Abstract
The RelB gene product is a member of the nuclear factor (NF)-κB family of transcription factors. It has been identified recently within mouse antigen-presenting cells and human monocyte-derived dendritic cells (DC). Disruption of the mouse RelB gene is accompanied, amongst other phenotypes, by abnormalities in the antigen-presenting cell lineages. In order to define RelB expression during human DC differentiation, we have analysed RelB mRNA by reverse transcriptase–polymerase chain reaction and RelB protein by intracellular staining in CD34+ precursors and different types of DC preparations. RelB mRNA was not detected in CD34+ precursor populations. Fresh blood DC (lineage−human leucocyte antigen-DR+ (lin−HLA-DR+)) lacked RelB mRNA and cytoplasmic RelB protein but a period of in vitro culture induced RelB expression in blood DC. Purified Langerhans’ cells (LC) (CD1a+ HLA-DR+) failed to express RelB mRNA. Immunocytochemical staining identified RelB protein in human skin epithelium. RelB protein was expressed in a very few CD1a+, CD83+ or CMRF-44+ dermal DC but was not present in CD1a+ LC. Tonsil DC (lin−HLA-DR+ CMRF-44+) were positive for RelB mRNA and RelB protein. Intestinal DC (HLA-DR+) also lacked immunoreactive RelB protein. The majority of interdigitating CD83+, CMRF-44+, CMRF-56+ or p55+ DC located in paracortical T-lymphocyte areas of lymph node and tonsil contained RelB protein. The expression of RelB mRNA and RelB protein correlates with the activated phase of blood DC and the postmigration cell (activated) stage of tissue DC development.
INTRODUCTION
Dendritic cells (DC) are specialist antigen-presenting cells (APC) derived, in common with other leukocytes, from bone marrow stem cells. Clear discrimination of the different lineages of DC differentiation and their subsets1 remains problematical because of a relative lack of DC lineage markers. For example, ‘myeloid DC’ and monocyte-derived DC (Mo-DC) may be the result of entirely different differentiation pathways.2 Human DC, at all stages of differentiation, express the CD45 and human leucocyte antigen (HLA)-DR antigens but not the lineage (lin)-restricted CD14, CD3, CD11b, CD16 or CD19 antigens. Langerhans’ cells (LC) express the HLA class I like molecule CD1a but blood and tonsil DC do not,1 although it is expressed on Mo-DC cultured in the presence of cytokines.3 Other cytoplasmic molecules such as p55 (fascin), which binds to a cytoskeleton-associated actin-bundling molecule,4 and the CD68 antigen, a lysosomal protein that has a perinuclear dot distribution in DC but a widespread cytoplasmic distribution in macrophages5 are useful for identifying DC in situ. Some new monoclonal antibodies (mAb) to surface antigens have enabled DC to be purified by positive selection protocols. These include the CD83 mAb, HB15a,6,7 and two other mAb produced in this laboratory, CMRF-448,9 and CMRF-5610 both of which recognize uncharacterized antigens on DC. The CMRF-44 mAb has been used to assess the Mo-DC generated from CD14+ cells cultured with cytokines2 and to identify DC in situ in rheumatoid joints.11 These reagents are becoming very useful in purifying and defining DC populations.
The precedents established from studies in other haemopoietic lineages would suggest that the differentiation of DC will be controlled by a range of transcriptional regulators.12–15 The nuclear factor (NF)-κB family of transcription factors plays a role in the regulation of expression of a wide number of genes throughout the haemopoietic system.16 NF-κB activity is regulated by both transcriptional and post-transcriptional mechanisms. To date, five members of this family, c-rel, RelA, RelB, p105/p50 and p100/p52 have been described. They form a variety of homo- and heterodimers with corresponding differences in NF-κB-like activity. In the mouse, RelB is restricted mainly to the interdigitating cells of lymphoid tissues and the thymic medulla,17 although it is also present in B220 enriched splenic B-lymphocyte fractions.18 Further studies have shown that murine RelB accounts for constitutive NF-κB activity in lymphoid tissue.19 Recent studies in mouse models have indicated that RelB plays an important role in DC differentiation and maturation. Two models have been described in which the RelB gene has been disrupted by insertion of a non-homologous gene15 or gene targeting.14 Disruption of the RelB gene resulted in mice with a phenotype that included an impaired APC function and an absence of thymic UEA-1+ cells (which show striking similarities to some human DC).15 The mice appeared to express normal levels of LC in the skin but excess levels of granulocytes and macrophages were produced.
Limited studies on RelB expression in man are available. RelB protein has been localized by immunohistochemistry to the medullary cells with a DC morphology in the human thymus.20 Constitutive nuclear RelB activity has been described in plasma cells16 and more recently in the Mo-DC derived from the culture of peripheral blood mononuclear cells in granulocyte–macrophage colony-stimulating factor (GM-CSF)21 or GM-CSF and interleukin-4 (IL-4).22 We anticipated that RelB expression would also be relevant to our understanding of human myeloid DC biology. Therefore, we used reverse transcription–polymerase chain reaction (RT–PCR), flow cytometry and immunohistology, combined with a relevant panel of new DC markers to document the expression of RelB mRNA and protein in DC populations at different stages of differentiation and maturation.1 Our laboratory has emphasized that the phenotype of DC is altered by their method of preparation. ‘Fresh’ blood DC populations isolated with minimal manipulation have low CD86 expression implying that these cells are not activated. These ‘fresh’ DC populations, prepared directly from peripheral blood by negative selection, are defined as CD45+ HLA-DR+ leukocytes that lack the lineage markers CD3, CD14, CD15, CD19 and CD16 and are able to initiate a primary T-lymphocyte response.23 In contrast, ‘activated’ blood DC prepared from fresh PBMC that have been incubated in media with 10% fetal calf serum (FCS) express CD86 in high density and other DC differentiation/activation antigens such as the CD836 CMRF-448 antigens and the recently identified CMRF-56 antigen.10
This report provides novel data, which correlates RelB expression with a differentiated/activated DC phenotype. The data also provides the first correlations relating the reactivity of several reagents that are becoming very common for defining DC, with each of the different stages (bone marrow, blood, tissue and lymph node) of DC differentiation/activation.
