Abstract
The kinetics of monocyte–macrophage differentiation was analysed using two Swine Workshop Cluster (SWC) CD molecules: SWC1 and SWC9. Myeloid cells were selected by labelling for the common myeloid antigen, SWC3. Confirmation of macrophage identification used acid phosphatase and phagocytosis activities. During differentiation, SWC1 was gradually lost. SWC9 was absent on monocytes but up-regulated early. Consequently, monocytes were SWC1+ SWC9− and macrophages were SWC1− SWC9+. An additional, intermediate, cell population was identified as SWC1+ SWC9+. Size and granularity characteristics mirrored the monocyte, macrophage and intermediate-cell phenotypes. Overall, SWC9 up-regulation was central in macrophage differentiation and dependent on plasma factors. The concomitant loss of SWC1 was independent of these factors, but always associated with mature macrophages. Upon up-regulation of SWC9, the SWC1+ SWC9+ intermediate monocytic cells became susceptible to African swine fever virus infection. These results demonstrate the heterogeneity of monocytic cell differentiation and the importance of these characteristics for interaction with monocytotropic viruses.
INTRODUCTION
Monocytic cells are a heterogeneous population, evident by the variety of functions that different subpopulations can display.1 When viruses infect such cells, immunological activity can be seriously impaired or modified. In this context, monocytic cells have been reported as susceptible target cells in vitro and in vivo (see refs 2–7). Interestingly, variations in the susceptibility of such cells to infection, or the capacity of virus to replicate therein, have been noted.4,6,7 One study reported that monocytic cell phenotype might be related to the susceptibility to virus infection.4
Comparative analyses on human blood monocytes, and the macrophages derived from them, have demonstrated a modulation of phenotype.8–14 Kreutz et al.15 stated that in vitro maturation of blood monocytes into macrophages could actually serve as a model for the in vivo phenomenon. Monocyte-derived macrophages could express surface markers not found on their monocyte precursors,8,9,12,14,15 with other markers being expressed preferentially on monocytes.10,13 Haverson et al.16 did not observe phenotypic differences between porcine monocytes and macrophages, although Gonzalez-Juarrero et al.17 did note an alteration in major histocompatibility complex (MHC) expression. Aller et al.18 and Wierda et al.19 also observed differences between porcine blood monocytes and alveolar macrophages, in terms of FcγRIII and its associated porcine natural killer cell-enhancing (PNK-E) molecule. Nevertheless, none of these groups analysed porcine monocytes during their differentiation into macrophages.
The Swine CD Workshop of the International Union of Immunological Societies (IUIS) identified a CD molecule, termed SWC9, on porcine alveolar macrophages.20 SWC refers to Swine Workshop Cluster, used to identify swine CD molecules for which no corresponding human molecule is known. Further analyses established the porcine monocyte phenotype as SWC9−, compared with the SWC9+ phenotype of porcine macrophages.21 Consequently, the present work aimed to analyse the population changes occurring as monocytes differentiated into macrophages. In order to achieve this, the study focused on the SWC9 macrophage marker, in combination with an additional marker, SWC1. The latter marker is known to be expressed on monocytes and T lymphocytes,22,23 but prior to this work had not been analysed on macrophages. Overall, the work had three aims. First, to ascertain whether cells intermediate in the differentiation from monocytes to macrophages could be identified. Second, to determine the relationship of porcine plasma factors to this differentiation. Third, to analyse the influence of the differentiation on susceptibility to infection with a monocytotropic virus.
MATERIALS AND METHODS
Media, buffers and medium supplements
Powdered Dulbecco’s modified Eagle’s medium (DMEM; phenol-red free) and ultra-pure water low in pyrogens were used. As medium supplements, porcine plasma from heparinized blood (Hep-Pl) was used at 30% (v/v) and glutamine (Gibco BRL, Life Technologies AG, Basel, Switzerland) at 2 mm to give ‘growth medium’. Phosphate-buffered saline (PBS; Dulbecco’s modification), PBS–EDTA (calcium/magnesium-free PBS containing 0·03% w/v EDTA) and Alsever’s solution were prepared from the basic chemicals. All glassware was sterilized at 180° for 6 hr.
Monocytic cells
Venous blood was collected from 100-kg specific pathogen-free (SPF) pigs (Swiss White Landrace) into Alsever’s solution.4 A total of 16 SPF pigs were used. Animals were bled a maximum of once per week. Peripheral blood mononuclear cells (PBMC) were isolated over Ficoll-Paque (Amersham Pharmacia Biotech AG, Dübendorf, Switzerland) in 50-ml Leukosep tubes.4 Bone marrow (BM) haematopoietic cells (BMHC) and lung lavage ‘alveolar’ macrophage preparations were prepared as described previously.4,24
Monocytic cell preparations were isolated as follows. The citrated blood was centrifuged at 1000 g for 25 min at room temperature, following which the buffy coat was removed, diluted 1:2 with PBS–EDTA and centrifuged over Ficoll-Paque at 800 g for 25 min at room temperature. Mononuclear cells (above the Ficoll) were diluted with cold (4°) PBS–EDTA, centrifuged at 350 g for 15 min at 4°, treated with 0·15 m NH4Cl, 10 mm NaHCO3, 1 mm disodium EDTA, pH 7·2 (for 5 min at 4°) and washed three times with PBS–EDTA (at 250 g for 10 min at 4°). The PBMC were resuspended in growth medium at 4×106 cells/ml. Non-adherent cells were removed following culture on plastic for 2 hr at 37°.
