Abstract
This study explores the expression and the function of major histocompatibility complex class II in the intestinal epithelial cell line CaCo-2, which has been widely used as a model for the human gastrointestinal epithelium. Human leucocyte antigen (HLA)-DR expression on CaCo-2 cells is induceable by interferon-γ (IFN-γ), but responsiveness to IFN-γ is dependent on cell differentiation and IFN-γ availability at the basolateral cell surface. HLA-DR expression is concentrated in apical cytoplasmic vesicles and on the basolateral cell surface. Invariant chain is expressed in apical vesicles but is absent from the cell surface. Immunoprecipitation studies show a slow rate of dissociation of HLA-DR from Ii. Double labelling shows some overlap between HLA-DR expression and basolateral endosomal markers but no overlap with apical endosomal markers. Functional studies show processing and presentation of lysozyme endocytosed from the basolateral, but not apical surfaces. CaCo-2 cells may provide a useful model with which to dissect the antigen-processing pathways in polarized epithelial cells. The regulated access of antigens taken up from the gut lumen to the processing compartments may prevent overloading the immune system with antigens derived from normal gut contents.
INTRODUCTION
The epithelium lining the gastrointestinal tract is one of the major sites at which foreign antigens (entering the body via the oral route) may gain access to the immune system, and thus induce an immune response or tolerance. There is very considerable interest in understanding the immunological events which occur at this site, for the purpose both of therapeutic intervention by the induction of tolerance,1 or prophylactic vaccination.2,3 Antigen uptake in the gut occurs preferentially through M cells overlying Peyer’s patches4 and presentation of transcytosed antigen is then presumed to involve ‘professional’ antigen-presenting cells (of dendritic lineage) in the underlying immunological tissue. However, intestinal epithelial cells other than M cells express major histocompatibility complex (MHC) class II molecules in both normal and inflamed tissue, suggesting that these cells can interact with CD4+ T cells,5–7 and epithelial antigen presentation, resulting in activation of antigen-specific T cells, has been reported both in vivo and in vitro.8–10 There is still controversy, however, over whether in vivo presentation by epithelial cells results in T-cell activation, in T-cell anergy, or in the induction of transforming growth factor-β (TGF-β) -producing ‘suppressor’ cells. It is also well established that epithelial cells can produce a whole variety of immunological mediators, including TGF-β, and thus play an active role in immunoregulation.11–13
This study focuses on the biosynthesis, distribution and function of class II MHC in epithelial cells. One major difference between intestinal epithelial cells and the much more extensively studied haematopoietic lineage antigen-presenting cells lies in the polarized, and hence vectorial, nature of the intracellular trafficking pathways in the epithelium. In order to analyse processing pathways in a meaningful way, an in vitro experimental model which retains the vectorial phenotype is therefore essential. One such model, which has been analysed extensively in studies of epithelial transport properties, is the human intestinal epithelial cell CaCo-2. Although this cell was initially derived from a colon carcinoma, it differentiates in vitro into a highly polarized cell exhibiting many of the characteristics of the small intestinal epithelium, including the development of microvilli at the luminal surface, tight junctions, and restricted and polarized distribution of many cell membrane receptors and brush border enzymes.14–16 A detailed analysis of MHC distribution and function in this single cell model identifies specialized features which relate to the polarized phenotype, and which should be further explored in future studies using other in vitro or in vivo models.
MATERIALS AND METHODS
Cell culture
CaCo-2 cells were maintained in stock culture and grown on Transwell inserts (6 mm filters; Costar U. K. Ltd, High Wycombe, Bucks, UK) as previously described.17 The differentiated cells were used for experiments after 10–14 days. Recombinant human interferon-γ (IFN-γ) at 1–3 ng/ml (PreproTech E. C. Ltd, London, UK) was added to the culture medium in either apical or basolateral compartments. IFN-γ was added for a certain time-interval only, unless otherwise stated (see the Results). T-cell hybridomas IC5.1 and AOIT were maintained in RPMI-1640 supplemented with 10% fetal calf serum (FCS), 2 mm glutamine, penicillin/streptomycin.
Antibodies
The following primary antibodies were used in this study: mouse monoclonal antibody (mAb) L243, specific for a monomorphic determinant expressed on mature MHC class II DR molecules;18 mouse mAb TAL14.1 Imperial Cancer Research Fund, specific for the monomorphic DRβ chain, which recognizes predominantly unfolded or immature DR dimers;19 mouse mAb LN2 (Biotest Serum, Dreieich, Germany) specific for the luminal portion of the invariant chain (carboxy terminal);20 mouse mAb BU45 (Binding Site, Birmingham, UK), specific for the luminal portion of Ii; Mab-11, mouse mAb against the apical marker of CaCo-2 cells;21 CII, mouse mAb against human collagen II (Dr R. Holmdahl, Lund University, Sweden) (used as a negative control); HBB2/614/88, mAb anti-human sucrase-isomaltase (kind gift from Dr H-P. Hauri, Basel, Switzerland); mouse mAb TIB93 (American Type Culture Collection, Rockville, MD), antimouse MHC class II I-Ak; rabbit anti-fluorescein isothiocyanate (FITC) antibody (Cambridge BioSciences, Cambridge, UK), rabbit antibodies against LMP-1 (gift of Dr S. Carlsson, University of Umea, Sweden), anti-mannose-6-phosphate receptor (gift of Dr B. Hoflack, Heidelberg, Germany). For immunofluorescence, secondary antibodies were a rabbit antimouse immunoglobulin G (IgG) Fc portion antibody; a swine antirabbit immunoglobulin conjugated to FITC (both from Dako Ltd, High Wycombe, Bucks., UK) and a goat antimouse IgG antibody conjugated to Texas Red (Cambridge BioScience). For the radioimmunoassay, an affinity-purified goat anti-mouse IgG antibody (Jackson ImmunoResearch, Inc., Bar Harbour, ME) was iodinated by the chloramine T (Sigma Chemical Co. Poole, UK) method using 125I (ICN, Thame, Oxfordshire, UK).
Immunofluorescence
Monolayers of CaCo-2 cells grown on filters were processed and labelled for single-colour or double-colour immunofluorescence as previously described,17 except that bovine serum albumin (BSA) was used as a blocking agent in place of gelatin. Labelling with Concanavalin-A Texas-red conjugate (100 μg/ml; Molecular Probes, Eugene, OR) was carried out on ice for 30 min, followed by three washes in phosphate-buffered saline (PBS). Slides were examined using a Zeiss fluorescence microscope with a MRC-600 scanning laser confocal apparatus (Bio-Rad Microscience Ltd, Hemel Hempstead, UK). Images were transferred to a PowerPC, processed using NIH-Image software and photographed using a Nikon FM camera.