MATERIALS AND METHODS
Reagents
The CD3 (OKT3, immunoglobulin G2a (IgG2a)), CD16 (HO-80, IgG2b), CD11b (OKM1,IgG2b) and HLA-DR (L243, IgG2a) mAb were produced from hybridomas obtained from the American Type Culture Collection (ATCC, Rockville, MD). CD19 (FMC63, IgG2a), the negative control mAb, Sal5 (IgG2a), Sal4 (IgG2b) and X63 (IgG1) were gifts from Professor H. Zola (Adelaide, Australia). The CD83 mAb (HB15a, IgG2b) was purchased from Immunotech (Miami, FL). The CD1a mAb (Na 1/34, IgG2a) was a gift from Professor A. McMichael (Institute of Molecular Medicine, Oxford, UK). BU63 (CD86, IgG1) was a gift from Dr D. Hardie (University of Birmingham, Birmingham, UK) and BB1 (CD80, IgM) was a gift from Dr J. Ledbetter (WA, USA). The CD68 mAb (KiM7, IgG1) was obtained from the third Human Leucocyte Workshop and the mAb to the p55 antigen (K-2, IgG1) was kindly provided by Dr E. Langhoff (Boston, MA). The CD45 (CMRF-12, IgG2a24), CD14 (CMRF-31, IgG2a25), CMRF-44 (IgM8), CMRF-50 (IgM), and CMRF-56 (IgG110) mAb were produced in this laboratory.
Affinity-purified rabbit polyclonal antibody specific for RelB (C-19) was purchased from Santa-Cruz (Santa Cruz, CA) and used at 20 μg/ml. Peroxidase-conjugated goat-antirabbit immunoglobulin (P-GAR) was purchased from Biosource International (Camarillo, CA). Biotinylated goat antimouse immunoglobulin (GAM), mouse immune serum for the nuclear protein Ki67 and the rabbit polyclonal antibody specific for mouse immunoglobulin (RAM) were purchased from Dako Corporation (Carpenteria, CA). Extravidin®– alkaline phosphatase was purchased from Sigma Chemical Company (St Louis, MO). The phycoerythrin (PE)-conjugated mAb; CD14, CD19, CD34 and HLA-DR were obtained from Becton Dickinson (San Jose, CA). Fluoroscein isothiocyanate (FITC)-conjugated sheep antirabbit Fab fragment (FITC-SAR) was obtained from Silenus (Melbourne, Australia).
Cells were cultured in RPMI-1640 supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mm l-glutamine and 10% heat-inactivated FCS (Life Technologies, Auckland, New Zealand). L428 cells were provided by V. Diehl (Klinik fur Innere Medizin., Cologne, Germany).
Cell preparation
CD34+ precursor cells
Cord blood and bone marrow samples (obtained with approval from the SRHA Ethical Committee) were used to isolate CD34+ haemopoietic precursors by the method of Sutherland.26 Mononuclear cells, obtained by density gradient centrifugation over Ficoll–Hypaque, were labelled with CD45 mAb and FITC-SAM. After blocking with 10% normal mouse serum, the cells were labelled with CD34-PE mAb. The CD34+, CD45+ precursor cells (>90% purity) were isolated by sorting on a FACS Vantage flow cytometer (Becton Dickinson, San Jose).
Fresh blood DC
Peripheral blood mononuclear cells (PBMC) were depleted of T lymphocytes by rosetting with neuraminidase treated sheep erythrocytes, Ficoll–Hypaque gradient centrifugation and ammonium chloride lysis to remove erythrocytes. T-lymphocyte depleted PBMC were labelled with a mix of CD3, CD11b, CD14, CD16 and CD19 mAb followed by goat antimouse immunoglobulin-coated magnetic beads (PerSeptive Biosystems, Framingham, MA). Labelled cells were depleted by magnetic immunodepletion, further labelled with FITC-SAM and HLA-DR-PE and sorted using a fluorescence-activated cell sorting (FACS) Vantage flow cytometer to collect the lin− HLA-DR+ fraction (>90% purity).27 Fresh DC for RT–PCR were frozen at −70° until required.
Cultured DC
T-lymphocyte depleted PBMC isolated as above were cultured at 2×107 cells/ml for 48 hr in RPMI/10% FCS (Life Technologies). Low-density cells were separated by gradient centrifugation over a Nycodenz (Nycomed, Pharma, Norway) gradient as previously described.28 Cultured DC were purified to >90% purity by FACS as CMRF-44+ CD14− CD19− or CMRF-56+ CD14− CD19− cells.9 These cells are not exposed to any cytokines or other factors other than those in FCS.
Langerhans’ cell preparation
LC were prepared from epidermal sheets that were treated overnight with Dispase II (0·5% in phosphate-buffered saline (PBS), Sigma) at 4° prior to separation of the epidermis and dermis. Epidermal fragments were disrupted by vigorous pipetting and passage through a cell dissociation cup in the presence of 0·25% trypsin and DNaseI (5 U/ml) in PBS at room temperature. The cells were collected immediately into HEPES-buffered RPMI supplemented with 10% FCS, filtered through nylon mesh, washed and resuspended in DNaseI/0·1% bovine serum albumin (BSA)/PBS. LC were enriched by Lymphoprep gradient centrifugation and further purified as the HLA-DR+ population by FACS.