Myeloid cells were identified by the porcine pan-myeloid marker, SWC3.23 With the adherent cells obtained from PBMC, the percentage of SWC3+ cells ranged from 60 to 75%, depending on the preparation. The remaining cells were lymphocytes. Within 24–48 hr of culture, this percentage of contaminating lymphocytes had fallen to <5%, many of the lymphocytes having detached during this time. In order to analyse monocyte-to-macrophage differentiation, the adherent cells were incubated at 37° for the different periods of time shown in the Results. (This was not necessary for the alveolar macrophage preparations, which were assumed to have already differentiated from monocytes into macrophages.) The cells were then labelled with monoclonal antibodies (mAbs) against particular CD molecules.
Determination of surface molecule expression on porcine monocytic cells
Surface molecule expression on porcine monocytic cells was analysed using a flow cytometer (FACScan Flow Cytometer; Becton-Dickinson AG, Basel, Switzerland) and mAbs reactive with particular cell determinants. The molecules recognized by the mAbs were given a CD nomenclature if related to known human CD molecules, or an SWC nomenclature (SWC=swine workshop cluster) if no relationship could be made. The mAbs used were: 11/8/1 or 76-6-7 anti-SWC1 (found on porcine monocytes and T lymphocytes);22–25 DH59 or 74-22-15 anti-SWC3 pan-myeloid marker;23 MIL-3 anti-SWC8 marker found on granulocytic populations;20,23,24 PM18-7 anti-SWC9 alveolar macrophage marker;21 and My4 anti-human CD14, cross-reactive with porcine CD14.26 DH59 was obtained from Veterinary Medical Research and Development (VMRD; Pullman, WA); My4 was from Coulter-Clone (Instrumenten Gesellschaft, Switzerland); and the other mAbs were prepared from hybridomas.
For single-antibody labelling, incubations were carried out for 20 min at 4° for the mAbs and fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 anti-mouse isotype-specific conjugates (Southern Biotechnology, Birmingham, AL). For double- and triple-antibody labelling, the mAbs employed were added simultaneously for the incubation period of 20 min at 4°. The anti-mouse isotype-specific conjugates were labelled with FITC, phycoerythrin (PE) or biotin (Southern Biotechnology). The biotinylated conjugate was detected using Spectralred-Streptavidin (incubation for 20 min at 4°). These labellings employed SWC3 to focus the analysis on the SWC3+ monocytic cell populations. With BMHC, triple labellings employed SWC3 and SWC8 to identify the SWC3+ SWC8− BM monocytic cells.24 Dead cells were excluded by labelling a separate sample of the cells with propidium iodide and gating on the region in the forward scatter/side scatter (FSC/SSC) plots in which the majority of these dead cells resided. As a result, >90% of the dead cells could be excluded without interfering with measurement of the living cells.
Determination of macrophage-like activity
Tartrate-resistant acid phosphatase (ACP) was measured using the kit from Sigma Diagnostics (Poole, Dorset, UK). Monocytes were cultured for 0, 1, 2 or 3 days (0 days corresponds to freshly isolated monocytes after 2 hr of adherence). Medium was replaced at these time-points, and the cells were cultured for a further 24 hr to detect de novo enzyme synthesis. Samples (1 ml) were added to the kit reagents, absorbance was measured at 405 nm using an LKB Ultrospec (Basel, Switzerland) values were converted into ACP activity (U/l) and medium-alone values (i.e. baseline levels) were subtracted.
Phagocytosis was measured using BODIPY-fluorescein-Zymosan (Molecular Probes, Holland), at a concentration of 50 particles/cell, opsonized with porcine serum (for 30 min at 37°), and incubated with monocytic cells for 30 min at 37°. Trypan Blue (0·04% v/v) was added to quench the signal from non-internalized zymosan.
Determination of cell susceptibility to virus infection
Analysis of monocytic cell susceptibility to African swine fever virus (ASF virus) (KWH strain, kindly provided by Dr Philip Wilkinson, BBSRC Institute for Animal Health, Pirbright, UK) infection was as described previously.4 The multiplicity of infection (MOI) was 10 infectious units per cell, virus adsorption was performed for 2 hr at 37° and residual unadsorbed virus was removed by washing five times. These cultures were incubated for 24 hr at 37° in growth medium, after which the cells were removed and fixed/permeabilized (Cell Permeabilisation Kit, Harlan Sera-Lab, Crawley Down, UK). The presence of de novo-synthesized virion antigen was detected with mAbs specific for the VP32 or VP73 proteins. This method of labelling permitted detection of de novo antigen synthesis, which was found to become detectable between 8 and 12 hr postinfection (data not shown); antibody labelling did not detect the input virus, thus allowing identification of the replicating virus only. Antibody labelling and flow cytometry procedures were as described above.
Statistical analyses
Each experiment was performed in triplicate, and repeated at least three times. Mean value (x¯) and standard deviation (SD) were calculated from:
and
For determination of statistical significance, the Student’s t-test was performed.