Double labelling for HLA-DR and endosomal/lysosomal compartments
Transferrin and BSA (Sigma) were conjugated to FITC as follows.15 One millilitre of 100 mm sodium carbonate buffer pH 9·2 was added to 10 mg protein in 1 ml water. FITC (dissolved in ethanol) was added to give a 20 molar excess. After 2 hr at room temperature, the protein–FITC conjugate was separated from unbound FITC by chromatography over Sephadex G25 (Pharmacia Biotech., St Albans, Herts., UK), and concentrated by dialysis against PEG 6000 (Sigma). For uptake of transferrin, filter-grown monolayers were washed and incubated in serum-free DMEM with 0·2% BSA for 2 hr at 37° to deplete any residual transferrin in the system. FITC-BSA or FITC-transferrin were diluted 1:5 in 0·2% BSA/Dulbecco’s modified Eagle’s minimal essential medium (DMEM) (final concentrations: transferrin-FITC, 1 mg/ml, BSA, 4 mg/ml) and added to either the apical or basal compartments for 90 min at 37°. At the end of the incubation, filters were put on ice, washed in ice-cold PBS, fixed, quenched and processed as described above. In some experiments, the FITC signal was amplified using an antibody against FITC, followed by a secondary antibody conjugated to FITC. For the experiments shown in Fig. 6(d), FITC-dextran (10 mg/ml, Molecular Probes) was added to the basolateral compartment of the filter-grown monolayers, and Mab-11 mAb (as unpurified culture supernatant) was added to the apical compartment. Cells were incubated at 37° for 2 hr to allow internalization of both markers, washed, fixed and processed for indirect immunofluorescence using goat antimouse conjugated with Texas-Red (Cambridge Biosciences) to visualize the Mab-11 staining.
Figure 6.
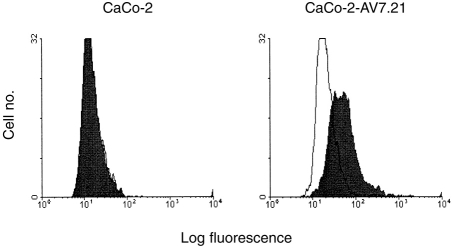
Flow cytometric analysis of I-Ak expression in CaCo-2–AV7.21 cells. CaCo-2 or CaCo-2–AV7.21 cells were stained with mAb TIB-93 (I-Ak; filled profile) or with non-specific antibody as control (CII; empty profile), and cells analysed by flow cytometry. Rabbit anti-mouse-FITC conjugate was used as second layer antibody. Staining with second layer antibody alone gave a similar profile to the control antibody.
Radioimmunoassay
Filters containing CaCo-2 monolayers were washed, fixed and quenched with 50 mm ammonium chloride as for immunofluorescence labelling, and then blocked and permeabilized with 1% BSA/0·5% saponin/PBS. The filters were then cut in half, and labelled with excess amounts of anti-class II antibody L243. The filter pieces were washed overnight in blocking solution on a shaker and then incubated with saturating amounts of 125I-labelled second antibody (2×106 c.p.m./filter piece) for 30 min. The filters were washed again extensively with PBS (4–5 hr) to remove any non-specifically bound antibody, and incorporated 125I was counted using a gamma-counter (Nuclear Enterprises NE1600, Edinburgh, UK) over 4 min.
Immunoprecipitation
Differentiated CaCo-2 monolayers grown in Transwell filters were treated for 10 days with IFN-γ (3 ng/ml). At the end of this period, cultures were washed thoroughly with PBS, and the medium was replaced with DMEM lacking methionine and cysteine, and supplemented with dialysed 2% FCS. Then, 250 μCi of 35S Translabel (a mixture of 80% methionine/20% cysteine, ICN Biomedicals Co., Oxfordshire, UK) was added to the basolateral compartment of each culture, and the cells incubated at 37° for 3 hr. Filters were washed to remove all medium containing free label, and the cultures were kept in complete medium, at 37°, for the chase period. Groups of four filters were used for each chase step (15 min, 3, 16 and 24 hr). After the chase, incubation filters were washed in PBS, and the cells lysed for 30 min at 4° in 0·5% nonidet P-40, 10 mm ethylenediamine tetraacetic acid (EDTA), 0·15 m Tris buffer, pH 7·4, containing the protease inhibitors leupeptin (50 μg/ml; Sigma), and PefaBloc (1 mm; Pentapharm A.G., Basel, Switzerland). After lysis, the nuclei were removed by centrifugation (1000 g, 20 min) and the cell lysates frozen until required. For immunoprecipitation, cell lysates were sequentially precipitated with 20 μl protein A–Sepharose (Sigma), preloaded with rabbit anti-mouse immunoglobulin (Dako), and either with mAb anti-HLA-DRα (TAL14.1); or mAb anti-Ii (BU45) or normal mouse serum (as negative control). The beads were washed extensively in lysis buffer, resuspended in sample buffer, and boiled for 5 min before loading on a 12·5% polyacrylamide gel.
Flow cytometry
CaCo-2 cells grown on Transwells were treated with IFN-γ (3 ng/ml) for 6–7 days. Cells were detached from filters using 1 mm EDTA, and washed in cold PBS. Approximately, 106 cells were used per staining. For analysis of surface expression of the IAk molecule on transfectants, CaCo-2 and CaCo-2–AV7.21 cells were stained with specific mAb TIB-93, and mAb CII as control, in PBS with 1% FCS/0·1% NaN3, at 4° for 30 min. For intracellular staining, cells were initially fixed in 4% paraformaldehyde, and permeabilized in PBS with 0·1% saponin/1% FCS/0·1% NaN3 (permeabilizing buffer). Cells were stained with anti-HLA-DR (L243); Ii (LN2) and intracellular adhesion molecule-1 (ICAM-1)22 diluted in permeabilizing buffer, at 4° for 30 min. A non-specific, isotype-matched antibody (anti-CII) was used as negative control. All washes were done in permeabilizing buffer. FITC-conjugated rabbit antimouse immunoglobulin (Dako) was used as a second layer antibody. Stained cells (5000 events) were analysed on a Becton and Dickinson fluorescence-activated cell sorter (FACScan) flow cytometer (Becton-Dickinson, Cowley, Oxford, UK).
Antigen processing and presentation assay
In order to assay antigen processing and presentation by CaCo-2 cells we transfected these cells with the I-Ak murine class II molecule. Cells were transfected by electroporation with an expressing vector containing both the I-Akα- and β-chains, each driven independently by the cytomegalovirus promoter,23 and also containing the hygromycin-resistance gene. Hygromycin-resistant cells were cloned by limiting dilution, and assayed for I-Ak expression by flow cytometry.
