm LY); LY294002 30 μm (XL+30 μm LY); LY294002 300 μm (XL+300 μm LY); LY294002 3 mm (XL+3 mm LY); or in untreated cells with no crosslinking antibody added (Basal).
Aggregation of FcγRI stimulates PLCγ1 that is dependent on p85-subunit of PI3-kinase
In order to define better the relationship between the activation of PI3-kinase and PLCγ1 and to rule out the possible direct inhibition of PLCγ1 activity by wortmannin or LY294002, the dominant negative p85 (U937:Δp85) cell line was used. Induction of the dominant negative p85 (Δp85) completely abolished the increase in InsP3 and DAG previously observed in the wild-type and non-induced (U937:Δp85) cells (Fig. 6).
Figure 6.
FcγRI stimulation of PLCγ1 is inhibited in U937:Δp85 cells when the dominant negative p85 (Δp85) is induced. (a) Total InsPs generation in wild-type U937 cells, with no crosslinking antibody added (Basal), or following FcγRI aggregation (XL); compared to the total InsPs generation in the U937:Δp85 cell line, when the Δp85 gene was uninduced with no crosslinking antibody added (Basal Δp85 uninduced), or following FcγRI aggregation (XL Δp85 uninduced); and when the Δp85 gene was induced with no crosslinking antibody added (Basal Δp85 induced); or following FcγRI aggregation (XL Δp85 induced). (b) Peak DAG production in wild-type U937 cells with no crosslinking antibody added (Basal); or following FcγRI aggregation (XL), compared to the peak DAG production in the U937:Δp85 cell line when the Δp85 gene was uninduced, with no crosslinking antibody added (Basal Δp85 uninduced), or following FcγRI aggregation (XL Δp85 uninduced); and when the Δp85 gene was induced, with no crosslinking antibody added (Basal Δp85 induced), or following FcγRI aggregation (XL Δp85 induced).
Aggregation of FcγRI stimulates PKC activity that is downstream of PI3-kinase activation
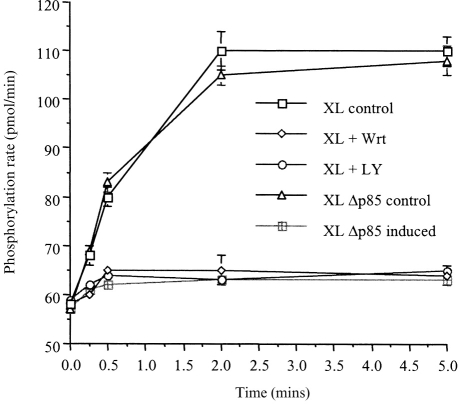
We have previously shown that in dbcAMP differentiated U937 cells, FcγRI aggregation stimulates conventional (calcium- and DAG-dependent) PKC activity.25 This knowledge, together with the observation that PI3-kinase is upstream of PLCγ1 in the signalling pathway initiated by FcγRI aggregation in these cells, leads us to suspect that PKC activity would also be downstream of PI3-kinase. PKC activity was therefore measured following FcγRI aggregation in cells pretreated with PI3-kinase inhibitors. PKC activity was indeed inhibited by the PI3-kinase inhibitors (Fig. 7). Consistent with this result, experiments carried out in the U937:Δp85 cells demonstrated that no PKC activity could be detected in cells where the dominant negative p85 (Δp85) was expressed (Fig. 7).
Figure 7.
Aggregation of FcγRI stimulates PKC enzyme activity that is downstream of PI3-kinase activation. PKC enzyme activity in wild-type U937 following FcγRI aggregation (XL); in U937 cells pretreated for 30 min with wortmannin (50 nm) prior to FcγRI aggregation (XL+Wrt), or U937 cells pretreated for 30 min with LY294002 (250 μm) prior to FcγRI aggregation (XL+LY). Compared to the PKC enzyme activity in the U937:Δp85 cell line following FcγRI aggregation in which the Δp85 gene has not been induced (XL Δp85 control), or following FcγRI aggregation in cells in which the Δp85 gene has been induced (XL Δp85 induced).
Taken together these results indicate that in this system, FcγRI aggregation results in the sequential activation of the tyrosine kinase dependent PI3-kinase, and that this activation is necessary for the subsequent activation of both PLCγ1, and PKC.
