Abstract
Bordetella pertussis interacts with very-late antigen-5 (VLA-5) receptors on the human monocyte resulting in cross-linking of these receptors followed by activation of complement receptor 3 (CR3) and firm adhesion of B. pertussis to these monocytes. In the present study we investigated whether protein tyrosine kinases are involved in the activation of CR3 on monocytes, which was assessed by the binding of C3bi-coated erythrocytes (EC3bi). Pre-incubation of monocytes with tyrphostin-A47, a specific protein tyrosine kinase inhibitor, before adherence of the cells to an anti-VLA-5 monoclonal antibody-coated surface, or addition of tyrphostin-A47 within 10 min of the adherence to such surface, reduced the binding of EC3bi to monocytes significantly. Pre-incubation of monocytes with tyrphostin-A47 reduced the binding of B. pertussis to such monocytes as well. Inhibitors of protein kinase A and/or C had no effect on EC3bi binding to monocytes. Cross-linking of VLA-5 on monocytes resulted in tyrosine phosphorylation of several proteins. Together, these results indicate that protein tyrosine kinases are involved in the VLA-5-induced activation of CR3 on human monocytes.
INTRODUCTION
Alveolar macrophages and exudate macrophages, which are monocyte-derived cells, play an important role in the defence against Bordetella pertussis infection. There is increasing evidence that B. pertussis ingested by mouse macrophages or human monocyte-derived macrophages can survive intracellularly.1–3
Bordetella pertussis can bind to, and subsequently become ingested by human monocytes or human monocyte-derived macrophages without the requirement of opsonization.3–8 By using mutant B. pertussis strains, it has been demonstrated that binding of non-opsonized B. pertussis to human monocytes is mediated by the virulence factors, such as filamentous haemagglutinin (FHA), fimbriae and pertactin.6 Recently, we have shown that both monoclonal antibodies (mAb) against the fibronectin receptor very-late antigen-5 receptor (VLA-5; α5β1; CD49e/CD29) on monocytes and soluble fibronectin inhibit the binding of non-opsonized B. pertussis to monocytes.4 This indicates that VLA-5 serves as a receptor for B. pertussis. Fimbriae, which are stable structures that are very resistant to disruption by various denaturing agents,9 cover the bacterial surface and upon binding, cluster VLA-5 receptors on monocytes.4,5 Next complement receptor type 3 (CR3; αMβ2; CD11b/CD18) on monocytes becomes activated, allowing FHA on B. pertussis to bind more firmly to these monocytes.4–7 The signals leading to CR3 activation after cross-linking of VLA-5, however, remain to be established.
The present contribution concerns a more detailed study on the involvement of protein tyrosine kinases in the activation of CR3 after cross-linking of VLA-5 on monocytes using, for convenience, C3bi-coated erythrocytes (EC3bi) as indicator.
MATERIALS AND METHODS
Isolation of monocytes
Mononuclear cells were isolated from buffycoats from healthy donors by Ficoll–Hypaque differential centrifugation.10 Further cell purification was performed by countercurrent centrifugal elutriation in a Beckmann J2–21 M/E centrifuge using a JE-6 Elutriation Rotor (Beckmann Instruments, Palo Alto, CA) as described.11 The resulting cell suspensions contained at least 80% monocytes, as determined by Giemsa staining, of which more than 95% were viable, as determined by trypan blue exclusion. The adherence of B. pertussis and EC3bi to cells was assessed microscopically, allowing distinction between adherence to monocytes and that to lymphocytes. The results include only the adherence of bacteria or erythrocytes to monocytes.
Monoclonal antibodies
The following mAb against human cell surface proteins were used in the form of culture supernatants or of purified immunoglobulin: SAM-112 [immunoglobulin G2b (IgG2b); 500 μg/ml] against the integrin α5 subunit (CD49e) of VLA-5 (Sanbio, Uden, the Netherlands), 15A813 (IgG1; 200 μg/ml) against the integrin α4 subunit (CD49d) of VLA-4, and NKI-M914 (IgG1; 200 μg/ml) against the integrin αV subunit (CD51) of the vitronectin receptor (CLB, Amsterdam, the Netherlands), 4G10 (IgG2b; 100 μg/ml) against phosphorylated tyrosine residues (Upstate Biotechnology Inc., Lake Placid, NY). Purified mouse IgG1 and IgG2b (Pharmingen, San Diego, CA) were used as isotype-matched controls. F(ab′)2 fragments of goat anti-mouse IgG (Cappel, Durham, NC) and peroxidase-conjugated goat anti-mouse immunoglobulin (Southern Biotechnology Associates Inc., Birmingham, AL). The final concentrations used were given in the relevant experiments.