For presentation experiments, the CaCo-2 I-Ak-expressing clone AV7.21, or untransfected parental cells were initially seeded onto Transwell inserts (12 mm; 3 μm pore size, coated with collagen), set normally the ‘right-way up’ in plates or set ‘upside-down’ in individual containers. For the ‘upside-down’ cultures, cells were allowed to attach to the Transwell membrane for a period of 24 hr before filters were then inverted and transferred to 12-well plates. Cells were cultured for a further 14 days, either with or without IFN-γ, with change of media every 2 days.
Confocal immunofluorescent staining of the 14 day cultures showed that by this time the CaCo-2 cells were confluent, but formed an inverted monolayer in which the basal surface of the cells was uppermost, and exposed to the upper chamber through the 3 μm holes in the filters. Hen egg white lysozyme (HEL, grade V, Sigma), or a synthetic peptide containing the amino acid residues 46–61 or 74–85 of lysozyme, was added to either the upper (basolateral) or lower (apical) chamber of the Transwell and cultured with the CaCo-2 cells for 18 hr, at 37°. Both chambers were then thoroughly washed, and 105 cells of the lysozyme-specific T hybridoma line IC5.1 (a gift of D.Wraith, Cambridge, UK), specific for the HEL 46–61 epitope, or the T hybridoma line AOIT, specific for the HEL 74–85 epitope, were added to the upper chamber. The cocultures were incubated for a further 24 hr, and supernatants were collected from both upper and lower chambers and tested for interleukin-2 (IL-2) using the IL-2-dependent CTLL line as previously described.24 In some experiments CaCo-2–AV7.21 monolayers were fixed with 0·05% gluteraldehyde for 30 seconds, followed by addition of 0·2 m lysine for 2 min and washed with PBS. In order to determine if the intestinal epithelial cells were non-specifically immunosuppressive for T-cell responses, murine (CBA) spleen cells (used as antigen-presenting cells), T hybridomas and antigen were cocultured with or without CaCo-2 monolayers and supernatants were assayed for IL-2. The presence of CaCo-2 cells did not alter IL-2 production in cultures.
RESULTS
MHC II expression by CaCo-2 cells requires induction by IFN-γ
Differentiated monolayers of CaCo-2 cells (after ±7–10 days of in vitro culture) showed the characteristic polarized distribution of the apical markers P100 (not shown) and sucrose-isomaltase (not shown) and expressed class I HLA at the basolateral surface (not shown). However, immunofluorescence using the monomorphic anti-human class II (HLA-DR) antibody L243, failed to detect expression of HLA-DR molecules on either undifferentiated CaCo-2 cultures (not shown) or fully differentiated CaCo-2 monolayers.
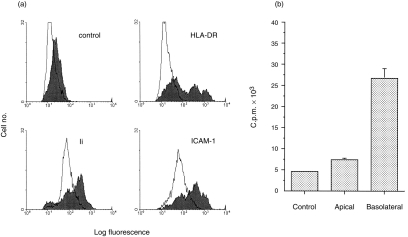
Since the cytokine IFN-γ has previously been shown to induce class II MHC on the non-polarized epithelial cell line HT-29,7,25 CaCo-2 monolayers were allowed to differentiate and were then incubated in the presence of 3 ng/ml IFN-γ. Limited expression was observed after 48–72 hr of culture, but expression continued to increase in intensity, and the strongest expression of HLA-DR became apparent after 6–7 days (Fig. 1a). In parallel, IFN-γ also increased the expression levels of invariant chain (Ii) and ICAM-1 (CD54), two molecules which are co-ordinately regulated by IFN-γ in other cell types (Fig. 1a).
Figure 1.
Expression of HLA-DR in CaCo-2 cells. (a) Flow cytometry of CaCo-2 cells. Differentiated CaCo-2 cell monolayers were treated with IFN-γ (3 ng/ml), for 6–7 days (filled profiles) or left untreated (open profiles). Cells from three filters were pooled, fixed, permeabilized and stained for expression of HLA-DR (L243 mAb); Ii (BU45 mAb), or ICAM-1. CII mAb was used as an non-specific isotype control (top left panel). Rabbit anti-mouse-FITC conjugate was used as second layer antibody. staining with second layer antibody alone gave a similar profile to that using the non-specific mAb (one experiment of three). (b) IFN-γ acts only at the basolateral surface of CaCo-2 cells. Cells were grown on filters and IFN-γ (1 ng/ml) was added either to the apical or basolateral chamber of duplicate filters, for 6–7 days. At the end of the culture period, the filters were fixed, cut in half, permeabilized and labelled with L243 mAb (HLA-DR), followed by iodinated anti-mouse IgG antibody. The bars show the range of duplicate samples (one experiment of four).
Importantly, IFN-γ only induced expression of HLA-DR when applied to the basolateral compartment of the cell, and not when added to the apical surface (Fig. 1b). The presence of IFN-γ for up to 7 days did not affect the permeability of the monolayers to horseradish peroxidase (not shown) consistent with previous reports that although IFN-γ increased membrane conductance it did not affect epithelial integrity when used at low doses.26
The induction of MHC class II by IFN-γ occurred only after CaCo-2 cells were allowed to differentiate, since HLA-DR expression was not observed in those cell monolayers exposed to IFN-γ within the first 6 days of seeding (not shown). A concentration of 1–3 ng/ml IFN-γ in the culture medium was optimal for HLA-DR induction, and 10 ng/ml showed no further increase in MHC class II induction, and appeared to be somewhat toxic to the cells (not shown).