DISCUSSION
The results presented in this study demonstrate that, in dbcAMP-differentiated U937 cells, FcγRI is coupled through tyrosine kinase to the activation of p85-dependent PI3-kinase, PLCγ1, and PKC. Furthermore the data presented here demonstrates that both PLCγ1 and PKC activation is downstream of PI3-kinase. The dependence of PLCγ1 on PI3-kinase is demonstrated by two observations: first, the inhibition of intracellular InsP3 and DAG generation following pretreatment with the PI3-kinase inhibitors wortmannin or LY294002 (Fig. 5); and second, inhibition of PLCγ1 activation by a dominant negative (Δp85) p85-subunit of PI3-kinase (Fig. 6a). Thus, in cells differentiated to a macrophage phenotype with dbcAMP, FcγRI is coupled solely to the tyrosine-kinase activated form of PI3-kinase and this is required for coupling signal transduction to PLCγ1 and PKC activation. FcγRI contains no tyrosine motifs in its cytoplasmic tail5 but rather, to initiate signal transduction, the receptor recruits an accessory molecule. Previous work has shown that this receptor utilizes a molecular switch to initiate different signal transduction cascades depending on the differentiation state of the cell.7 The finding here that, in differentiated cells, FcγRI is coupled solely to p85 tyrosine-kinase activated form of PI3-kinase differs from that previous findings that, in cytokine primed cells this receptor is coupled to both tyrosine-kinase and G-protein-regulated PI3-kinase isoforms13 and provides further evidence for our proposal that FcγRI is able to switch signalling pathways in a differentiation-dependent fashion.9
In the dbcAMP differentiated cells, FcγRI is coupled to the activation of PLCγ17 and the conventional PKCs, α, β and γ.25 Here, we show that activation of both these signalling components is downstream of the activation of the tyrosine-kinase activated form of PI3-kinase.
PI3-kinase-dependent PLCγ activation has also been demonstrated in experiments carried out using the platelet-derived growth factor receptor (PDGFR).24 PDGF was shown to activate PI3-kinase and subsequently PLCγ in this report. Wortmannin and LY294002 inhibited PIP3 and InsP3 generation without preventing PI3-kinase association with the receptor.24 Of interest, Bae et al.22 reported that constitutive expression of the p110 catalytic subunit of PI3-kinase caused sustained increases in cellular InsP3 levels, without the necessity for receptor activation. This also supports the notion that PI3-kinase can be upstream of PLCγ activation. Moreover, in the same study it was reported that PIP3 can directly activate PLCγ hydrolytic activity in vitro and that this activation can be blocked by the addition of isolated SH2 domains of PLCγ. Various studies have shown that PIP3 can directly associate with SH2 domain-containing proteins and regulate their activity.18 Furthermore, recent in vitro data has demonstrated that PIP3 binds to the C-terminal SH2 domain of PLCγ1.24 However, recent work has shown that PIP3 also binds to a number of pleckstrin homology (PH) domains of proteins and that this binding is associated with the translocation of the protein to the membranes. Falasca et al.14 demonstrated that the N-terminal PH domain of PLCγ can bind PIP3 and that mutations of this domain diminished recruitment of PLCγ to the cell membrane, raising the possibility that simultaneous binding of the PH and SH2 domains to PIP3 may be involved in PLCγ enzyme activation. Regulating the translocation of the enzyme PLCγ provides a dual method of controlling its activation as translocation normally approximates the enzyme to various membrane-anchored kinases, but also localizes the enzyme to a cellular site rich in its substrate.
In these dbcAMP-differentiated cells, FcγRI has also been shown to be coupled to the activation of the conventional PKCs, α, β and γ. Here, this coupling was disrupted by overexpression of the dominant negative form of p85 indicating that this activation is also downstream of PI3-kinase activation. As conventional PKCs are activated by DAG and calcium, it seems likely that the failure of FcγRI to couple to PKC activation in these cells is secondary to the lack of PLC activation. However, PI3-kinase has also been shown to regulate activation of novel (calcium independent) and atypical PKCs that are independent of both calcium and DAG. In these studies PI3-kinase operates through the PDK1 regulatory domain by regulating the phosphorylation of PKCs δ and ζ.29 The results presented in this paper together with the recent observations that PIP2 and/or PIP3 can activate the serine/threonine protein kinases Akt/PKB and PDK125 and that PIP3 can stimulate the Arf1 nucleotide exchange protein, Grp1,26 suggest that PI3-kinase may regulate multiple and complex signalling pathways.