RESULTS
Expression of the SWC1 monocyte/T-lymphocyte marker on differentiating monocytic cells
Both blood- and BM-derived monocytes (freshly isolated) expressed high levels of SWC1 (Fig. 1a, 1b: 2 hr). The level of SWC1 was higher than that found on T lymphocytes separated from monocytic cells using the SWC3 myeloid marker: monocytes were SWC1bright SWC3+, while T cells were SWC1+ SWC3−.22 Upon differentiation of the monocytes in culture, SWC1 expression was down-regulated (Fig. 1a, 1b). This was notable by a 10-fold decrease during the initial 24 hr of culture (Fig. 1a, 1 day). Thereafter, the rate of down-regulation was more gradual. This culminated in the eventual loss of SWC1 expression on the majority of monocyte-derived macrophages to give an SWC1− or an SWC1dim phenotype (Fig. 1a, 4 days; Fig. 1b, 10 days). Alveolar macrophages were also dominated by an SWC1− phenotype, although a minority of cells from particular preparations showed an SWC1dim phenotype (Fig. 1b).
Figure 1.
Relative expression of the porcine monocyte/T-lymphocyte marker SWC1 (a–c), the porcine macrophage marker SWC9 (d–f) and the common myeloid antigen CD14 (g–i) on porcine blood monocytic cells (a,d,g), bone marrow (BM) monocytic cells (b,e,h) and lung lavage monocytic cells (c,f,i). The results show blood monocytic cells at 2 hr, and at 1, 2, 4 and 10 days, after isolation and culture in Dulbecco’s modified Eagle’s medium (DMEM) containing 30% (v/v) porcine plasma (a,d,e). Such culturing permits the differentiation of macrophage-like cells from blood monocytes. The BM monocytic cells were also analysed at 2 hr and at 10 days after isolation and culture in DMEM containing 30% (v/v) porcine plasma (b,e,h). Only the results for the 10-day BM monocytic cell cultures are shown for the SWC9 labelling (e) because no SWC9+ cells were detectable amongst recently isolated BM monocytic cells. The results with the lung lavage cells are shown for the cells only after isolation, but for different preparations from different animals (c,f,i). All results are presented with reference to an appropriate isotype-control reaction (myeloma cell protein, MOPC). (MDM, monocyte-derived macrophages.)
Expression of the SWC9 macrophage marker on monocytes differentiating in vitro
In contrast to SWC1, both blood- and BM-derived monocytes were mainly negative for SWC9 (Fig. 1d, 2 hr). As the monocytes differentiated in vitro, there was a rapid up-regulation of SWC9 expression within 24 hr (Fig. 1d, 1 day). The relative intensity of expression on the SWC9+ cells increased after a further 24 hr (Fig. 1d, 2 days), following which it remained relatively stable. Both blood- and BM-derived monocytic cells behaved similarly (Fig. 1d, 1e). The resultant SWC9 phenotype on the monocyte-derived macrophages was similar to that of alveolar macrophages (Fig. 1f). Both the in vitro- and in vivo-derived macrophages also had similar microscopic morphology and flow cytometric FSC/SSC characteristics (data not shown).
In comparison, the CD14 myeloid antigen was down-regulated as monocytic cells differentiated into macrophages, both in vitro (Fig. 1g, 1hr) and in vivo (Fig. 1i). Unlike SWC1, however, the macrophages remained CD14+, albeit CD14low compared with expression on monocytes.
Dual expression of SWC1 and SWC9
Different preparations of alveolar macrophages, gated as SWC3+, were dominated by SWC1− SWC9+ cells (Fig. 2a, 2b). Of those preparations containing cells with an SWC1dim expression (Fig. 1c), the SWC1dim cells were also positive for SWC9 (Fig. 2a). Neither SWC1− SWC9− nor SWC1+SWC9− cells were found amongst the myeloid cells within lung lavages. When SWC1dim SWC9+ cells were present, the SWC9 expression tended to be lower on all myeloid cells therein, compared with lavages in which no SWC1+ cells could be identified (Fig. 2a, Fig. 2b).
Figure 2.
Triple immunofluorescence labelling (SWC9/SWC1/SWC3) and flow cytometric analysis of two different representative samples of freshly isolated porcine lung lavage monocytic cells (a,b) and splenocytes (c). Gating was on SWC3+ cells to show the expression of SWC1 (y-axis) and SWC9 (x-axis).
Freshly isolated SWC3+ splenocytes contained both SWC1+ SWC9− and SWC1+ SWC9+ cells (Fig. 2c). SWC1− SWC9+ cells were also present, but less common than the other two populations. These phenotypes did not form distinct sets, but presented a considerable overlap. Such an image was suggestive of an active process of differentiation: from SWC1+ monocytes, to intermediate cells and, finally, to SWC9+ macrophages.
Intermediate cells in monocytic cell differentiation express both monocytic and macrophage markers
Multiple labellings were also performed on PBMC to determine how the SWC1 marker, dominant on monocytes, related to that of the macrophage SWC9 marker. The SWC3+ monocytic cells in freshly isolated PBMC, as well as those which had been left to adhere for 2 hr, were dominated by cells with an SWC1bright SWC9− phenotype (Fig. 3a shows the 2 hr-adherent monocytic cells). After 24 hr of culture, 35% of the cells remained SWC1+ SWC9−, but had down-regulated SWC1 (Fig. 3b). The remainder of the cells had also down-regulated SWC1, but concomitantly started to express SWC9 (Fig. 3b). At 48 hr and 72 hr of culture, both the SWC1+ SWC9− and SWC1+ SWC9+ cells continued to down-regulate SWC1 (Fig. 3c, 3d). In addition, an SWC1− SWC9+ subpopulation was clearly identifiable wherein the SWC9 marker showed a tendency towards a higher level of expression (Fig. 3c, 3d). With cells cultured for 4 days, the majority were now SWC1− SWC9+ (Fig. 3e). A minority of viable cells were SWC1− SWC9−, but few were double positive owing to SWC1 down-regulation (Fig. 3e). Between days 4 and 10 of culture, depending on the experiment, all viable myeloid cells became SWC1− SWC9+ (Fig. 3f). This phenotype was maintained with prolonged culture. It is unclear whether the SWC1− SWC9− cells shown in Fig. 3(e) had also up-regulated SWC9 by day 10.