Protein kinase inhibitors
Monocytes were treated with the following protein kinase inhibitors: 450 nm staurosporine (Calbiochem, La Jolla, CA) which is a broad-spectrum inhibitor of protein kinases,15 10 μm tyrphostin-A47 (Sigma, St. Louis, MO) which selectively inhibits protein tyrosine kinase activity,16 10 μm tyrphostin-1 (Sigma), an ineffective analogue of tyrphostin-A47, 50 μm H7 (Seikagaku Koguo Co. Ltd, Tokyo, Japan) which selectively inhibits protein kinases A and C,17 and 30 μm H89 (Calbiochem) which is a selective inhibitor of protein kinase A.18 The concentrations used were those found to be optimally effective as reported in the respective references; they did not affect cell viability as determined by trypan blue exclusion (data not shown).
Adherence of B. pertussis to monocytes
Wild-type B. pertussis strain Welcome 28 (W28)19 was labelled with fluorescein isothiocyanate (FITC; Sigma), as described.1,20 Briefly, 1×108 bacteria/ml were incubated in a solution of 1 mg FITC per ml, 50 mm sodium carbonate and 100 mm NaCl (pH 9·0) for 20 min at room temperature, washed four times and resuspended in HAP medium to a final concentration of 1×108 bacteria/ml. The bacteria were kept for 30 min at 37° until use.
Adherence of B. pertussis to cultured monocytes was assessed as described4 with some minor modifications. Wells of Terasaki plates (Greiner Labortechnik, Frickenhausen, Germany) were coated with HAP medium, i.e. phosphate-buffered saline (PBS) containing 3 mm glucose, 150 nm CaCl2, 500 nm MgCl2, 0·3 U/ml aprotinin and 0·05% (w/v) human serum albumin (HSA), to which mAb against VLA-5, VLA-4, VNR, or non-specific isotype-matched antibodies were added, or not, to a final concentration of 5 μg/ml. Plates were then incubated for 1 hr at room temperature. After three washes with PBS at room temperature, 5×103 monocytes per well were allowed to adhere for 90 min at 37°. Wells were washed three times with PBS of 37° and 5×105 FITC-labelled B. pertussis was added to each well with or without mAb against VLA-5, VLA-4, VNR, or non-specific isotype-matched antibodies to a final concentration of 5 μg/ml. After incubating for 30 min at 37°, the plates were then washed five times with PBS (37°) and then fixed for 15 min with 0·05% glutaraldehyde (Polyscience Inc., Warrington, PA). After washing twice with PBS the number of bacteria adhering to 100 monocytes was counted at a magnification of ×400. In some experiments, monocytes were preincubated for 10 min at 37° with the protein tyrosine kinase inhibitor or its inactive analogue before incubation in Terasaki plates. The inhibitors remained in the medium during the entire assay. Wells of Terasaki plates were incubated with HAP medium for 1 hr at room temperature.
Adherence of EC3bi to monocytes
Sheep erythrocytes were coated with C3bi (EC3bi) as described.21 Elutriation-purified monocytes were used to avoid interference of contaminating lymphocytes. Wells of Terasaki plates (Greiner Labortechnik) were coated with HAP medium, with or without mAb against VLA-5 to a final concentration of 5 μg/ml. Plates were then incubated for 1 hr at room temperature. Next, wells were washed three times with PBS at room temperature before 5×103 monocytes per well were allowed to adhere for 90 min at 37°. After three washes with PBS at 37°, 5×105 EC3bi were added to each well with the remaining monocytes and incubated for 45 min at 37°. Plates were then washed five times with PBS (37°) and then fixed for 15 min with 0·05% glutaraldehyde (Polyscience Inc.). After washing twice with PBS the number of EC3bi cells adhering to 100 monocytes was counted at a magnification of ×400. In some experiments, monocytes were preincubated for 10 min at 37° with various protein kinase inhibitors before incubation in Terasaki plates. The inhibitors remained in the medium during the entire assay.