Our present data suggest that FcγRI-mediated activation of PI3-kinase is linked to the enhanced activation of protein kinase C through the stimulation of PLCγ1. We speculate that PI3-kinase, through the activation of PLCγ1, may also be linked to the release of intracellular calcium from InsP3-sensitive stores and activation of calcium entry. We have previously shown that in dbcAMP differentiated U937 cells, FcγRI mobilize intracellular calcium transients that are long lasting and that at the single cell level oscillates.7 The possibility that the magnitude and/or duration of intracellular calcium transients can moderate cell fate has recently been confirmed by the work of Dolmetsch et al.,30 in which they show that activation of nuclear factor (NF)-κB and c-Jun N-terminal kinase occurred with rapid high calcium transients, whereas nuclear factor of activated T cells (NFAT) was preferentially activated by low, more sustained calcium release. The differentiation-dependent switch in signalling pathways initiated by FcγRI therefore likely regulates the cell fate after immune complex activation.
Acknowledgments
This work was funded by a grant from the MacFeat bequest, University of Glasgow.
Abbreviations
- FcγRI
high affinity immunoglobulin G receptor
- PI3-kinase
phosphatidylinositol-3-kinase
- PIP3
phosphatidylinositol-trisphosphate
- PLCγ1
phospholipase Cγ1
- InsP3
inositol-1,4,5-trisphosphate
- InsPs
inositol-phosphate
- DAG
diacylglycerol
- PKC
protein kinase C
- dbcAMP
dibutyryl cyclic AMP.
References
- 1.Ravetch JV, Kinet J-P. Fc receptors. Ann Rev Immunol. 1991;9:457. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 2.Graziano RF, Fanger MW. FcγRI and FcγRII on monocytes and granulocytes are cytotoxic triggere molecules for tumor cells. J Immunol. 1987;139:3536. [PubMed] [Google Scholar]
- 3.Fanger MW, Shen L, Graziano RF, Guyre PM. Cytotoxicity mediated by human Fc receptors for IgG. Immunol Today. 1989;10:92. doi: 10.1016/0167-5699(89)90234-X. [DOI] [PubMed] [Google Scholar]
- 4.Ely P, Wallace PK, Givan AL, Guyre PM, Fanger MW. Bispecific-armed, interferon gamma-primed macrophage-mediated phagocytosis of malignant non-Hodgkin’s lymphoma. Blood. 1996;87:3813. [PubMed] [Google Scholar]
- 5.Allen JM, Seed B. Isolation and expression of functional hihg-affinity receptor for IgG complementary cDNAs. Science. 1989;243:378. doi: 10.1126/science.2911749. [DOI] [PubMed] [Google Scholar]
- 6.Scholl PR, Geha RS. Physical association between the high-affinity IgG receptor (FcγRI) and the γ subunit of the high affinity IgE receptor (FcγRI) Proc Natl Acad Sci USA. 1993;90:8847. doi: 10.1073/pnas.90.19.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melendez A, Floto RA, Cameron AJ, Gillooly DJ, Harnett MM, Allen JM. A molecular switch changes the signalling pathway used by the FcγRI antibody receptor to mobilise calcium. Curr Biol. 1998;80:210. doi: 10.1016/s0960-9822(98)70085-5. [DOI] [PubMed] [Google Scholar]
- 8.Liao F, Shin HS, Rhee SG. Tyrosine phosphorylation of phospholipase C-γ1 induced by cross-linking of the high-affinity or low affinity Fc receptor for IgG in U937 cells. Proc Natl Acad Sci USA. 1992;89:3659. doi: 10.1073/pnas.89.8.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholl PR, Ahern D, Geha RS. Protein tyrosine phosphorylation induced via the IgG receptors FcγRI and FcγRII in the human monocytic cell line THP1. J Immunol. 1992;149:1751. [PubMed] [Google Scholar]
- 10.Lin C-T, Shen Z, Boros P, Unkeless JC. Fc receptor-mediated signal transduction. J Clin Immunol. 1994;14:1. doi: 10.1007/BF01541170. [DOI] [PubMed] [Google Scholar]
- 11.Davis W, Sage SO, Allen JM. Cytosolic calcium elevation in response to Fc receptor cross-linking in undifferentiated and differentiated U937 cells. Cell Calcium. 1994;16:29. doi: 10.1016/s0143-4160(05)80005-3. [DOI] [PubMed] [Google Scholar]