Distribution of HLA-DR and Ii in CaCo-2 cells
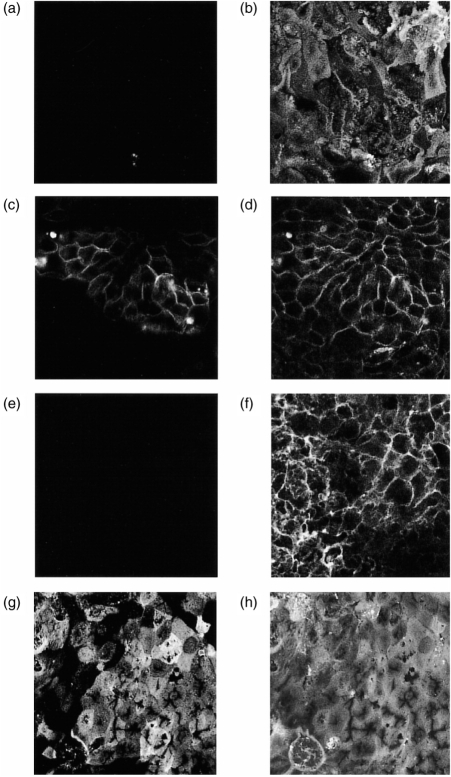
The distribution of HLA-DR at the CaCo-2 cell membrane is examined in detail in Fig. 2. HLA-DR expression on the basolateral membrane (Fig. 2c), adjacent to the filters (equivalent to the serosal side of the intestinal mucosa), colocalized with basolateral Concanavalin A staining (Fig. 2d), although expression was heterogeneous within the monolayer. In contrast, no expression was observed at the apical (or luminal) surface (Fig. 2a). The reverse pattern was observed for the apical cell surface marker recognized by mAb Mab-1121 (Fig. 2e–h), indicating that there was no intrinsic problem in staining the apical surface of the monolayer.
Figure 2.
Distribution of HLA-DR on the membranes of CaCo-2 cells.Cells were grown on filters in the presence of IFN-γ as described in the Materials and Methods, and labelled by immunofluorescence for the presence of HLA-DR with L243 antibody (a and c) or Mab-11 (e and g) together with Concanavalin A Texas Red conjugate (b, d, f and h). Confocal sections were taken parallel to the monolayer, and representative sections at the apical (a/b and g/h) and basolateral (c/d and e/f) are shown. The Concanavalin A staining labelled all cell membranes non-specifically, and was useful in identifying the plane of the membranes, especially when staining for a specific marker was negative. Note that no apical staining for HLA-DR was detected, while a ‘cobblestone’ pattern is seen on the basolateral membrane. In contrast, Mab-11 stained only the apical but not the basolateral surface. Results are from one experiment of eight.
A substantial amount of HLA-DR staining was found intracellularly within the CaCo-2 cells, predominantly in a group of vesicles situated in the apical cytoplasmic region of the cell, lying just above the nucleus (Fig. 3a). Ii detected using antibody (BU45) which recognizes an epitope on the luminal portion of Ii, which is lost during the proteolytic cleavage which precedes the dissociation of Ii from the mature MHC class II dimer, also stained apical cytoplasmic vesicles similar to those stained with anti-HLA-DR antibodies (Fig. 3b). In addition, Ii was found in a tubular, vesicular structure underlying the basolateral membrane (Fig. 3c), but no colocalization with the plasma membrane itself was observed. Although it proved difficult to colocalize Ii and HLA-DR by confocal microscopy (due to low-intensity staining), the association of these two molecules was demonstrated using immunoprecipitation.
Figure 3.
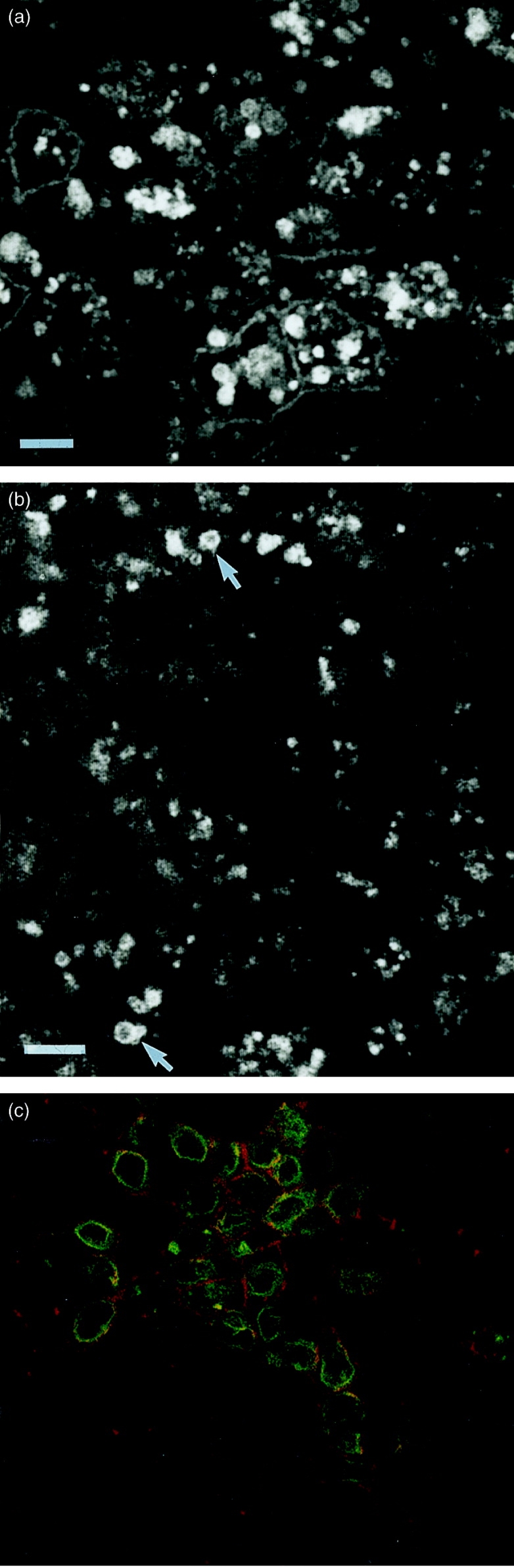
Intracellular distribution of HLA-DR and Ii. Cells were grown on filters in the presence of IFN-γ as described in the Materials and Methods, and labelled by immunofluorescence for the presence of HLA-DR (a) or Ii (b and c). (a) and (b) show optical sections taken though the cells above the nucleus, where the cytoplasm is full of heterogeneous vesicular structures. (c) is taken just above the filter, showing the basolateral surface labelled with Concanavalin A Texas Red, together with submembrane tubular vesicular structures stained for Ii (green). Bars represent 1 μm. The results are from one experiment of four.
Immunoprecipitation of class II/Ii trimers from CaCo-2
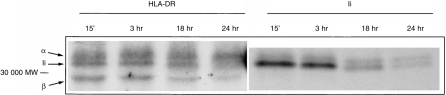
CaCo-2 monolayers were incubated in the presence of IFN-γ, and the biosynthesis of HLA-DR and Ii investigated by pulse-chase immunoprecipitation (Fig. 4). An antibody to the β-chain of HLA-DR precipitated a trimer which consisted of the two chains of HLA-DR, together with an associated invariant chain.27 Some Ii was still associated with HLA-DR after 16 hr, indicating a rather slow rate of HLA-DR maturation, but by 24 hr, substantially less HLA-DR was bound to Ii (see lane 4).The identity of the 33 000 MW middle band was confirmed by direct immunoprecipitation with anti-Ii antibody (lanes 5–8), which also precipitated a minor band probably corresponding to an alternative splicing variant of Ii.27 The Ii chain did not coprecipitate HLA-DR since this antibody does not efficiently recognize Ii complexed to HLA-DR.