Assessment of tyrosine phosphorylation of proteins after cross-linking of VLA-5
After cross-linking of VLA-5 on monocytes in suspension with antibodies, the tyrosine-phosphorylated proteins of monocytes were determined as described22,23 with minor modifications. In short, purified monocytes were suspended in RPMI-1640 medium containing 10 mm HEPES to a concentration of 5×107 cells per ml. These cells were then incubated with mAb against VLA-5 to a final concentration of 5 μg/ml for 10 min at room temperature. Next, goat anti-mouse F(ab′)2 was added to a concentration of 25 μg/ml to the medium and the cells were incubated at 37° for the indicated periods. Then, to stop activation, 80 μl of the cell suspension was mixed with 30 μl of sodium dodecyl sulphate (SDS) buffer (40% SDS, 0·2 m dithiothreitol, 20%β-mercaptoethanol, 20% glycerol and 0·01% bromophenol blue in 10 mm Tris buffer, pH 7·0), followed by incubation at 100° for 5 min. Next, 10 μl cell-lysate (5 μg protein) per lane were run on an SDS–7·5% polyacrylamide gel and the proteins were electrophoretically transferred to nitrocellulose paper (Schleicher & Scheull GmbH, Dassel, Germany). These blots were incubated with 5% dried non- fat milk (Elk; Campina Melkunie bv., Eindhoven, the Netherlands) and 0·1% Tween-20 in PBS for 1 hr at room temperature to reduce non-specific binding of antibodies. After washing with PBS containing 0·1% Tween-20, the blot was incubated for 1 hr with 1 μg of antiphosphotyrosine mAb 4G10 per ml in PBS containing 0·5% Elk and 0·1% Tween-20 for 90 min at room temperature. After washing with PBS containing 0·1% Tween-20 peroxidase-conjugated goat anti-mouse immunoglobulin antibodies were added, and the blot was then incubated for 1 hr at room temperature. The binding of the antibody to tyrosine-phosphorylated proteins was assessed using ECL Western blotting detection reagents (Amersham Life Science, Buckinghamshire, UK).
Statistical analysis
Differences between the results of the various experiments were evaluated by means of a paired two-tailed t-test. Results are shown as means and standard deviation (SD).
RESULTS
Involvement of VLA-5 on the adherence of B. pertussis to monocytes
Previously we have shown that mAb against fibronectin receptor VLA-5 on monocytes and soluble fibronectin inhibit the binding of non-opsonized B. pertussis to these cells.4 To address whether other receptors with binding affinity for fibronectin are involved in the adherence of B. pertussis on monocytes, we investigated the VLA-4 receptor and the vitronectin receptor (VNR).
When monocytes, adherent to surfaces pretreated with HAP medium, were incubated with B. pertussis in the presence of mAb against VLA-5, the bacteria bound significantly less (P<0·01) in comparison with B. pertussis in the presence of HAP medium or the isotype-matched antibody control (Table 1). The presence of anti-VLA-4 or anti-VNR mAb did not reduce the adherence of B. pertussis to such monocytes, suggesting that neither the VLA-4 nor the vitronectin receptor are involved in adherence of B. pertussis to monocytes.
Table 1.
Involvement of VLA-5 on the adherence of B. pertussis to monocytes
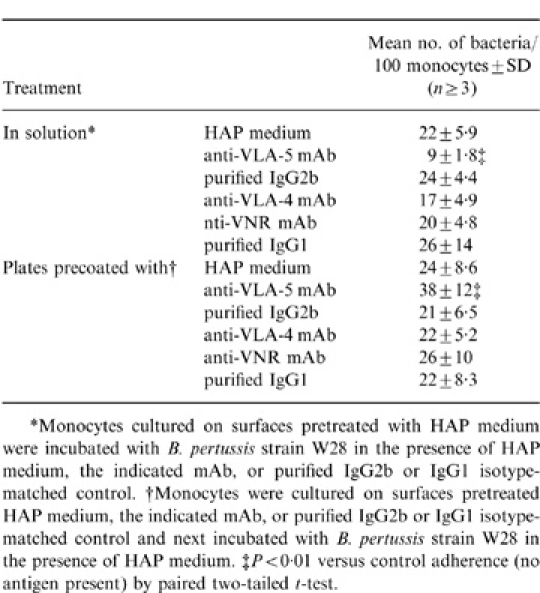 |
Monocytes cultured on surfaces coated with anti-VLA-5 mAb revealed a significantly (P<0·01) higher binding of B. pertussis (38±12 bacteria/100 cells) in comparison to monocytes cultured on surfaces without anti-VLA-5 mAb (24×8·6 bacteria/100 cells; Table 1). When monocytes were cultured on surfaces coated with anti-VLA-4, anti-VNR mAb, or isotype-matched antibody controls, the adherence of B. pertussis was not increased. Together, these results indicate that not VLA-4 and VNR, but VLA-5 is involved in the adherence of B. pertussis to monocytes.