Tonsil IDC
A single-cell suspension was prepared in cold 10% FCS/RPMI media containing 5 mm ethylenediamine tetra-acetic acid (EDTA) from fresh tonsil tissue obtained with ethical permission at routine tonsillectomy. Mononuclear cells were isolated by Ficoll–Hypaque density gradient centrifugation and depleted of T lymphocytes as above.5,29 Mononuclear non-T lymphocytes were labelled with a mix of CD3, CD14, CD16 and CD19 mAb followed by goat antimouse IgG-coated magnetic beads. Labelled cells were removed by magnetic immunodepletion. The mAb negative cells were then double labelled with FITC-SAM and HLA-DR-PE and further purified by sorting using a FACS Vantage flow cytometer (>90% purity). After sorting, the lin− HLA-DR+ cells re-presented an enriched population of tonsil interdigitating DC.
RT–PCR
Total RNA was prepared from frozen cell pellets using either an NP40 lysis protocol,30 if >5×104 cells were available or RNeasy kit (QIAGEN, Melbourne, Australia) according to manufacturer’s instructions. Cells were stored frozen for short periods and for different lengths of time. The results were consistent and did not suggest any artefacts caused by frozen storage of cell pellets. Total RNA from cellular equivalents was annealed to oligo-dT primers and reverse transcribed using Superscript II (Life Technologies) at 42° for 1 hr. RNA was removed by digestion with RNase H at 37° for 30 min.
RT–PCR was performed with Taq polymerase (Boehringer Mannheim, Auckland, NZ) using the cDNA template and conditions described by the manufacturer. The RelB specific primers were RelBU1; 5′-CATCCTGGACCACTTCCTGCC-3′ (nts 1464–1485) and RelBD1; 5′-GAACATGTTGCTGCCCACAAG-3′ (nts 1798–1818).31 Amplification of human β2-microglobulin (β2M) was performed to normalize the RT–PCR. The specific primers were β2MU1; 5′-TTAGCTGTGCTCGCGCTACTCTCT-3′ (nts 1–24) and β2MD1; 5′-TGTCGGATTGATGAAACCCAGAGA-3′ (nts 113–136).32
The PCR products were fractionated through a 2% agarose gel, transferred to Hybond N+ and probed with digoxigenin-labelled internal oligonucleotides according to the manufacturer’s recommendations (Boehringer Mannheim). The oligonucleotide sequence used for RelB hybridizations was RelBi; 5′-CACTCTTCACCATGCTG-3′ (nts 1634–1650) and the sequence for β2-M was β2Mi; 5′-TTGAGGCTATCCAGCGTACT-3′ (nts 35–54).
Flow cytometry
Surface staining was performed by standard techniques. Briefly, cells were incubated with primary mAb for 20 min at 4°, washed, incubated with FITC-SAM for 20 min at 4°, washed, blocked with 10% normal mouse serum for 5 min and then incubated with PE-conjugated mAb.
Intracellular staining was performed using a Fix & Perm kit (Caltag Laboratories, Burlinghame, CA). Briefly, cells were labelled with the primary mAb for 20 min at 4° then incubated in fixation medium for 30 min at room temperature, washed and resuspended in permeabilization medium. The treated cells were double labelled with RelB antibody or purified rabbit antimouse polyclonal antibody followed by FITC-SAR and CD83, CMRF-44 and CMRF-56 mAb and analysed on a FACS Vantage flow cytometer. Data was processed with CellQuest software.
Immunoalkaline phosphatase peroxidase double staining
Normal lymph nodes were obtained from biopsy specimens that proved to show reactive changes only. Normal tonsils and skin were obtained after informed consent from patients undergoing routine tonsillectomy or reduction mammoplasty, respectively. Cryostat-cut sections (4–6 μm) were placed on gelatin coated slides and air dried overnight, prior to fixing in cold acetone (4°). Sections were preblocked with 10% human AB serum for 30 min at room temperature followed by the application of the primary mouse mAb for 30 min at room temperature. Sections were washed three times with PBS then incubated with biotinylated GAM (1:200) for a further 30 min before again washing three times with PBS. Extravidin®–alkaline phosphatase (1:200) was applied for 30 min and the sections were washed twice with PBS and once with Tris–HCl pH 7·6 before being developed with Fast Blue (Sigma) for 5–10 min. Development was monitored using a microscope and stopped by washing with PBS. Sections were then incubated with the RelB antibody (1:50) for 45 min, washed three times with PBS and incubated with horseradish-peroxidase conjugated goat antirabbit immunoglobulin (1:100) for 30 min. Following two washes in PBS and one with Tris–HCl pH 7·6, sections were developed with 0·1% 3,3,diaminobenzidine for 3–10 min. The reaction was terminated, by washing in PBS, before mounting in glycerol gelatine.
All sections were analysed at 400× using an Olympus Bx50 microscope. T-lymphocyte areas in both lymph nodes and tonsils were identified by CD3 staining and their failure to stain for CD19. DC were counted in the T-lymphocyte areas of serial sections. Three staining patterns were counted; cells staining with RelB antibody alone, cells staining with the other mAb alone, and cells staining with both antibodies. Counts were performed in five different T-lymphocyte areas of each specimen and the areas were matched as closely as possible for each mAb under study. It was then possible to determine the proportions of RelB+ cells that coexpressed DC, macrophage and costimulator molecules. Conversely, the proportion of cells identified by these mAb and double labelling with RelB could also be determined. The results for the other tissues studied are reported in a descriptive fashion.