Figure 4.
Immunoprecipitation of class II/Ii trimers in CaCo-2 cells. Filter-grown cells, induced with IFN-γ, were incubated with Translabel for 1 hr, and then in ‘cold’ medium for various times as shown. Samples were immunoprecipitated with either TAL14.1 (anti HLA-DRβ) or BU45 (anti-Ii) and analysed by SDS–PAGE. The arrows show, from top to bottom, the position of the α-chain of HLA-DR (34 000 MW), the major invariant chain isoform (31 000 MW), and the β-chain of HLA-DR (29 000 MW). No bands were precipitated in this region by a control antibody. Results are from one experiment of three.
The position of HLA-DR molecules in the endocytic pathway
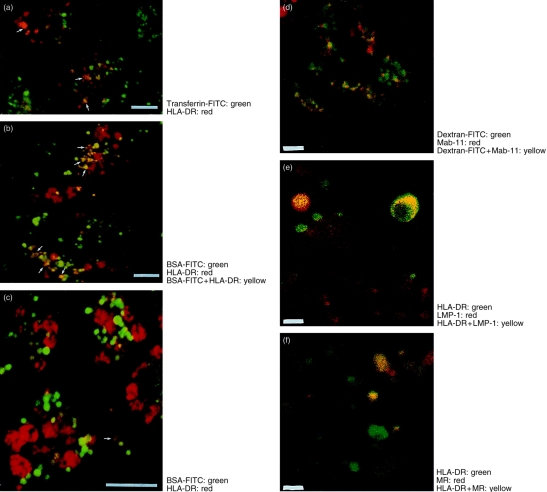
CaCo-2 cells contain two independent endocytic pathways, accessed from the apical and basolateral surfaces although both pathways have been shown to share a common late endosomal/lysosomal compartment.21 The transferrin receptor is expressed exclusively at the basolateral surface, and is consequently a convenient marker for the early basolateral endocytic pathway. FITC-transferrin labelled a population of endosomes close to the basal plasma membrane which are class II MHC negative. However, some labelling of vesicles in the apical cytoplasm was also observed and a small proportion of these vesicles colocalized with class II MHC (Fig. 5a). A similar degree of colocalization was observed when the basolateral endocytic pathway was labelled with FITC-BSA, a generalized fluid-phase marker of the endosomal/lysosomal compartment (Fig. 5b). When FITC-BSA was applied only to the apical surface this marker labelled a heterogeneous population of vesicles in the apical cytoplasm of the cell. Although these vesicles were sometimes very closely juxtaposed to Class II DR-positive vesicles (Fig. 5c), overlap of the two labels was not seen. Similar results were obtained using FITC-dextran labelling for various time periods (not shown), indicating that the lack of colocalization was not due to rapid BSA degradation.
Figure 5.
The distribution of HLA-DR and endocytic tracers in CaCo-2 cells. Induced CaCo-2 monolayers were labelled as described in Materials and Methods. Two-colour optical sections were taken through the cells above the nucleus. Labelling for both markers is indicated by yellow pseudocolour. Experiments shown in (a)–(c) were carried out five times, while those in (d)–(f) were carried out twice. (a) Transferrin-FITC (green) endocytosed at the basolateral surface, followed by immunolabelling with HLA-DR (red). Note occasional colocalization. (b) BSA-FITC endocytosed at the basolateral surface followed by immunolabelling with HLA-DR (red). (c) BSA-FITC endocytosed at the apical surface followed by immunolabelling with HLA-DR (red). Note lack of colocalization. (d) FITC-Dextran (green) endocytosed at the basolateral surface, and Mab-11 antibody endocytosed from the apical surface for 1 hr. Endocytosed Mab-11 was visualized using anti-mouse immunoglobulin Texas-Red conjugate (red). Note extensive colocalization. (e) Immunolabelling of HLA-DR (green) and the lysosomal marker LMP-1 (red). Note the presence of small red structures which are HLA-DR negative, and the presence of larger HLA-DR-containing vesicles which also contain LMP-1. (f) Immunolabelling of HLA-DR (green) and the mannose-6-phosphate receptor (red). Both singly and doubly labelled vesicles are visible. Bars represent 1 μm.
In order to confirm that the confocal technique we were using was indeed capable of detecting true overlapping populations of vesicles, cells were loaded from the basolateral surface with FITC-dextran, and apically with the mAb Mab-11. Previous studies using electron microscopy have shown that under these conditions both markers colocalize to a common population of lysosomes.15,21 As shown in Fig. 5(d), under these conditions extensive colocalization of the two markers was observed, labelling vesicular structures which were quite different in appearance from the larger HLA-DR-containing vesicles.
In order to identify further the nature of the HLA-DR-containing vesicles, the CaCo-2 monolayers were double stained for HLA-DR and either the lysosomal marker LMP-1 (Fig. 5e) or the cation-independent mannose-6-phosphate receptor (Fig. 5f). Antibodies to LMP-1 labelled small HLA-DR-negative structures which are likely to represent lysosomes, as well as showing some limited colocalization with HLA-DR. The mannose-6-phosphate receptor antibody labelled many of the large characteristic HLA-DR vesicles, although populations which expressed HLA-DR, but not mannose-6-phosphate receptor, and vice versa could be observed.