Involvement of protein kinases in the VLA-5-induced activation of CR3 on monocytes
In monocytes cultured on surfaces coated with anti-VLA-5 mAb, CR3 become activated by cross-linking of VLA-5 receptors at the basal site of these adherent cells.24 Activated CR3 of monocytes cultured on surfaces coated with anti-VLA-5 mAb bound significantly (P<0·05) more EC3bi (43±24 EC3bi/100 cells) in comparison to CR3 of monocytes cultured on surfaces pretreated with HAP medium (31±15 EC3bi/100 cells) (Fig. 1a,b). Pre-incubation of monocytes for 10 min with the non-specific protein kinase inhibitor, staurosporin, or with the specific protein tyrosine kinase inhibitor, tyrphostin-A47, significantly inhibited the binding of EC3bi to monocytes cultured on either surfaces coated with anti-VLA-5 mAb, or pretreated with HAP medium, compared to indicated controls (Fig. 1a,b). Neither tyrphostin-1, the inactive analogue of tyrphostin-A47, nor protein kinase A and C inhibitor H7 nor the protein kinases A inhibitor H89 reduced the binding of EC3bi to monocytes adhering to a surface coated with mAb against VLA-5 or a surface without mAb coating. These results show that tyrosine kinases are involved in the VLA-5-induced activation of CR3 on monocytes.
Figure 1.
Effect of protein kinase inhibitors on the binding of EC3bi to adherent monocytes. Monocytes, preincubated with protein kinase inhibitors tyrphostin-1 (tyr1), tyrphostin-A47 (tyrA47), staurosporine (stauro), H7 or H89 were plated on surfaces pretreated with HAP medium (a) or precoated with anti-VLA-5 mAb (b). Next, EC3bi were allowed to adhere to these cells. Values are the mean±SD of at least five separate experiments. Difference in adherence of B. pertussis to monocytes and indicated control was determined by paired two-tailed t-test: *P<0·05, **P<0·01 versus indicated control.
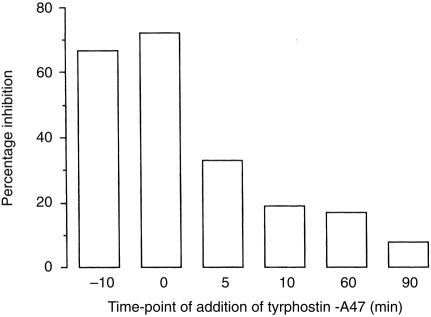
Effect of tyrphostin on activation of CR3 on monocytes at different time-points
The kinetics of tyrosine phosphorylation by protein tyrosine kinases after cross-linking of VLA-5 on monocytes adhering to a surface coated with the mAb against VLA-5, was assessed by adding tyrphostin-A47 at various time-points. Incubation of monocytes with tyrphostin-A47 for 10 min before the adherence assay or addition of this compound at the start of this assay resulted in 60–70% inhibition of the binding of EC3bi to monocytes (Fig. 2). Addition of tyrphostin-A47 5 min after the start of the adherence assay, inhibited the binding by only 30%; addition at 10 min or later had almost no effect. Addition of the inactive analogue tyrphostin-1 at the start of this assay did not inhibit the binding of EC3bi to monocytes (data not shown). Thus, protein tyrosine kinases have only an effect during a limited period after cross-linking of VLA-5 on monocytes, indicating that activation via these kinases is a transient process.
Figure 2.