RESULTS
Expression of RelB mRNA in purified precursors and DC populations
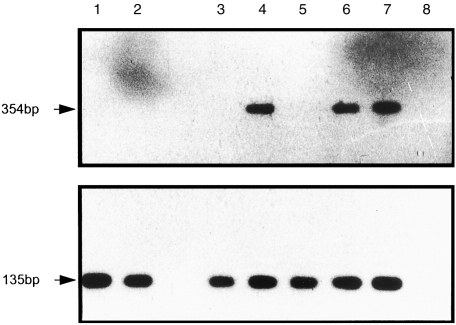
CD34+ haemopoietic precursors and cells representing different stages of the maturation and differentiation of the DC lineage were analysed by RT–PCR for expression of RelB mRNA (Fig. 1). The CD34+ haemopoietic precursor population is heterogeneous and includes a subpopulation of cells with allostimulatory activity.33 Studies have indicated that a CD34+ subpopulation can differentiate into cells that have a DC phenotype, including expression of CMRF-44 antigen34 and function,35 indicating that this population is likely to include a subpopulation of DC progenitors. RelB mRNA was not expressed by CD34+ cord blood or bone marrow cells (Fig. 1). Fresh blood DC (lin− HLA-DR+) represent the immature circulating population of DC, which after brief periods of culture, have a phenotype associated with activated DC. The immature blood DC population lacked RelB mRNA (Fig. 1). Likewise epithelial tissue DC, i.e. the next stage of a DC differentiation pathway, were studied as an example of purified LC (lin− HLA-DR+) and these also lacked RelB mRNA.
Figure 1.
Expression of RelB transcripts in CD34+ precursors and DC populations.cDNA prepared from total RNA from purified cell populations was subjected to RT–PCR for RelB (upper panel) and β2-microglobulin (lower panel) and subsequently probed with the digoxigenin labelled gene-specific internal oligonucleotides. The molecular size of the RT–PCR products is indicated. Lane 1; CD34+ cord blood precursors Lane 2; CD34+ bone marrow precursors, Lane 3; Freshly isolated blood DC (lin− HLA-DR+), Lane 4; Cultured blood DC (CMRF-44+), Lane 5; Purified LC (lin− HLA-DR+), Lane 6; Purified tonsil DC (lin− HLA-DR+), Lane 7; L428 cell line, Lane 8; no cDNA control. The results shown are typical of three independent cell preparations and RT–PCR analysis.
Short-term in vitro culture of fresh blood DC, in RPMI/10% FCS induces the expression of a number of antigens (e.g. CD83, CMRF-44 and CMRF-56), which are associated with an activated DC phenotype. A short period of culture of fresh blood DC induced these antigens (the presence of FCS increased their expression) and also induced expression of RelB mRNA (Fig. 1). The RT–PCR analysis indicated translational control of RelB mRNA throughout differentiation and activation of the DC lineage. The more mature lin− HLA-DR+ lymphoid tissue DC purified from the tonsil express RelB mRNA. The lin− HLA-DR+ purified tonsil DC include two phenotypically distinct populations of allostimulatory cells determined by the expression of the CMRF-44 antigen (K. Summers et al. in preparation). We further analysed the HLA-DR+ CMRF-44+ and HLA-DR+ CMRF-44− populations purified from tonsil and found RelB mRNA expressed in both populations (data not shown).
Intracellular expression of RelB in blood DC
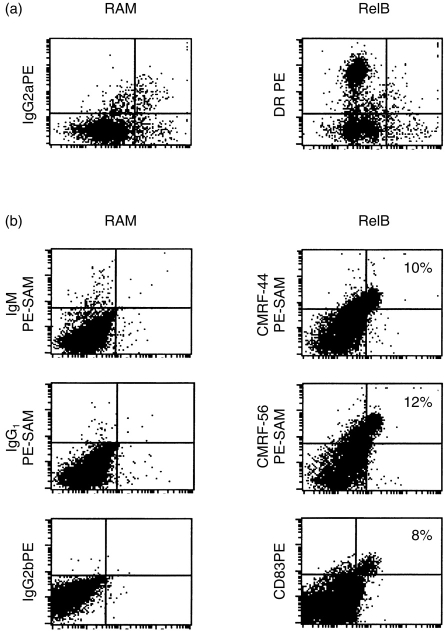
We next assessed the intracellular expression of RelB protein by flow cytometry using purified blood DC populations. Although the backgrounds were high, there was no staining above background with the RelB antibody in lin− HLA-DR+ fresh blood DC (Fig. 2a). Adequate permeabilization of the cells was demonstrated in the positive controls (data not shown). In contrast, blood DC which had been cultured for 48 hr clearly contained cytoplasmic RelB protein (Fig. 2b). Cultured blood DC also express a number of activation antigens9 and double-labelling studies confirmed that the majority of CMRF-44+, CMRF-56+ and CD83+ blood DC expressed the RelB protein (Fig. 2b).
Figure 2.
Detection of intracellular RelB protein in blood DC.Blood DC were isolated and stained for intracellular RelB proteins as described in Materials and Methods. The amount of RelB staining is expressed as a dot plot for (a) fresh lin− HLA-DR+ and (b) cultured CD83+, CMRF-44+ and CMRF-56+ blood DC. Typical results for one of three experiments are shown.
These results establish that the presence of RelB mRNA correlated closely with intracellular RelB protein expression as both are present in the blood DC population, which had an activated phenotype.