Figure 3.
Triple immunofluorescence labelling (SWC9/SWC1/SWC3) and flow cytometric analysis of porcine blood monocytic cells. Gating was on SWC3+ cells to show the expression of SWC1 (y-axis) and SWC9 (x-axis), at 2 hr (a), and at 1 (b), 2 (c), 3 (d), 4 (e) and 10 (f) days after isolation and culture in Dulbecco’s modified Eagle’s medium (DMEM) containing 30% (v/v) porcine plasma.
The relatively high proportion of SWC1+ SWC9− cells, observed during differentiation and shown in Fig. 3(b), 3(c), 3(d), was not always observed with different preparations of cells. These results presented in Fig. 3 were chosen to facilitate the visualization of the SWC1+ SWC9− subpopulation. The most consistent observation throughout was the early appearance of a dominant SWC1+ SWC9+ subpopulation during the differentiation process. Moreover, the gradual progression towards an SWC1− SWC9+ phenotype was also characteristic of monocyte-to-macrophage differentiation.
Morphology of differentiating monocytic cell subpopulations
Following labelling of SWC3+ cells for SWC1 and SWC9 expression, gates were placed around the different subpopulations as shown in Fig. 4(a), (4e), (4i). Considering the monocytic cells after 24 hr of differentiation (Fig. 4a, 4b, 4c, 4d), the SWC1+ SWC9−cells (Fig. 4a, R1) retained the FSClow SSClow monocyte morphology (Fig. 4b). This contrasted with cells that had concomitantly up-regulated SWC9 to become SWC1+ SWC9+ (Fig. 4a, R2). Such cells had clearly shifted towards larger size and higher granularity (Fig. 4c). In addition to these two populations, it was suggested that a minor (3%) population was becoming SWC1− (Fig. 4a, R3). This population also showed a tendency towards higher FSC/SSC (Fig. 4d).
Figure 4.
Subpopulations of porcine monocytic cells after 1 (a), 2 (e) and 3 (i) days of culture, gated as SWC1+ SWC9− (R1), SWC1+ SWC9+ (R2) or SWC1− SWC9+ (R3). These subpopulations were analysed in terms of their forward scatter (FSC) and side scatter (SSC) characteristics (b,f,j: R1; c,g,k: R2; d,h,l: R3). Identical photomultiplier tube (PMT)/compensation set-up was employed in order to visualize and relate the FSC/SSC characteristics of the cells after different periods of time in culture.
By 48 hr of the differentiation process, the SWC1+ SWC9− population (Fig. 4e, R1) was again dominated by cells of smaller size and lower granularity (Fig. 4f). However, there was a small increase in the FSC and SSC compared with cells after 24 hr in culture (the same set-up was employed for all preparations). The SWC1+ SWC9+ population (Fig. 4e, R2) was now clearly modulated in terms of increased FSC and SSC signals (Fig. 4g). Therein, the tendency towards larger cells of increased granularity was unmistakable. Moreover, cells with higher FSC/SSC (Fig. 4hr) also dominated the now clearly identifiable SWC1− SWC9+ population (Fig. 4e, R3). Interestingly, there was little difference in the FSC/SSC characteristics of the SWC1+ SWC9+ and SWC1− SWC9+ subpopulations (Fig. 4g, 4hr).
When the 3-day-old differentiating cells were analysed, the above results, on the morphological development of the subpopulations during differentiation towards macrophages, were confirmed. SWC1+ SWC9− cells (Fig. 4i, R1) retained the lowest FSC/SSC signals (Fig. 4j). The tendency towards higher FSC/SSC, suggested by the results from the 48-hr-old cells, was again noted. SWC1+ SWC9+ cells (Fig. 4i, R2) clearly displayed increased FSC and SSC (Fig. 4k). With the SWC1− SWC9+ subpopulation (Fig. 4i, R3), this was unmistakably different from the SWC1+ SWC9+ subpopulation (Fig. 4k, 4l). Many cells in Fig. 4l gave signals that accumulated to the right of the FSC/SSC plot (the maintained set-up was designed to visualize, simultaneously and comparatively, monocytes and macrophages).
Determination of macrophage-like functional activity in the monocytic cell cultures
The phenotypic and morphological analyses demonstrated gradual differentiation through a number of stages. In order to confirm that these cells were indeed progressing towards macrophages, two tests were performed for typical macrophage functions. ACP activity was noted to increase as the phenotypic and morphological changes progressed (Fig. 5a). Similarly, phagocytic activity – internalization of zymosan particles – increased in a manner related to the modulation of cell phenotype and morphology (Fig. 5b). Interestingly, the increase in the relative intensity of zymosan/cell was greatest between monocytes and cells after 2 days in culture (Fig. 5b, filled circles). Concerning the percentage of cells that were phagocytosing the zymosan, this was already relatively high with monocytes (Fig. 5b, open circles, day 0). The highest increase in this percentage occurred between 2 and 4 days of culture (P<0·001).