Antigen processing of basolateral versus apical antigen
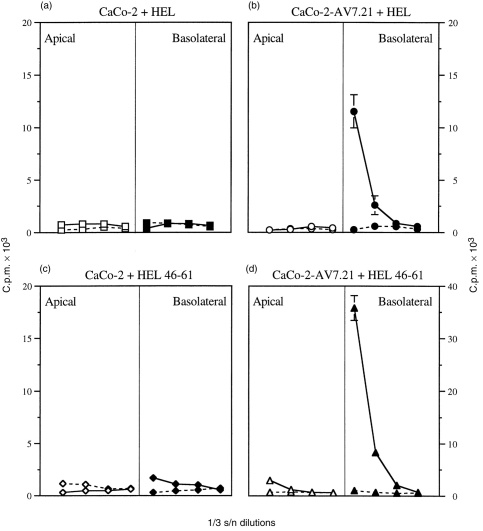
CaCo-2 cells were transfected with a plasmid expressing both chains of the murine H-2 I-Ak molecule in order to study antigen processing and presentation of a nominal antigen (HEL) by CaCo-2 cells, using the response of murine T-cell hybridomas as a read out. As shown in Fig. 6 the transfectants, but not the parental cells, expressed H-2 I-Ak as detected by flow cytometry. Confocal immunofluorescence analysis showed that surface expression and intracellular distribution of I-Ak were very similar to that of the endogenous IFN-γ-induced HLA-DR (not shown). Preliminary immunoprecipitation experiments have also established that the murine MHC class II molecule associates normally with the human invariant chain (data not shown). For the functional studies, differentiated ‘upside-down’ monolayers of either the transfectants (CaCo-2-AV7·21), or the parental CaCo-2 cells were incubated with HEL or HEL peptides and subsequently cocultured with HEL-specific T-cell hybridomas (added at the basolateral surface of CaCo-2 cells). As shown in Fig. 7, CaCo-2–AV7.21 pulsed overnight either with whole HEL or HEL 46–61 peptide at the basolateral side were able to stimulate the T cells, resulting in release of IL-2 into the upper chamber. Surprisingly however, no antigen presentation was observed when HEL or HEL peptides were added to the apical compartment of CaCo-2–AV7.21 cultures (Fig. 7b,d). The parental CaCo-2 cells failed to present either of the forms of the antigen added to the cultures from either of the compartments (Fig. 7a,c). The low levels of measurable IL-2 in the lower chamber confirm that during the culture period the CaCo-2 cells have formed a tight monolayer and no macromolecular leakage between compartments was seen.
Figure 7.
Antigen processing and presentation of antigen added to apical or basolateral compartments of CaCo-2 cells. Antigen processing was assayed as described in the Materials and Methods. (a) and (c) show assay of the CaCo-2 parental line, and (b) and (d) show assay of the I-Ak-transfected CaCo-2–AV7.21 cells. HEL (200 μm, a,b) or HEL 46–61 (2 μm; c,d) was added to either the apical or basolateral chamber of Transwell cultures of CaCo-2.The antigen was added to the CaCo-2 cells (parental or transfectant) for 18 hr prior to addition of the HEL-specific T-cell hybridoma IC5·1. Culture supernatants were collected from both basolateral (solid line) and apical (broken line) chambers, and tested for IL-2 by titration on CTLL cells as described. The results show mean±SEM of [3H]thymidine incorporation from triplicate CTLL IL-2 indicator cultures and represent one experiment of five. Supernatants from CaCo-2 or CaCo-2–AV7.21 cultures incubated with medium alone gave c.p.m. of less than 2000.
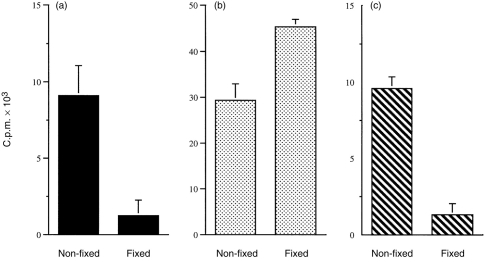
The degree of stimulation by CaCo-2–AV7.21 was, in general, lower if HEL was added as intact/native rather than in the peptide form, and much higher concentrations of intact lysozyme were required for processing/presentation. Similar results were obtained in the absence or presence of IFN-γ (not shown), perhaps reflecting the presence of some Ii even in unstimulated CaCo-2 cells (Fig. 1a). However, basolateral presentation of intact HEL was abrogated by prior fixation of the cells, while fixed monolayers still presented peptides effectively, confirming the ability of CaCo-2 cells to process HEL (Fig. 8a,b).
Figure 8.
Fixation abrogates antigen processing by CaCo-2–AV7.21 cells. Antigen processing by non-fixed and fixed CaCo-2–AV7.21 cells was assayed as described in the Materials and Methods. Data are from cell cultures pulsed with antigen at the basolateral compartment, for 18 hr. (a) HEL (200 μm); (b) HEL 46–61 (2 μm); (c) HEL 74–85 (2 μm). In (a) and (b), CaCo-2–AV7.21 cells were cocultured with IC5.1 hybridoma cells specific for the 46–61 epitope, while in (c) the T-cell hybridoma AOIT, specific for 74–85, was used. The results show mean±SEM of [3H]thymidine incorporation from triplicate CTLL IL-2 indicator cultures in presence of supernatants collected from basolateral chamber only. Supernatants collected from the apical side of the same cultures showed counts < 2000, as did supernatants from cultures incubated in the presence of medium alone.
CaCo-2–AV7.21 cells were further tested for antigen presentation using another T hybridoma line – AOIT, specific for an epitope sequence within the HEL 74–85 peptide. As previously shown (unpublished information), this peptide requires some limited further processing to stimulate AOIT cells. As shown in Fig. 8(c), HEL 74–85 peptide added at the basolateral compartment of CaCo-2–AV7.21 cultures was also presented to AOIT cells, and such presentation was also abrogated by previous fixation of the monolayer.
In contrast to the experiments above, in which ‘upside-down’ monolayers were used, when CaCo-2–AV7.21 monolayers were set up in the ‘right-way up’, so that T cells added to the Transwells were in contact with the apical cell surface, no stimulation was observed from filters pulsed with HEL or peptides added from either compartment (c.p.m. < 1000 with or without antigen).
DISCUSSION
This study is a detailed analysis of antigen processing in a cell of human intestinal epithelial origin. The results highlight a number of unusual features of the antigen-processing machinery in this cell line, which are discussed in detail below.
A first striking feature observed was the polarized nature of class II HLA-DR expression, with surface expression almost entirely restricted to the basolateral surface. This distribution is consistent with the site of T-cell/epithelial cell interaction in vivo, and has been reported in some electron microscope studies of HLA distribution,28,29 as well as in a recent study on T84 cells.9 However, other studies using light immunohistochemistry have reported HLA-DR on both surfaces of the intestinal epithelium, and this question clearly needs further study to resolve these apparent contradictions. The results further suggest that the functional IFN-γ receptor, as well as HLA-DR, is expressed only at the basolateral surface. Such a polarized distribution has already been documented for the human intestinal cell lines T84 and HT29,26,30 and is consistent with the in vivo microenvironment, where T-cell-derived cytokines are presumably found only at the basolateral surface of the epithelium.
It was also interesting that HLA-DR expression could not be induced in undifferentiated CaCo-2 cells. A linkage between acquisition of the mature enterocyte phenotype, and HLA-DR expression is also observed in vivo, where it results in the well-documented role in the graded distribution of class II MHC observed along a small intestine villus.31 Further studies will determine whether this linkage is due to graded expression of the IFN-γ receptor, or to differentiation-linked expression of some intracellular component of the IFN-γ signalling pathway.