RelB expression in non-lymphoid tissues
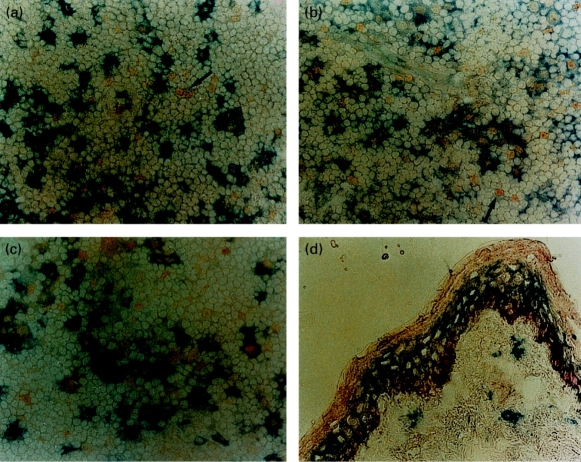
The expression of RelB in tissues was assessed by immunohistology. RelB expression in skin was localized to the epidermis, where strong perinuclear staining was observed in the basal cell layer. RelB was also present in the more superficial epidermal layers, although at much lower levels. LC, readily identified by CD1a staining did not coexpress RelB (Fig. 3d). This pattern was also seen in other epithelial tissues such as bladder mucosa and prostatic glands (data not shown). Immunohistological examination of other nonlymphoid tissues revealed no staining for RelB in the interstitium of both kidney and liver (data not shown), sites that are known to contain interstitial DC.36,37 This data, in conjunction with the RelB mRNA data, establishes that RelB is not expressed in the immature DC in the skin and interstitial DC populations.
Figure 3.
Immunohistological detection of RelB in skin and lymph nodes.In the T-lymphocyte areas of lymph nodes most RelB staining cells (yellow nuclear pattern) coexpress (a) CD83 (b) HLA-DR and (c) CD86 (blue staining). Occasional RelB staining cells are present which do not express any of these markers (arrowed). In skin (d), RelB stains the basal epidermal layer (red) but is not expressed by the LC, which are labelled with CD1a (blue, all ×400). These results are typical of the six skin and six lymph node sections studied.
RelB expression in lymphoid tissues
Lymph node and tonsil sections were analysed for coexpression of RelB and a series of antigens associated with DC differentiation/activation. The CD83, CMRF-44, CMRF-56, p55, CD1a and HLA-DR antigens have all been used separately for the immunological identification of DC. Coexpression of these antigens as well as CD80 and CD86 costimulator molecules was, therefore, studied. RelB expression in monocytes/macrophages, as identified by CD14 and CD68, was also investigated in an effort to clarify the stage of DC differentiation at which these molecules were expressed.
In the lymph node RelB expression was identified in scattered nuclei in both the T lymphocyte and B lymphocyte areas, although its density was several times greater in the T-lymphocyte areas. In the T-lymphocyte areas the majority of RelB+ cells coexpressed the DC activation antigen CD83 (Fig. 3a) and HLA-DR (Fig. 3b). Similar results were seen with CMRF-44, CMRF-56 and p55 (data not shown). A similar proportion were positive for the costimulator molecule CD86 (Fig. 3c) whereas CD80 expression was less frequent and weak when present (data not shown). A small number of RelB+ cells coexpressed the monocyte/macrophage markers CD14 and CD68. Almost all activated DC showed obvious nuclear expression of RelB.
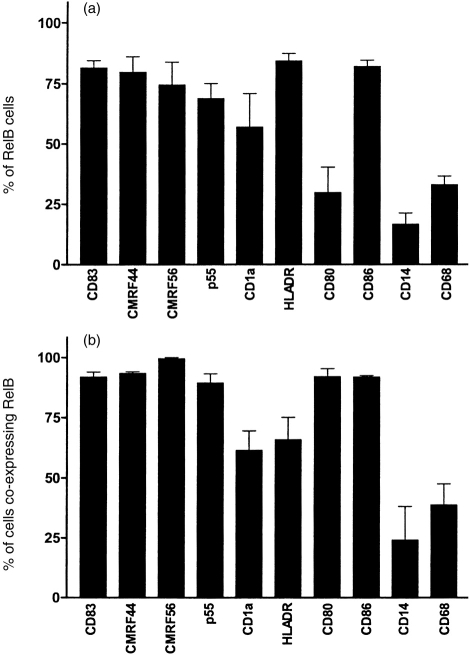
As summarized in Fig. 4, RelB is expressed by the majority of tissue DC in the T-lymphocyte area of the lymph nodes identified by DC associated activation antigens, including CD83, CMRF-44 and CMRF-56 and the costimulator molecule CD86. Thus, RelB expression in DC correlates with the post-migration activated stage.
Figure 4.
Graphical representation of the coexpression of RelB and other antigens by lymphoid DC and monocyte/macrophages in lymph node. (a) The proportion of RelB+ cells costaining with DC-associated antigens (CD83, CMRF-44, CMRF-56, p55, CD1a and HLA-DR) or the monocyte/macrophage antigens (CD14 and CD68) clearly indicated that not all RelB+ cells are DC. (b) The proportion of DC identified by the DC-associated antigens; CD83, CMRF-44, CMRF-56, p55, CD1a and HLA-DR or monocyte/macrophages identified by CD14 and CD68 indicates that most activated DC found in the T-lymphocyte areas of the lymph node express RelB. These results were collated from six samples.
The identity of the RelB+ cells present in B-lymphocyte follicles could not accurately be determined. This was because of the diffuse staining of germinal centre B lymphocytes with CD83, CMRF-44, CMRF-56 and HLA-DR mAb.
Very similar findings were observed in the tonsils studied. The majority of cells expressing DC associated antigens CD83, CMRF-44, CMRF-56 and p55 coexpressed RelB (data not shown). Few of the CMRF-44− tonsil DC appeared to express RelB protein. Heavy expression of RelB in the tonsillar epithelium was also noted. This expression tended to be perinuclear rather than nuclear and was consistently stronger in the basal layers.