Figure 5.
Determination of activities associated with the development of macrophages. (a) The presence of secreted acid phosphatase (ACP) activity (y-axis) associated with porcine blood monocytic cells at different time-points (x-axis) after isolation and culture in Dulbecco’s modified Eagle’s medium (DMEM) containing 30% (v/v) porcine plasma. (b) The level of cellular phagocytic activity associated with porcine blood monocytic cells at different time-points (x-axis) after isolation and culture in DMEM containing 30% (v/v) porcine plasma. Phagocytic activity was analysed in terms of the amount of phagocytosed fluorescein isothiocyanate (FITC)–zymosan (filled circles, solid line, y1-axis) and the percentage of cells positive for the presence of FITC–zymosan (open circles, broken line, y2-axis). The error bars show the standard deviation about the mean of results obtained from three replicate experiments, using different preparations of peripheral blood mononuclear cells (PBMC), as described in the Materials and methods.
Dependence of macrophage differentiation on plasma factors
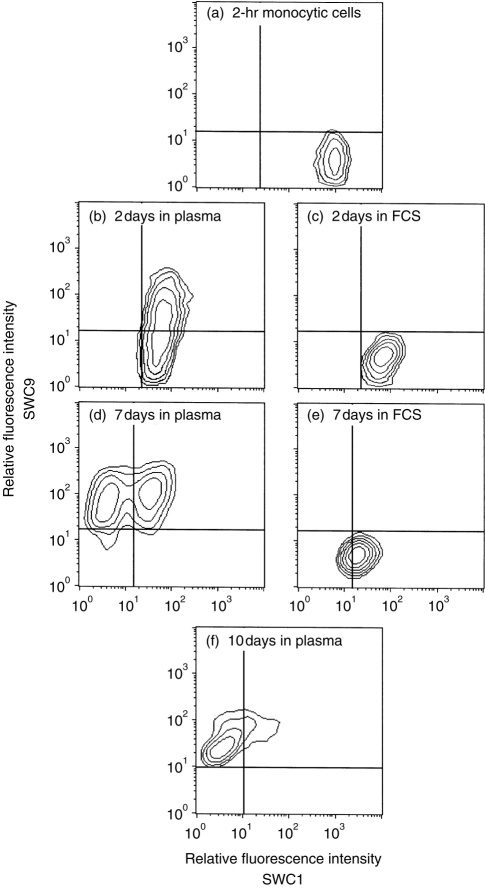
Considering the significance of SWC1 and SWC9 in macrophage differentiation, it was of interest to determine the relevance of in vivo factors therein. Comparison was made of blood monocyte cultures in serum-free medium with those in medium containing fetal calf serum (FCS) or porcine plasma. The serum-free cultures did not survive. With the FCS-supplemented cultures, the monocytes adhered in a manner similar to that observed with porcine plasma-supplemented cultures (data not shown). The difference between these two systems was in the modulation of SWC1/SWC9.
Cells in both FCS- and plasma-supplemented cultures began with the expected SWC1+ SWC9− phenotype (Fig. 6a). Down-regulation of SWC1 with time in culture was observed in the FCS-containing samples (Fig. 6c, 6e). Analysis of different batches of FCS showed that the majority facilitated a gradual down-regulation of SWC1 on monocytic cells with time in culture. SWC9 expression was poor on cells cultured in the presence of FCS (Fig. 6c, 6e), although a few batches did permit a degree of up-regulation after ≥10 days (data not shown). In contrast, most plasma-supplemented cultures gave the image shown in Fig. 6(b), 6(d), 6(f) – concomitant down-regulation of SWC1 and up-regulation of SWC9. This progression resulted in the SWC1− SWC9+ phenotype, typical of differentiated macrophages (Fig. 6f). Moreover, cells cultured in FCS-supplemented medium remained morphologically closer to monocytes, whereas cells in porcine plasma-containing medium acquired morphological and functional activities of macrophages (data not shown).
Figure 6.
The influence of porcine plasma factors (b,d,f) compared with fetal calf serum (FCS; c,e), at 30% (v/v) in Dulbecco’s modified Eagle’s medium (DMEM), on the modulation of SWC1 (x-axis) and SWC9 (y-axis) expression. Cultured porcine monocytic cells were analysed at 2 hr after isolation (a), and after 2 (b,c), 7 (d,e) and 10 (f) days of culture.
Interestingly, different batches of blood monocytes showed variations in the kinetics of SWC1/SWC9 modulation. When the kinetics for cells in ‘plasma’ medium was as shown in Fig. 6, cells in ‘FCS’ cultures also behaved as in Fig. 6. With kinetics in ‘plasma’ medium similar to that in Fig. 3, the cells in ‘FCS’ medium could display an SWC9dim expression after ≥10 days in culture. Considering all of these results, SWC1 down-regulation was a phenomenon common to both ‘plasma’ and ‘FCS’ cultures. In contrast, the rapid up-regulation of SWC9, concomitant with down-regulation of SWC1, was only observed in the presence of porcine plasma.
Varying the concentrations of serum or plasma between 10 and 50% did not alter these results. Only when the porcine monocytic cells were cultured in 100% porcine plasma was there a modification of the kinetics. This was observed as an increase in the rate at which the cells expressed SWC9, but had no effect on SWC1 modulation (data not shown).