Effect of tyrphostin-A47 on activation of CR3 on monocytes at different time-points. Tyrphostin-A47 was added before or during adherence of monocytes on surfaces coated with mAb against VLA-5 and the binding of EC3bi was assessed. Results of a representative experiment are expressed as the percentage inhibition of binding of EC3bi to monocytes relative to control (no tyrphostin added).
Tyrosine phosphorylation of proteins in monocytes after cross-linking VLA-5
Since tyrphostin-A47 was only effective in inhibiting CR3 activation on monocytes for a limited period after cross-linking of VLA-5, the role of protein tyrosine kinases was further assessed by phosphorylation of proteins on their tyrosine residues.
Monocytes in suspension were incubated with anti-VLA-5 mAb. Thereafter, at various intervals cross-linking was induced by adding goat anti-mouse F(ab′)2. The tyrosine-phosphorylated protein content of total monocyte-lysates was determined after their separation on a polyacrylamide gel and transfer to nitrocellulose paper, using an anti-phosphotyrosine mAb. The results showed that cross-linking of VLA-5 for 1 min led to tyrosine phosphorylation of several proteins with a molecular weight between 55 000–86 000 and 100 000–120 000, which was maximal between 2 and 10 min after cross-linking of VLA-5 (Fig. 3). Dephosphorylation of tyrosine on other proteins, for instance a 90 000 MW protein, was also observed. One of the signals which can occur upon activation of monocytes is the increase of the intracellular calcium (Ca2+) concentration. Cross-linking of VLA-5 on monocytes in suspension with mAb against VLA-5 and goat anti-mouse F(ab′)2 did not result in an increase in intracellular Ca2+ for at least 20 min (data not shown).
Figure 3.

Phosphorylation of proteins on tyrosine residues after cross-linking of VLA-5 on monocytes. Cross-linking of VLA-5 receptors on monocytes in suspension was assessed by incubation of the cells with anti-VLA-5 mAb before the addition of goat anti-mouse F(ab′)2. At the indicated time-points cells were lysed and tyrosine phosphorylation in total monocyte-lysates was determined (representative experiment).
Effect of protein tyrosine kinase inhibitor in the adherence of B. pertussis to monocytes
Adherence of B. pertussis fimbriae to VLA-5 on monocytes leads to cross-linking of these receptors which results in the activation of CR3 and firm binding of bacteria via FHA to their surface.5 Cross-linking of VLA-5 on monocytes with anti-VLA-5 mAb leads also to CR3 activation which involves protein tyrosine kinases. These findings raised the question whether protein tyrosine kinases are involved in the adherence of B. pertussis to monocytes. When monocytes were preincubated with tyrphostin-A47 before allowing adherence on HAP medium pretreated surfaces, binding of B. pertussis was significantly inhibited (P<0·01) compared to monocytes incubated with the inactive analogue tyrphostin-1 or HAP medium (Fig. 4).
Figure 4.
Effect of a protein tyrosine kinase inhibitor on binding of B. pertussis to adherent monocytes. Monocytes were preincubated with tyrphostin-A47 (tyrA47), tyrphostin-1 (tyr1), or HAP medium before being allowed to adhere on HAP medium-treated surfaces. Next, B. pertussis was allowed to adhere to these cells. Values are the mean±SD of at least six separate experiments. Difference in adherence of B. pertussis to monocytes and indicated control was determined by paired two-tailed t-test: **P<0·01 versus indicated control.
DISCUSSION
From the present study we conclude that protein tyrosine kinases are involved in VLA-5-mediated activation of CR3 on human monocytes. Furthermore, these kinases mediate the adherence of B. pertussis to human monocytes. These conclusions are based on the inhibition of adherence of B. pertussis and EC3bi to activated CR3 in the presence of staurosporin, a non-specific protein kinase, and tyrphostin-A47, a specific protein kinase inhibitor. Protein kinase A and C inhibitor (H7) and protein kinase A inhibitor (H89) had no effect on EC3bi binding.
Tyrphostin-A47 was most effective when added before, or at the start of, assays with monocytes adhering to a surface coated with anti-VLA-5 mAb. This indicates that tyrosine kinase-mediated activation of CR3 after cross-linking of VLA-5 is a rapid, but transient, process. This was supported by the rapid tyrosine phosphorylation of several proteins with a molecular weight between 55 000–86 000 and 100 000–120 000, which was maximal between 2 and 10 min after cross-linking of VLA-5. It remains to be established, which proteins phosphorylated on their tyrosine residues are involved in CR3 activation.