- 12.Melendez A, Floto RA, Gillooly DJ, Harnett MM, Allen JM. FcγRI coupling to phospholipase D initiates sphingosine kinase-mediated calcium mobilization and vesicular trafficking. J Biol Chem. 1998;273:9393. doi: 10.1074/jbc.273.16.9393. [DOI] [PubMed] [Google Scholar]
- 13.Melendez AJ, Gillooly DJ, Harnett MM, Allen JM. Aggregation of the human high affinity immunoglobulin G receptor (FcγRI) activates both tyrosine kinase and G protein-coupled phosphoinositide 3-kinase isoforms. Proc Natl Acad Sci USA. 1998;95:2169. doi: 10.1073/pnas.95.5.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J. Activation of phospholipase C gamma by PI3-kinase-induced PH-domain-mediated targetting. EMBO J. 1998;17:414. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephens LR, Hughes KT, Irvine RF. Pathway of phosphatidylinositol-(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991;351:33. doi: 10.1038/351033a0. [DOI] [PubMed] [Google Scholar]
- 16.Kotani K, Yonezawa K, Hara K, et al. Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J. 1994;13:2313. doi: 10.1002/j.1460-2075.1994.tb06515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer RH, Schonwasser DC, Rahman D, Papin DJC, Herget T, Parker PJ. PRK1 phosphorylates MARCKS at the PKC sites: serine 152, serine 153 and serine 163. FEBS Lett. 1996;378:281. doi: 10.1016/0014-5793(95)01454-3. [DOI] [PubMed] [Google Scholar]
- 18.Inukai K, Anai M, van Breda E, et al. A novel 55 kDa regulatory subunit for phosphatidylinositol 3-kinase structurally similar to p55PIk is generated by alternative splicing of the p85 alpha gene. J Biol Chem. 1996;271:5317. doi: 10.1074/jbc.271.10.5317. [DOI] [PubMed] [Google Scholar]
- 19.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3-bisphosphate. Science. 1997;275:665. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 20.Alessi DR, James SR, Downes CP, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr Biol. 1997;7:261. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 21.Chardin P, Paris S, Antony B, et al. A human exchange factor for ARF contains Sec7-and plescktrin-homology domains. Nature. 1996;384:481. doi: 10.1038/384481a0. [DOI] [PubMed] [Google Scholar]
- 22.Bae Y, Cantley L, Chen C-S, Kim S-R, Kwon K-S, Rhee S. Activation of phospholipase C gamma by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:4465. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- 23.Nakanishi S, Catt KJ, Balla T. Inhibition of agonist-stimulated inositol 1,4,5-trisphosphate production and calcium signalling by the myosin light chain kinase inhibitor, wortmannin. J Biol Chem. 1994;269:6528. [PubMed] [Google Scholar]
- 24.Rameh LE, Rhee SG, Spokes K, Kaslauskas A, Cantley LC, Cantley LG. Phosphoinositide 3-kinase regulates phospholipase C gamma mediated calcium signalling. J Biol Chem. 1998;273:23750. doi: 10.1074/jbc.273.37.23750. [DOI] [PubMed] [Google Scholar]
- 25.Melendez AJ, Harnett MM, Allen JM. Differentiation-dependent switch in protein kinase C isoenzyme activation by FcγRI, the human high-affinity receptor for immunoglobulin G. Immunology. 1999;93:457. doi: 10.1046/j.1365-2567.1999.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coggeshall KM, McHugh JC, Altman A. Predominant expression and activation-induced tyrosine phosphorylation of phospholipase Cγ2 in B lymphocytes. Proc Natl Acad Sci USA. 1992;82:5660. doi: 10.1073/pnas.89.12.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harnett W, Harnett MM. Inhibition of murine B cell proliferation and down-regulation of protein kinase C levels by a phosphorylcholine-containing filarial excretory–secretory product. J Immunol. 1993;151:4829. [PubMed] [Google Scholar]
- 28.Hara K, Yonezawa K, Sakaue H, et al. 1-Phosphatidylinositol 3-kinase activity is required for insulin-stimulated glucose transport but not for RAS activation in CHO cells. Proc Natl Acad Sci USA. 1994;91:7415. doi: 10.1073/pnas.91.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 30.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]