Variable susceptibility to virus infection by monocytes differentiating into macrophages
One particular consequence of monocyte differentiation into macrophages relates to the functioning of immune defences. Consequently, it was decided to analyse how in vitro differentiation might modify the characteristics of porcine monocytic cell susceptibility to virus infection. For this analysis, ASF virus was chosen, owing to its known predilection for macrophages as a target cell.2,4 The results demonstrated that monocytes were in fact primarily resistant to virus infection, detected in terms of de novo synthesis of virion proteins (Fig. 7). As the cells differentiated towards the SWC1− SWC9+ macrophage phenotype, there was a gradual and significant increase in susceptibility (Fig. 7). Variation occurred between experiments, particularly concerning infection of the intermediate differentiating cells. Nevertheless, susceptibility of the cells clearly increased as differentiation progressed. This gave the relative level of susceptibility as: day 3 cells>day 2 cells>day 1 cells>day 0 cells.
Figure 7.
Susceptibility of porcine blood monocytes and monocyte-derived macrophages to infection with African swine fever virus (at a multiplicity of infection of 10 infectious units/cell). The monocytic cells were employed at 2 hr, and at 1, 2, or 3 days, after isolation and culture in Dulbecco’s modified Eagle’s medium (DMEM) containing 30% (v/v) porcine plasma (x-axis). At these time-points, the cells were infected as described in the Materials and Methods. Detection of virus-specific proteins was performed at 24 hr after infection, as described in the Materials and Methods (at this multiplicity of infection, input virus could not be detected). The percentage of cells positive, at each time-point, for VP73 virus protein synthesis (VP32 analysis gave similar profiles) is shown (y-axis). Error bars associated with each value represent the standard deviation about the mean of triplicate results obtained from each of the three replicate experiments.
DISCUSSION
The in vitro differentiation of blood monocytes to macrophages has been used as a model for studying macrophage differentiation.8–10,12 Analyses have demonstrated modulation of the CD phenotype,8,9,12,13,15 although the characteristics of the kinetics of this modulation have received less attention. Currently, emphasis on the porcine immune system has been towards a potential model for human research, as well as being of interest to porcine immunology itself.27,28 This has therefore required an increased understanding of the porcine immune system. Related to this are studies on the susceptibility of monocytic cells to virus infection, and how this might alter during differentiation.4,6,7,29 Consequently, the objective of the present work was to analyse monocytic cells as they differentiated into macrophages. The aim was to determine the characteristics of this process, with respect to both the cell subpopulations involved and their susceptibility to virus infection.
Porcine monocytes have been characterized as CD14+.26 They also share a CD molecule, termed SWC1, with T lymphocytes.22–25 The present work has shown that SWC1 is down-regulated and eventually lost as blood- and bone marrow-derived monocytes differentiated in vitro into macrophages. In contrast, the SWC9 macrophage marker, absent on monocytes, was rapidly up-regulated. Alveolar macrophages were also found to be dominated by SWC1− SWC9+ cells, although a minor population of SWC1low cells was found in certain preparations. These SWC1low cells concomitantly expressed SWC9. Splenocyte analyses also demonstrated that SWC1 and SWC9 were co-expressed on SWC3+ myeloid cells. Within these splenocytes, subpopulations were clearly definable. SWC1+ SWC9− and SWC1+ SWC9+ cells predominated, although a few SWC1− SWC9+ cells could also be found. The double-positive splenocytes actually ranged from SWC1high SWC9+ to SWC1low SWC9+, and were the major subpopulation in the preparations. Moreover, the considerable overlap between these splenic myeloid subpopulations would suggest an active process of concomitant SWC1 down-regulation and SWC9 up-regulation.
Further analysis of the differentiation of blood monocytes into macrophages in vitro confirmed the existence of a gradual process of concomitant SWC1/SWC9 modulation. All populations, from SWC1+ SWC9− through SWC1+ SWC9+ to SWC1− SWC9+, merged. None appeared as distinct entities, similar to the situation observed in the spleen. The up-regulation of SWC9 expression was seen to be an early marker for differentiation. In addition, the process was characterized by the gradual and continuous SWC1 down-regulation, which was co-existent on the same cell as the SWC9 up-regulation.
Considering the SWC1 and SWC9 modulation on differentiating porcine monocytes, relationships can be made with reports from human cells. Markers such as B148.4 on human monocytes10 are similar to SWC1, but are not identical. B148.4 does not show the initial rapid loss observed with SWC1, and lymphocytes are negative. The SWC9 marker is comparable to human carboxypeptidase M (MAX.1),30 MAX.331 and TOMS-1,32 but again is not identical. SWC9 is an early-differentiation marker, rapidly up-regulated on differentiating cells. MAX.1 and MAX.3 are late-differentiation markers, as are CD16, CD51 and CD71, not appearing until after 4–7 days.8,10,30,31 Even TOMS-132 and MAX.118 do not up-regulate before 48 hr or 72 hr, respectively, and then show heterogeneity in expression.32
The concomitant down-regulation of SWC1 and up- regulation of SWC9 permitted the identification of subpopulations of myeloid cells that were intermediate in the differentiation process. These subpopulations were also associated with the morphological maturation of the macrophage. SWC1+ SWC9− cells were always smaller and less granular, being most closely related to monocytes. The SWC1+ SWC9+ intermediate differentiating cells were larger and more granular than monocytes. Finally, the SWC1− SWC9+ macrophages were the largest and most granular of all. Comparing the rate of phenotypic modulation with the morphological changes suggested that the former, at least in terms of SWC1 and SWC9, would precede morphological maturation.