Monocytes cultured on a surface not coated with anti-VLA-5 mAb bind EC3bi via CR3, which could be inhibited by staurosporin and tyrphostin-A47. This binding activity might be due to monocyte activation as a result of the isolation procedure of these cells. For neutrophils it has been demonstrated that the Arg-Gly-Asp amino acid-containing region of C3bi binds to the β3-integrin leucocyte response integrin on these cells, followed by activation of the β2-integrin receptor CR3, which then binds another region of C3bi.25 It might be that such auto-activation also occurs in monocytes.
Besides the observed up-regulation of the expression of CR3 on neutrophils after clustering of VLA-5 and the laminin receptor VLA-6 on these cells,26 recent reports indicate also that cross-linking of various β1-integrins on several cell types induces intracellular signals which are mediated by tyrosine phosphorylation. For instance, the interaction of VLA-4 with fibronectin in combination with VLA-5 activates natural killer cells via protein tyrosine kinases,27,28 whereas cross-linking of VLA-3 on human epidermal carcinoma cells29 and cross-linking of VLA-4 on B lymphocytes as well as T lymphocytes30,31 resulted in an enhanced tyrosine kinase activity.
Our results showed that B. pertussis did not bind to two other fibronectin-binding receptors, VLA-4 and VNR, and cross-linking of these receptors on monocytes did not enhance the binding of B. pertussis. Thus, although these three integrin receptors can bind the same ligand, activation of the cell via these integrin receptors can still be specific.
Motivation for the present study was the activation of CR3 on monocytes after interaction of B. pertussis with the β1-integrin receptor VLA-5.4,5 This interaction of B. pertussis with VLA-5 on monocytes is not unique for these bacteria. Yersinia pseudotuberculosis32 and Y. enterocolitica33 can also bind to various β1-integrins on several mammalian cells via their virulence factor invasin and consequently promote their ingestion. Furthermore, Trypanosoma cruzi,34,35Leishmania sp.,36Staphylococcus aureus37,38 and various mycobacterial species39,40 can bind fibronectin and micro-organisms with bound fibronectin interact with VLA-5 and other fibronectin receptors. In this regard it is interesting that fibronectin and fimbriae of B. pertussis have similar binding specificities.41 Whether binding of micro-organisms to VLA-5 results in activation of CR3 is not known as yet.
Based on our previous studies4–7 together with the present results, we conclude that cross-linking of VLA-5 on the monocyte by B. pertussis resulting in activation of CR3 is mediated via protein tyrosine kinases.
Acknowledgments
The authors wish to express their gratitude to Peter N. Nibbering and Henry Beekhuizen for helpful suggestions. This work was supported by the National Institute of Public Health and the Environment, Bilthoven, the Netherlands.
References
- 1.Cheers C, Gray DF. Macrophage behaviour during the complaisant phase of murine pertussis. Immunology. 1969;17:875. [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman RL, Nordensson K, Wilson L, Akporiaye ET, Yocum DE. Uptake and intracellular survival of Bordetella pertussis in human macrophages. Infect Immun. 1992;60:4578. doi: 10.1128/iai.60.11.4578-4585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saukkonen K, Cabellos C, Burroughs M, Prasad S, Tuomanen E. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J Exp Med. 1991;173:1143. doi: 10.1084/jem.173.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hazenbos WLW, van den Berg BM, van Furth R. Very late antigen-5 and complement receptor type 3 cooperatively mediate the interaction between Bordetella pertussis and human monocytes. J Immunol. 1993;151:6274. [PubMed] [Google Scholar]
- 5.Hazenbos WLW, van den Berg BM, Geuijen CW, Mooi FR, van Furth R. Binding of fimD on Bordetella pertussis to very late antigen-5 on monocytes activates complement receptor type 3 via protein tyrosine kinases. J Immunol. 1995;155:3972. [PubMed] [Google Scholar]