DISCUSSION
We have studied the expression of the RelB transcription factor at different stages of differentiation/activation within the human myeloid DC lineage using RT–PCR, flow cytometry and immunohistochemistry. It was important to examine the ‘natural’ DC differentiation pathway as well as what may be an alternative ‘boost’ pathway of Mo-DC differentiation.1 No RelB mRNA or protein was found in the earlier stages of DC differentiation, including CD34+ precursors from cord blood and bone marrow, fresh blood DC and interstitial tissue and epithelial associated LC. In contrast, RelB was expressed in the later stages of DC differentiation, i.e. the postmigration (activated) interdigitating DC associated with lymphoid tissue. The in vitro differentiation/activation of blood DC resulting from 48 hr culture in media containing serum, was associated with the expression of RelB. This treatment has been well documented to be associated with marked phenotypic and functional changes thought to mimic differentiation/activation events in vivo.9,38 Although RelB was expressed in nearly all mature human DC, it was also expressed by cells other than DC.
The distribution of human RelB appears to be similar to the mouse gene product, suggesting an important role for RelB in the control of DC differentiation and function. In mice, RelB is expressed predominantly in the thymic medulla and the interdigitating cells of the lymphoid tissue but not in interstitial DC or LC. The disruption of RelB expression by gene targeting or insertional mutagenesis did not effect the LC population14,15 but had profound influences on APC activity. It appears that only the mature myeloid DC markers and not the lymphoid DC markers are reduced in RelB knockout mice, a result which emphasizes the need to study RelB carefully in relation to DC differentiation pathways and subsets. Our new data indicates that RelB contributes during the late stages of human myeloid DC differentiation/activation in a manner entirely compatible with this murine data.
The correlation of the CMRF-44, CMRF-56 and CD83 differentiation/activation markers with RelB has not been described previously. In human thymus RelB was localized to medullary cells with a dendritic morphology.39 Nuclear localization of the p50, p52 and RelB members of the NF-κB family of proteins in normal human tonsils and lymph nodes with follicular hyperplasia has been described.20 A panel of antibodies including anti-HLA-DR, CD68 and CD1a antibodies failed to localize the expression of the transcription factors to a particular cell type although it was noted that the distribution may indicate localization to a type of APC. Using the recently available antibodies to DC activation/differentiation markers we were able to localize the expression of RelB to the interdigitating (CD83+, CMRF-44+, CMRF-56+) DC. A small subset of RelB positive cells appear to be macrophages as defined by CD14 or CD68 staining, but the majority of macrophages do not stain with RelB. We have recently identified a subset of CMRF-44− tonsil DC and these appear to express low levels of RelB mRNA.
A recent study40 using RT–PCR, noted RelB mRNA to be present in most human blood lymphoid cell populations, whereas immunohistological analysis colocalized RelB expression to HLA-DR+ cells within lymphoid (tonsil) tissue section. Human skin was reported not to stain for RelB. Electrophoretic mobilization shift assays demonstrated RelB nuclear activity in Mo-DC but not in peripheral blood DC isolated without a period of in vitro culture. Our data extends these findings and makes the critical (new) observation that RelB expression correlates with markers that identify mature differentiated or activated DC populations in both cultured blood preparations and lymphoid tissues. The isolated DC purified directly from fresh blood were negative, but cultured blood upregulates RelB expression in parallel with other DC markers. This appears to mimic in vivo events associated with myeloid DC differentiation and suggests that RelB may be important in human in vivo DC differentiation. Monocytes cultured in the presence of cytokines, notably GM-CSF/IL-4, generate cells with an activated DC-like phenotype.2 These cells, which clearly differ in their molecular characteristics from blood cultured DC (in preparation), also express RelB, as has been described by others.
The presence of RelB in activated, but not resting DC, suggests that it is involved in DC functional APC activity. CD40 is one cell-surface molecule that plays a crucial role in B-lymphocyte activation41 and in the induction of DC costimulator activity.27 Recently, it was shown that stimulation of B lymphocytes with CD40 ligand expressed on L cell transfectants resulted in the constitutive accumulation of RelB in the nucleus.42 This effect was not seen when B lymphocytes were stimulated with lipopolysaccharide (LPS) or anti-IgM. In addition, stimulation through CD40 induced the transcription of new RelB mRNA. It has been shown that fresh lin− blood DC express very little CD40 however, CD40 expression is rapidly upregulated by a brief (8 hr) culture period.27 These fresh lin− blood DC also fail to express RelB mRNA and protein which are induced following culture. The correlation of RelB and CD40 expression suggests that CD40 plays a role in the regulation of RelB within blood DC populations.
It is not known which genes, in either mouse or human DC, are regulated by RelB or other NF-κB family members but the mouse knockout data implies they are highly relevant to DC function. The promoter region of the human CD80 gene43 contains a NF-κB binding site with which homo- and heterodimers containing p50, c-Rel and RelB are able to interact. The CD80 costimulator molecule is expressed by activated blood DC and lymphoid tissue DC but not by fresh blood DC or interstitial DC.44 Our results show that this expression correlates with the presence of RelB mRNA and protein. The immunohistology performed in these studies confirms that very few RelB+ cells express CD80; however, the majority of CD80+ cells (>90%) coexpress RelB. Thus, expression of the human CD80 gene by DC may be regulated by RelB. It may contribute directly or indirectly to expression of the CD83, CMRF-44 and CMRF-56 antigens.