In vitro analyses also identified a subpopulation on which SWC9 did not initially up-regulate, but on which SWC1 did down-regulate. These were the cells that remained morphologically closer to monocytes. During human monocyte-to-macrophage differentiation, a minor subpopulation with a monocyte phenotype has also been reported.33 In the pig spleen, in contrast, SWC1+ SWC9− cells were always SWC1high, typical of freshly isolated blood monocytes. These differences between the in vitro and in vivo results may relate to the influence of different co-factors and adherence strata on macrophage differentiation.8,12,31–39 Furthermore, the loss of SWC1 was common to all viable porcine monocytic cell cultures, even when bovine serum was employed in place of porcine plasma. In contrast, efficient differentiation to SWC9+ macrophages required the presence of porcine plasma. Consequently, it was the concomitant down-regulation of SWC1 with up-regulation of SWC9, dependent on porcine plasma factors, which was common to differentiation in vitro and in vivo.
The identification of intermediate cell populations, arising during monocyte-to-macrophage differentiation, permitted a closer examination of monocytotropic virus infection. Analysis of monocytic cell susceptibility to infection by ASF virus demonstrated that monocytes were impervious to that infection. The main target cell was the macrophage that differentiated from the monocytes. Particularly interesting was the observation that the intermediate SWC1+ SWC9+ cells were the first to display this susceptibility. Although the up- regulation of the SWC9 marker coincided with increased susceptibility to virus infection, blocking experiments have shown that SWC9 is not the receptor for the binding of ASF virus to the monocytic cell plasma membrane (data not shown). Cytomegalovirus, another large DNA monocytotropic virus, has also been reported to permissively infect a particular subset of human mononuclear cells.7 With this latter work, it was unclear as to when, during macrophage differentiation, the susceptibility increased. Current interest is now focusing on the functional activity of these subpopulations of monocytic cells. In particular, differentiation-dependent modulations of cell activity are being analysed in terms of increased susceptibility to infection. Additionally, the manner in which the virus infection modulates the immunological activity of the different subpopulations is under study, especially in the context of disease immunopathological characteristics.
Acknowledgments
The authors are grateful to the Swiss National Science Foundation (grant nos 31-33646.92, 31-40887.94 and 31-47239.96) and the Swiss Federal Veterinary Office for financial support of this work, to Robert Tschudin for technical assistance, and to Daniel Brechbühl and Peter Zulliger for animal care and bleedings.
References
- 1.Lewis CD, Mcgee JO’D. The Macrophage. Oxford: IRL Press; 1992. [Google Scholar]
- 2.Becker Y, editor. African Swine Fever. Boston: Martinus-Nijhoff Publishing; 1987. [Google Scholar]
- 3.Liess B, editor. Classical Swine Fever Virus and Related Viral Infections. Boston: Martinus-Nijhoff Publishing; 1988. [Google Scholar]
- 4.McCullough KC, Schaffner R, Fraefel W, Kihm U. The relative density of CD44-positive monocytic cell populations varies between isolations and upon culture and influences the susceptibility to infection by African swine fever virus. Immunol Lett. 1993;37:83. doi: 10.1016/0165-2478(93)90136-p. [DOI] [PubMed] [Google Scholar]
- 5.Summerfield A, Knötig SM, Mccullough KC. Lymphocyte apoptosis during classical swine fever: implication of activation-induced cell death. J Virol. 1998;72:1853. doi: 10.1128/jvi.72.3.1853-1861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maciejewski JP, Bruening EE, Donahue RE, et al. Infection of mononucleated phagocytes with human cytomegalovirus. Virology. 1993;196:327. doi: 10.1006/viro.1993.1383. [DOI] [PubMed] [Google Scholar]
- 7.Söderberg C, Larsson S, Bergstedt-Lindqvist S, Möller E. Definition of a subset of human peripheral blood mononuclear cells that are permissive to human cytomegalovirus infection. J Virol. 1993;67:3166. doi: 10.1128/jvi.67.6.3166-3175.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andreesen R, Bross KJ, Osterholz J, Emmrich F. Human macrophage maturation and heterogeneity: analysis with a newly generated set of monoclonal antibodies to differentiation antigens. Blood. 1986;67:1257. [PubMed] [Google Scholar]
- 9.Andreesen R, Brugger W, Scheibenbogen C, et al. Surface phenotype analysis of human monocyte to macrophage maturation. J Leukocyte Biol. 1990;47:490. doi: 10.1002/jlb.47.6.490. [DOI] [PubMed] [Google Scholar]
- 10.Anegon I, Blottiere H, Cuturi MC, et al. Characterization of a human monocyte antigen, B148.4, regulated during cell differentiation and activation. J Leukocyte Biol. 1993;53:390. doi: 10.1002/jlb.53.4.390. [DOI] [PubMed] [Google Scholar]