- 6.Hazenbos WLW, van den Berg BM, ′t Wout JW, van Furth R. Virulence factors determine attachment and ingestion of nonopsonized and opsonized Bordetella pertussis by human monocytes. Infect Immun. 1994;62:4818. doi: 10.1128/iai.62.11.4818-4824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hazenbos WLW, Geuijen CAW, van den Berg BM, Mooi FR, van Furth R. Bordetella pertussis fimbriae bind to human monocytes via the minor fimbrial subunit fimD. J Infect Dis. 1995;171:924. doi: 10.1093/infdis/171.4.924. [DOI] [PubMed] [Google Scholar]
- 8.Relman D, Tuomanen E, Falkow S, Golenbock DT, Saukkonen K, Wright SD. Recognition of a bacterial adhesin by an integrin: macrophage CR3 (′M′2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990;61:1375. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- 9.Zhang JM, Cowel JL, Steven AC, Carter PH, McGrath PP, Manclark CR. Purification and characterization of fimbriae isolated from Bordetella pertussis. Infect Immun. 1985;48:422. doi: 10.1128/iai.48.2.422-427.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyum A. Separation of leukocytes from blood and bone marrow. Scan J Clin Lab Invest. 1968;21(Suppl. 97):7. [PubMed] [Google Scholar]
- 11.Beekhuizen H, Corsel-van Tilburg AJ, van Furth R. Characterization of monocyte adherence to human macrovascular and microvascular endothelial cells. J Immunol. 1990;145:510. [PubMed] [Google Scholar]
- 12.te Velde AA, Klomp JPG, Yard BA, de Vries JE, Figdor C. Modulation of phenotypic and functional properties of human peripheral blood monocytes by IL-4. J Immunol. 1988;140:1548. [PubMed] [Google Scholar]
- 13.Humphries MJ. The molecular basis and specificity of integrin–ligand interactions. J Cell Sci. 1990;97:585. doi: 10.1242/jcs.97.4.585. [DOI] [PubMed] [Google Scholar]
- 14.de Vries JE, Keizer GD, te Velde AA, et al. Characterization of melanoma-associated surface antigens involved in the adhesion and motility of human melanoma cells. Int J Cancer. 1986;38:465. doi: 10.1002/ijc.2910380403. [DOI] [PubMed] [Google Scholar]
- 15.Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++ dependent protein kinase. Biochem Biophys Res Comm. 1986;135:379. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- 16.Levitzki A. Tyrphostins: tyrosine kinase blockers as novel antiproliferative agents and dissectors of signal transduction. FASEB J. 1992;6:3275. doi: 10.1096/fasebj.6.14.1426765. [DOI] [PubMed] [Google Scholar]
- 17.Kawamoto S, Hidaka H. 1-(5-isoquinolinesulfonyl)-2-methylpiperazine (H-7) is a selective inhibitor of protein kinase C in rabbit platelets. Biochem Biophys Res Comm. 1984;125:258. doi: 10.1016/s0006-291x(84)80362-9. [DOI] [PubMed] [Google Scholar]
- 18.Chijiwa T, Mishima A, Hagiwara M, et al. Inhibition of Forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-Bromocinnamylamino) ethyl]-5- soquinolinesulfon-amide (H-89), of PC12D Pheochromocytoma cells. J Biol Chem. 1990;265:5267. [PubMed] [Google Scholar]
- 19.Robinson A, Ashworth LAE, Baskerville A, Irons LI. Protection against intranasal infection of mice with Bordetella pertussis. Dev Biol Stand. 1985;61:165. [PubMed] [Google Scholar]
- 20.Wright SD, Jong MTC. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med. 1986;164:1876. doi: 10.1084/jem.164.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Vliet KE, De Graaf-Miltenburg LAM, Verhoef J, van Strijp JAG. A flow cytometric rosetting assay for the analysis of Fc receptors and C3 receptors on HSV-infected cells. J Immunol Meth. 1993;157:57. doi: 10.1016/0022-1759(93)90070-n. [DOI] [PubMed] [Google Scholar]
- 22.Connelly PA, Farrell CA, Merenda JM, Conklyn MJ, Showell HS. Tyrosine phosphorylation is an early signaling event common to Fc receptor cross-linking in human neutrophils and rat basophilic leukemia cell (RBL-2H3) Biochem Biophys Res Commun. 1991;177:192. doi: 10.1016/0006-291x(91)91967-h. [DOI] [PubMed] [Google Scholar]