The majority of the MHC class II molecules were apparently localized to a population of vesicles in the apical cytoplasm of the cell, which were similar to those identified by antibody to Ii. It seems likely that these vesicles are a compartment within the HLA-DR biosynthetic pathway, from which mature HLA dimers are then transported to the basolateral cell surface (the equivalent to the antigen-loading compartment described in other conventional antigen-presenting cell types32). The results of the immunoprecipitation experiments, however, as well as the relative amounts of class II intracellularly and at the cell surface, suggest that formation of mature class II dimers is a relatively slow process.
The results of colocalization studies also suggest that the HLA-DR-containing compartments are part of the biosynthesis pathway, and distinct from the major endocytic pathways of either the basolateral or apical surfaces. Thus the basolateral endosomal compartment, identified with the markers to transferrin (early endosome) or BSA-FITC or dextran-FITC (later endosomal markers), showed only a limited degree of overlap with the class II-containing vesicles. No significant overlap could be detected between class II expression and BSA or dextran-FITC loaded through the apical compartment. Nevertheless, as described previously, the apical and endosomal endocytic pathways do share a common lysosomal end-point.15,21 In CaCo-2 cells therefore, as in many other cell types, the intracellular MHC Class II compartments are part of the biosynthetic, as opposed to the endocytic, pathways and although sharing some markers, are distinct from the lysosomal degradative organelle (identified in this study as LMP-1-positive, HLA-DR-negative vesicles).
CaCo-2 cells seem to differ from conventional antigen-presenting cells, however, in that the degree of access allowed between the endocytic pathway and the class II-containing compartments is much more limited, and particularly that apical endosomal traffic on its way to the lysosome does not intersect significantly with the MHC-containing vesicles. In contrast, a previous study,32,33 using ex vivo intestinal explants, did report colocalization of protein taken up from the luminal surface and endogenous HLA Class II. It seems likely that, in vivo, the uptake and processing by epithelial cells are regulated by other factors, perhaps from the underlying lymphocyte population. Increased apical antigen uptake and transport by CaCo-2 cells in response to signals from mucosal lymphocytes have in fact been recently reported.34 Similarly, the nature of the antigen may also determine the efficiency of apical or basolateral processing.9,35
Both immunoprecipitation and immunofluorescence give only indirect evidence on the antigen-processing function of CaCo-2 cells. In order to analyse the processing and presentation by CaCo-2 cells directly, we transfected CaCo-2 cells with an exogenous murine MHC molecule. Although the introduction of a murine restriction model into a human cell line might potentially complicate the interpretation of our studies, previous studies have demonstrated that murine class II molecules associate normally with human invariant chain and present normally to mouse cells.36
One advantage to this approach, rather than using the endogenous HLA-DR molecules, is that we could then use the very well-characterized, I-Ak-restricted, lysozyme-specific hybridomas as a read-out of antigen presentation. However, the more important advantage of our system is that these murine hybridoma T cells are much less dependent on ‘second signals’ via accessory molecules on their surface, and therefore our experiments focus more precisely on the antigen-processing abilities of the CaCo-2 cells, rather than on their subsequent efficiency of antigen presentation. Our results using this model of epithelial antigen are consistent with the conclusions drawn from the immunofluorescence experiments described above. Antigen taken up basolaterally is processed and presented to responding T cells. The concentrations of antigen required are high, as for other non-professional antigen-presenting cells which use non-specific fluid-phase endocytosis for antigen uptake. However, it was not possible to detect presentation of antigen taken up from the apical surface. The mechanisms for this vectorial processing have not been completely resolved; in part, they may reflect the lower rates of endocytosis from the apical surface. However, the results are also consistent with the results discussed above which suggested that apical endosomal traffic on its way to the lysosome does not intersect significantly with the MHC-containing vesicles. The results are also consistent with another recent study suggesting the existence of two distinct pathways for antigen presentation by intestinal epithelial cells.8
This study demonstrates that the CaCo-2 cell line, when cultured in the presence of IFN-γ, contains the major components of the class II antigen-processing pathway, but differs in several significant respects from conventional antigen-presenting cells. This conclusion raises two important questions. The first is the degree to which the results obtained on the CaCo-2 cell line can be generalized to the physiological situation in vivo. Although similar studies on other cell lines9 may provide some further information, this question can only be satisfactorily answered by studies on antigen processing in the intestinal epithelium in vivo, which are limited by available technology. The second question is the physiological significance of these specializations, and to what extent they reflect adaptations to the epithelial microenvironment. The polarized expression of the MHC, the molecular signals for which remain completely unknown, is consistent with a functional interaction between the epithelium and CD4 T cells in the underlying lamina propria. A further specialization may result in a high level of segregation of the apical fluid-phase endocytic pathway from the antigen-processing pathway: such a segregation would make sense in avoiding the wholesale presentation of normal luminal gut contents to the immune system. In contrast, when the epithelial integrity is broken, either by pathogens which infect the epithelial cells themselves, or under conditions when antigens can leak through the epithelial barrier and gain access to the basal side of the epithelium, antigen can be processed more efficiently and in these situations processing and presentation by the epithelial cells may contribute to an enhanced local immune response.
Acknowledgments
We thank Dr K. Rutault for help with immunoprecipitation gels. This work was supported by the Arthritis and Rheumatism Council, the Wellcome Trust and the Sir Jules Thorne Foundation.