In this paper, our RT–PCR data demonstrate that transcriptional regulation of RelB is clearly an important contribution to DC maturation. Our methodology concentrated on documenting the transcription and translation of the RelB gene in human myeloid DC differentiation. Two important observations resulted. First, RelB expression is associated with normal myeloid DC differentiation/activation at the post-tissue-migration phase. Thus RelB mRNA and protein product are induced in activated blood DC and interdigitating lymphoid DC. Second, we have shown that RelB expression in human myeloid DC correlates very closely with expression of the CD83, CMRF-44 and CMRF-56 antigens, an observation which will help interpret the phenotypic data now emerging using these markers.
Acknowledgments
Andrew Troy was supported by a Schering Plough Fellowship from the Urological Society of Australasia. We are grateful to Lisa Whyte for technical support on the flow cytometer and Dr D. Fearnley for helpful advice and we thank Mrs J.Merry-Martin for help in preparing this manuscript.
Abbreviations
- APC
antigen-presenting cells
- β2M
β2 microglobulin
- DC
dendritic cells
- LC
Langerhans’ cells
- lin
lineage
- mAb
monoclonal antibodies
- Mo-DC
monocyte-derived dendritic cells
- PBMC
peripheral blood mononuclear cells, PE, phycoerythrin
- GAM
goat antimouse
- SAR
sheep antirabbit
- GAR
goat antirabbit
References
- 1.Hart DNJ. Dendritic cells: unique leucocyte populations which control the primary immune response. Blood. 1997;90:3245. [PubMed] [Google Scholar]
- 2.Vuckovic S, Fearnley DB, Mannering SI, Dekker LF, Whyte L, Hart DNJ. Generation of CMRF-44+ monocyte derived dendritic cells: Insights into phenotype and function. Exp Hematol. 1998;26:1. [PubMed] [Google Scholar]
- 3.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosialos G, Birkenbach M, Ayehunie S, et al. Circulating human dendritic cells differentially express high levels of a 55-kd actin-bundling protein. Am J Pathol. 1996;148:593. [PMC free article] [PubMed] [Google Scholar]
- 5.Hart DNJ, McKenzie JL. Isolation and characterization of human tonsil dendritic cells. J Exp Med. 1988;168:157. doi: 10.1084/jem.168.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou LJ, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821. [PubMed] [Google Scholar]
- 7.Zhou LJ, Tedder TF. A distinct pattern of cytokine gene expression by human CD83+ blood dendritic cells. Blood. 1996;86:3295. [PubMed] [Google Scholar]
- 8.Hock BD, Starling GC, Daniel PB, Hart DNJ. Characterisation of CMRF-44, a novel monoclonal antibody to an activation antigen expressed by the allostimulatory cells within peripheral blood, including dendritic cells. Immunology. 1994;83:573. [PMC free article] [PubMed] [Google Scholar]
- 9.Fearnley DB, McLellan AD, Mannering SI, Hock BD, Hart DNJ. Isolation of human blood dendritic cells using the CMRF-44 monoclonal antibody: implications for studies on antigen presenting cell function and immunotherapy. Blood. 1997;89:3708. [PubMed] [Google Scholar]
- 10.Hock BD, Fearnley DB, Boyce A, et al. Human dendritic cells express a 95kda activation/differentiation antigen defined by CMRF-56. Tissue Antigens. 1999;53:320. doi: 10.1034/j.1399-0039.1999.530402.x. [DOI] [PubMed] [Google Scholar]
- 11.Thomas R, Lipsky PE. Could endogenous self-peptides presented by dendritic cells initiate rheumatoid arthritis? Immunol Today. 1996;17:559. doi: 10.1016/s0167-5699(96)20030-1. [DOI] [PubMed] [Google Scholar]
- 12.Kerhl JH. Haematopoietic lineage commitment: role of transcription factors. Stem Cells. 1996;13:223. doi: 10.1002/stem.5530130304. [DOI] [PubMed] [Google Scholar]
- 13.Shivdasani RA, Orkin SH. The transcriptional control of hematopoiesis. Blood. 1996;87:4025. [PubMed] [Google Scholar]
- 14.Weih F, Carrasco D, Durham SK, et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κB/Rel family. Cell. 1995;80:331. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 15.Burkly L, Hession C, Ogata L, et al. Expression of RelB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin AS. The NF-κB and I-Kb proteins: new discoveries and insights. Ann Rev Immunol. 1996;14:649. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 17.Carrasco D, Ryseck R-P, Bravo R. Expression of RelB transcripts during lymphoid organ development: specific expression in dendritic antigen-presenting cells. Development. 1993;118:1221. doi: 10.1242/dev.118.4.1221. [DOI] [PubMed] [Google Scholar]
- 18.Lernbecher T, Muller U, Wirth T. Distinct NFκB/Rel transcription factors are responsible for tissue-specific and inducible gene activation. Nature. 1993;365:767. doi: 10.1038/365767a0. [DOI] [PubMed] [Google Scholar]
- 19.Lernbecher T, Kistler B, Wirth T. Two distinct mechanisms contribute to the constitutive activation of RelB in lymphoid cells. EMBO J. 1994;13:4060. doi: 10.1002/j.1460-2075.1994.tb06723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feuillard J, Dargemont C, Ferreira V, et al. Nuclear Rel-a and C-Rel protein complexes are differentially distributed within human thymocytes. J Immunol. 1997;158:2585. [PubMed] [Google Scholar]