- 23.Zheng L, Nibbering PH, Zomerdijk TPL, van Furth R. Protein tyrosine kinase activity is required for FcγR-stimulated intracellular killing of Staphylococcus aureus by human monocytes. Infect Immun. 1994;62:4296. doi: 10.1128/iai.62.10.4296-4303.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright SD, Rao PE, van Voorhis WC, et al. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci USA. 1983;80:5699. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Strijp JAG, Russel DG, Tuomanen E, Brown EJ, Wright SD. Ligand specificity of purified complement receptor type three (CD11b/CD18, αmβ2, Mac-1): Indirect effect of an ARG-GLY-ASP (RGD) sequence. J Immunol. 1993;151:3324. [PubMed] [Google Scholar]
- 26.Simms H, D’Amico R. Regulation of polymorphonuclear neutrophil CD16 and CD11b/CD18 expression by matrix proteins during hypoxia is VLA-5, VLA-6 dependent. J Immunol. 1995;155:4970. [PubMed] [Google Scholar]
- 27.Gismondi A, Milella M, Palmieri G, Piccoli M, Frati L, Santoni A. Stimulation of protein tyrosine phophorylation by interaction of NK cells with fibronectin via α4β1 and α5β1. J Immunol. 1995;154:3128. [PubMed] [Google Scholar]
- 28.Palmieri G, Serra A, De Maria R, et al. Cross-linking of α4β1 and α5β1 fibronectin receptors enhances natural killer cell cytotoxic activity. J Immunol. 1995;155:5314. [PubMed] [Google Scholar]
- 29.Kornberg LJ, Earp HS, Turner CE, Prockop C, Juliano RL. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of β1 integrins. Proc Natl Acad Sci USA. 1991;88:8392. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedman AS, Rhynhart K, Nojima Y, et al. Stimulation of protein tyrosine phosphorylation in human B cells after ligation of the β1 integrin VLA-4. J Immunol. 1993;150:1645. [PubMed] [Google Scholar]
- 31.Nojima Y, Rothstein DM, Sugita K, Schlossman SF, Morimoto C. Ligation of VLA-4 on T cells stimulates tyrosine phosphorylation of a 105-kD protein. J Exp Med. 1992;175:1045. doi: 10.1084/jem.175.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isberg RR, Leong JM. Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 33.Young VB, Falkow S, Schoolnik GK. The invasin protein of Yersinia enterocolitica: internalization of invasin-bearing bacteria by eukaryotic cells is associated with reorganization of the cytoskeleton. J Cell Biol. 1992;166:197. doi: 10.1083/jcb.116.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouaissi MA, Afchain D, Capron A, Grimaud JA. Fibronectin receptors on Trypanosoma cruzi trypomastigotes and their biological function. Nature. 1984;308:380. doi: 10.1038/308380a0. [DOI] [PubMed] [Google Scholar]
- 35.Wirth JJ, Kierszenbaum F. Fibronectin enhances macrophage association with invasive forms of Trypanosoma cruzi. J Immunol. 1984;133:460. [PubMed] [Google Scholar]
- 36.Wyler DJ, Sypek JP, McDonald JA. In vitro parasite–monocyte interactions in human leishmaniasis: possible role of fibronectin in parasite attachment. Infect Immun. 1985;49:305. doi: 10.1128/iai.49.2.305-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978;276:718. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- 38.Proctor RA, Mosher DF, Olbrantz PJ. Fibronectin binding to Staphylococcus aureus. J Biol Chem. 1982;257:14788. [PubMed] [Google Scholar]
- 39.Ratliff TL, McGarr JA, Abou-Zeid C, et al. Attachment of mycobacteria to fibronectin-coated surfaces. J Gen Microbiol. 1988;134:1307. doi: 10.1099/00221287-134-5-1307. [DOI] [PubMed] [Google Scholar]
- 40.Thole JER, Schöningh R, Janson AAM, et al. Molecular and immunological analysis of a fibronectin-binding protein antigen secreted by Mycobacterium leprae. Mol Microbiol. 1992;6:153. doi: 10.1111/j.1365-2958.1992.tb01996.x. [DOI] [PubMed] [Google Scholar]
- 41.Geuijen CAW, Willems RJL, Bongaerts M, Top J, Gielen H, Mooi FR. Role of the Bordetella pertussis minor fimbrial subunit, FimD, in colonization of the mouse respiratory tract. Infect Immun. 1997;65:4222. doi: 10.1128/iai.65.10.4222-4228.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]