References
- 1.Weiner HL, Friedman A, Miller A, et al. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 2.Chatfield SN, Strugnell RA, Dougan G. Live Salmonella as vaccines and carriers of foreign antigenic determinants. Vaccine. 1989;7:495. doi: 10.1016/0264-410x(89)90271-5. [DOI] [PubMed] [Google Scholar]
- 3.Michalek SM, Childers NK, Katz J, et al. Liposomes as oral adjuvants. Curr Top Microbiol Immunol. 1989;146:51. doi: 10.1007/978-3-642-74529-4_5. [DOI] [PubMed] [Google Scholar]
- 4.Neutra MR, Kraehenbuhl JP. Transepithelial transport and mucosal defense – the role of M cells. Trends in Cell Biology. 1991;2:134. doi: 10.1016/0962-8924(92)90099-9. [DOI] [PubMed] [Google Scholar]
- 5.Mayer L, Shlien R. Evidence for the function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987;166:1471. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bland PW, Warren LG. Antigen presentation by epithelial cells of the rat small intestine. Immunology. 1986;58:1. [PMC free article] [PubMed] [Google Scholar]
- 7.MacDonald TT, Weinel A, Spencer J. HLA-DR expression in human fetal intestinal epithelium. Gut. 1988;29:1342. doi: 10.1136/gut.29.10.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershberg RM, Framson PE, Cho DH, et al. Intestinal epithelial cells use two distinct pathways for HLA class II antigen presentation. J Immunol. 1997;200:204. doi: 10.1172/JCI119514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hershberg RM, Cho SH, Youakim A, et al. Highly polarized HLA class II antigen processing and presentation by human intestinal epithelial cells. J Clin Invest. 1998;102:792. doi: 10.1172/JCI3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaiserlian D, Vidal K, Revillard JP, Griffiths CEM. Murine enterocytes can present soluble antigen to specific class II-restricted CD4+ T cells. Eur J Immunol. 1989;19:1513. doi: 10.1002/eji.1830190827. [DOI] [PubMed] [Google Scholar]
- 11.Panja AE, Siden E, Mayer L. Synthesis and regulation of accessory/proinflammatory cytokines by intestinal epithelial cells. Clin Exp Immunol. 1995;100:298. doi: 10.1111/j.1365-2249.1995.tb03668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnard JA, Beauchamp RD, Coffey RJ, Moses HL. Regulation of intestinal epithelial cell growth by transforming growth factor type beta. Proc Natl Acad Sci USA. 1989;86:1578. doi: 10.1073/pnas.86.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung HC, Eckmann L, Yang SK, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinto M, Robine-Leon S, Appay M, et al. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line CaCo-2 in culture. Biol Cell. 1983;47:323. [Google Scholar]
- 15.Hughson EJ, Hopkins CR. Endocytic pathways in polarized Caco-2 cells: identification of an endosomal compartment accessible from both apical and basolateral surfaces. J Cell Biol. 1990;110:337. doi: 10.1083/jcb.110.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa De Beauregard MA, Pringault E, Robine S, Louvard D. Suppression of villin expression by antisense RNA impairs brush border assembly in polarized epithelial intestinal cells. EMBO J. 1995;14:409. doi: 10.1002/j.1460-2075.1995.tb07017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughson EJ, Cutler DF, Hopkins CR. Basolateral secretion of kappa light chain in the polarised epithelial cell line, Caco-2. J Cell Sci. 1989;94:327. doi: 10.1242/jcs.94.2.327. [DOI] [PubMed] [Google Scholar]
- 18.Lampson LA, Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980;125:293. [PubMed] [Google Scholar]
- 19.Adams TE, Bodmer JG, Bodmer WF. Production and characterization of monoclonal antibodies recognizing the alpha-chain subunits of human ia alloantigens. Immunology. 1983;50:613. [PMC free article] [PubMed] [Google Scholar]
- 20.Quaranta V, Majdic O, Stingl G, Liszka K, Honigsmann H, Knapp W. A human Ia cytoplasmic determinant located on multiple forms of invariant chain (gamma, gamma 2, gamma 3) J Immunol. 1984;132:1900. [PubMed] [Google Scholar]
- 21.Knight AM, Hughson EJ, Hopkins CR, Cutler DC. Membrane protein trafficking through the common apical compartment of polarised CaCo-2 cells. Mol Cell Biol. 1995;6:597. doi: 10.1091/mbc.6.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai K, Tsujisaki M, Hareyama M, et al. CD45 workshop panel report. In: Kishimoto T, editor. Leukocyte Typing VI. London, UK: Garland; 1997. p. 409. [Google Scholar]
- 23.Venkitaraman AR, Culbert EJ, Feldmann M. A phenotypically dominant regulatory mechanism suppresses major histocompatibility complex class II gene expression in a murine plasmacytoma. Eur J Immunol. 1987;17:1441. doi: 10.1002/eji.1830171009. [DOI] [PubMed] [Google Scholar]
- 24.Bennett K, Levine T, Ellis JS, Peanasky RJ, Kay J, Chain BM. Antigen processing for presentation by class II MHC requires cleavage by cathepsin E. Eur J Immunol. 1992;22:1519. doi: 10.1002/eji.1830220626. [DOI] [PubMed] [Google Scholar]
- 25.Lowes JR, Radwan P, Priddle JD, Jewell DP. Characterisation and quantification of mucosal cytokine that induces epithelial histocompatibility locus antigen-DR expression in inflammatory bowel disease. Gut. 1992;33:315. doi: 10.1136/gut.33.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams RB, Planchon SM, Roche JK. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol. 1993;150:2356. [PubMed] [Google Scholar]
- 27.Teyton L, O’sullivan D, Dickson PW, et al. Invariant chain distinguishes between the exogenous and endogenous antigen presentation pathways. Nature. 1990;348:39. doi: 10.1038/348039a0. [DOI] [PubMed] [Google Scholar]
- 28.Hirata I, Austin LL, Blackwell WH, Weber JR, Dobbins WO. Immunoelectron microscopic localization of HLA-DR antigen in control small intestine and colon and in inflammatory bowel disease. Dig Dis Sci. 1986;31:1317. doi: 10.1007/BF01299810. [DOI] [PubMed] [Google Scholar]
- 29.Gorvel JP, Sarles J, Maroux S, Olive D, Mawas C. Cellular localization of class I (HLA-A, B, C) and class II (HLA-DR and DQ) MHC antigens on the epithelial cells of normal human jejunum. Biol Cell. 1984;52:249. doi: 10.1111/j.1768-322x.1985.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 30.Terpend K, Boisgerault F, Blanton MA, Desjeux JF, Heyman M. Protein transport and processing by human HT29A intestinal cells: effect of IFN-γ. Gut. 1998;42:538. doi: 10.1136/gut.42.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bland PW, Kambarage DM. Antigen handling by the epithelium and lamina propria macrophages. Gastroenterol Clin North Am. 1991;20:577. [PubMed] [Google Scholar]
- 32.Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 33.Gonella PA, Wilmore DW. Co-localisation of class II antigen and exogenous antigen in the rat. J Cell Sci. 1993;103:937. doi: 10.1242/jcs.106.3.937. [DOI] [PubMed] [Google Scholar]
- 34.Kerneis S, Bogdanova A, Kraehenbuhl J, Pringault E. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- 35.Zimmer KP, Poremba C, Webber P, Ciclitira PJ, Harms E. Translocation of gliadin into HLA-DR antigen containing lysosomes in coeliac disease enterocytes. Gut. 1995;36:703. doi: 10.1136/gut.36.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brooks AG, Campbell PL, Reynolds P, Gautam AM, McCluskey J. Antigen presentation and assembly by mouse I-Ak class II molecules in human APC containing deleted or mutated HLA DM genes. J Immunol. 1994;153:5382. [PubMed] [Google Scholar]