- 21.Granelli-Piperno A, Pope M, Inaba K, Steinman RM. Coexpression of NF-κB/Rel and Sp1 transcription factors in human immunodeficiency virus 1-induced, dendritic cell-T-cell syncytia. Proc Natl Acad Sci USA. 1995;92:10 944. doi: 10.1073/pnas.92.24.10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akagawa KS, Takasuka N, Nozaki Y, et al. Generation of CD1+ RelB+ dendritic cells and tartrate-resistant acid phosphatase-positive osteoclast-like multinucleated giant cells from human monocytes. Blood. 1996;88:4029. [PubMed] [Google Scholar]
- 23.Egner W, Andreesen R, Hart DNJ. Allostimulatory cells in fresh human blood: heterogeneity in antigen presenting cell populations. Transplantation. 1993;56:945. doi: 10.1097/00007890-199310000-00032. [DOI] [PubMed] [Google Scholar]
- 24.Starling GC, Davidson SE, McKenzie JL, Hart DNJ. Inhibition of natural killer cell mediated cytolysis with monoclonal antibodies. Immunology. 1987;61:351. [PMC free article] [PubMed] [Google Scholar]
- 25.Summers KL, O’Donnell JL, Sew Hoy M, et al. Monocyte–macrophage antigen expression on chondrocytes. J Rheumatol. 1995;22:1326. [PubMed] [Google Scholar]
- 26.Sutherland DR, Armand K, Nayar R, Anania S, Stewart AK. Sensitive detection and enumeration of CD34+ cells in peripheral and cord blood by flow cytometry. Exp Hematol. 1994;22:1003. [PubMed] [Google Scholar]
- 27.McLellan AD, Sorg RV, Williams LA, Hart DNJ. Human dendritic cells activate T lymphocytes via a CD40:CD40 ligand-dependent pathway. Eur J Immunol. 1996;26:1204. doi: 10.1002/eji.1830260603. [DOI] [PubMed] [Google Scholar]
- 28.McLellan AD, Starling GC, Hart DNJ. Isolation of human blood dendritic cells by Nycodenz discontinuous gradient centrifugation. J Immunol Methods. 1995;184:81. doi: 10.1016/0022-1759(95)00077-n. [DOI] [PubMed] [Google Scholar]
- 29.Prickett TCR, McKenzie JL, Hart DNJ. Adhesion molecules on human tonsil dendritic cells. Transplantation. 1992;53:483. doi: 10.1097/00007890-199202010-00041. [DOI] [PubMed] [Google Scholar]
- 30.Dallman MJ, Porter ACG. Analysis of cytokine gene expression by semi-quantitative PCR. In: McPherson MJ, Quirke P, Taylor GR, editors. PCR: a Practical Approach. Oxford: OUP; 1991. p. 215. [Google Scholar]
- 31.Bours V, Azarenko V, Dejardin E, Siebenlist U. Human RelB (I-Rel) functions as a KB site-dependent transactivating member of the family of Rel-related proteins. Oncogene. 1994;9:1703. [PubMed] [Google Scholar]
- 32.Suggs SV, Wallace RB, Hirose T, Kawashima EH, Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human β2-microglobulin. Proc Natl Acad Sci USA. 1981;78:6613. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egner W, Hart DNJ. The phenotype of freshly isolated and cultured human bone marrow allostimulatory cells: heterogeneity in dendritic cell populations. Immunology. 1995;85:611. [PMC free article] [PubMed] [Google Scholar]
- 34.Luft T, Pang KC, Thomas E, et al. A serum-free culture model for studying the differentiation of human dendritic cells from adult CD34+ progenitor cells. Exp Hematol. 1998;26:489. [PubMed] [Google Scholar]
- 35.Szabolcs P, Moore MAS, Young JW. Expansion of immunostimulatory dendritic cells among the myeloid progeny of human CD34+ bone marrow precursors cultured with C-kit ligand, granulocyte-macrophage colony-stimulating factor, and TNF-α. J Immunol. 1995;154:5851. [PubMed] [Google Scholar]
- 36.Hart DNJ, McKenzie JL. Interstitial dendritic cells. Int Rev Immunol. 1990;6:127. doi: 10.3109/08830189009056624. [DOI] [PubMed] [Google Scholar]
- 37.Prickett TCR, McKenzie JL, Hart DNJ. Characterization of human interstitial dendritic cells in liver. Transplantation. 1988;46:754. doi: 10.1097/00007890-198811000-00024. [DOI] [PubMed] [Google Scholar]
- 38.Mannering SI, McKenzie JL, Hart DNJ. Optimisation of the conditions for generating human DC initiated primary antigen specific T lymphocyte lines in vitro. J Immunol Methods. 1998;219:69. doi: 10.1016/s0022-1759(98)00125-2. [DOI] [PubMed] [Google Scholar]
- 39.Feuillard J, Korner M, Israel A, Vassy J, Raphael M. Differential nuclear localization of p50, p52 and RelB proteins in human accessory cells of the immune response in situ. Eur J Immunol. 1996;26:2547. doi: 10.1002/eji.1830261102. [DOI] [PubMed] [Google Scholar]
- 40.Pettit A, Quinn C, MacDonald KPA, et al. Nuclear localization of RelB is associated with effective antigen-presenting cell function. J Immunol. 1997;159:3681. [PubMed] [Google Scholar]
- 41.Banchereau J, Bazan F, Blanchard D, et al. The CD40 antigens and its ligand. Annu Rev Immunol. 1994;12:881. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 42.Neumann M, Wohlleben G, Chuvpilo S, et al. CD40, but not lipopolysaccharide and anti-IgM stimulation of primary B lymphocytes, leads to persistent nuclear accumulation of RelB. J Immunol. 1996;157:4862. [PubMed] [Google Scholar]
- 43.Zhao J-L, Freeman GJ, Gray GS, Nadler LM, Glimcher LH. A cell type-specific enchancer in the human B7.1 gene regulated by NF-κB. J Exp Med. 1996;183:777. doi: 10.1084/jem.183.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hart DNJ, Starling GC, Calder VL, Fernando NJ. B7/BB-1 is a leucocyte differentiation antigen on human dendritic cells induced by activation. Immunology. 1993;79:616. [PMC free article] [PubMed] [Google Scholar]