- 11.Huh HY, Pearce SF, Yesner LM, Schindler JL, Silverstein RL. Regulated expression of CD36 during monocyte-to-macrophage differentiation: potential role of CD36 in foam cell formation. Blood. 1996;87:2020. [PubMed] [Google Scholar]
- 12.Kreutz M, Krause SW, Hennemann B, Rehm A, Andreesen R. Macrophage heterogeneity and differentiation: defined serum-free culture conditions induce different types of macrophages in vitro. Res Immunol. 1992;143:107. doi: 10.1016/0923-2494(92)80087-2. [DOI] [PubMed] [Google Scholar]
- 13.Prieto J, Eklund A, Patarroyo M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol. 1994;156:1912. doi: 10.1006/cimm.1994.1164. [DOI] [PubMed] [Google Scholar]
- 14.Poli G, Wang JM, Ruco L, et al. Expression and modulation of a mononuclear phagocyte differentiation antigen (PAM-1) during in vitro maturation of peripheral blood monocytes. Int J Clin Lab Res. 1993;23:83. doi: 10.1007/BF02592288. [DOI] [PubMed] [Google Scholar]
- 15.Kreutz M, Andreesen R, Krause SW, Szabo A, Ritz E, Reichel H. 1,25-dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood. 1993;82:1300. [PubMed] [Google Scholar]
- 16.Haverson K, Bailey M, Higgins VR, Bland PW, Stokes CR. Characterization of monoclonal antibodies specific for monocytes, macrophages and granulocytes from porcine peripheral blood and mucosal tissues. J Immunol Methods. 1994;170:233. doi: 10.1016/0022-1759(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Juarrero M, Medus CA, Pan R, Revilla Y, Alonso JM, Lunney JK. Swine leukocyte antigen and macrophage marker expression on both African swine fever virus-infected and non-infected primary porcine macrophage cultures. Vet Immunol Immunopathol. 1992;32:243. doi: 10.1016/0165-2427(92)90049-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aller SC, Cho D, Kim YB. Characterization of the cytolytic trigger molecules G7/PNK-E as a molecular complex on the surface of porcine phagocytes. Cell Immunol. 1995;161:270. doi: 10.1006/cimm.1995.1036. [DOI] [PubMed] [Google Scholar]
- 19.Wierda WG, Johnson BD, Dato ME, Kim YB. Induction of porcine granulocyte-mediated tumor cytotoxicity by two distinct monoclonal antibodies against lytic trigger molecules (PNK-E/G7) J Immunol. 1993;151:7117. [PubMed] [Google Scholar]
- 20.Saalmüller A. Characterization of swine leukocyte differentiation antigens. Immunol Today. 1996;17:352. doi: 10.1016/S0167-5699(96)90273-X. [DOI] [PubMed] [Google Scholar]
- 21.Dominguez J, Ezquerra A, Alonso F, et al. Workshop studies with monoclonal antibodies identifying a novel porcine macrophage-specific differentiation antigen SWC9. Vet Immunol Immunopathol. 1998;60:343. doi: 10.1016/s0165-2427(97)00110-4. [DOI] [PubMed] [Google Scholar]
- 22.Saalmüller A, Reddehase MJ. Immune system of swine: dissection of mononuclear leukocyte sub-populations by means of two-colour cytofluorometric analysis. Res Vet Sci. 1988;45:311. [PubMed] [Google Scholar]
- 23.Lunney JK. Characterization of swine leukocyte differentiation antigens. Immunol Today. 1993;14:147. doi: 10.1016/0167-5699(93)90227-C. [DOI] [PubMed] [Google Scholar]
- 24.Summerfield A, Mccullough KC. Porcine myeloid bone marrow cells: phenotype and adhesion molecule expression. J Leukocyte Biol. 1997;62:176. doi: 10.1002/jlb.62.2.176. [DOI] [PubMed] [Google Scholar]
- 25.Saalmüller A, Aasted B, Canals A, et al. Analysis of monoclonal antibodies reactive with the porcine SWC1. Vet Immunol Immunopathol. 1994;43:255. doi: 10.1016/0165-2427(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler-Heitrock HWI, Appl B, Kafferlein E, et al. The antibody MY4 recognizes CD14 on porcine monocytes and macrophages. Scand J Immunol. 1994;40:509. doi: 10.1111/j.1365-3083.1994.tb03497.x. [DOI] [PubMed] [Google Scholar]
- 27.Kenmochi T, Mullen Y, Miyamoto M, Stein E. Swine as allotransplantation model. Vet Immunol Immunopathol. 1994;43:177. doi: 10.1016/0165-2427(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 28.Tumbleson M, Schook L. Swine in Biomedical Research. New York: Plenum Press; 1996. [Google Scholar]
- 29.McCullough KC, Schaffner R, Natale V. Phenotype of porcine mononuclear cells in relation to susceptibility to infection by African swine fever virus (ASFV) In: Schwyzer M, Ackermann M, Bertoni G, editors. Immunobiology of Viral Infections. Lyon, France: Fondation Marcel Mérieux/ European Society for Veterinary Virology; 1995. p. 79. [Google Scholar]
- 30.Rehli M, Krause SW, Kreutz M, Andreesen R. Carboxypeptidase M is identical to the MAX.1 antigen and its expression is associated with monocyte to macrophage differentiation. J Biol Chem. 1995;270:15644. doi: 10.1074/jbc.270.26.15644. [DOI] [PubMed] [Google Scholar]
- 31.Brugger W, Kreutz M, Andreesen R. Macrophage colony-stimulating factor is required for human monocyte survival and acts as a cofactor for their terminal differentiation to macrophages in vitro. J Leukocyte Biol. 1991;49:483. doi: 10.1002/jlb.49.5.483. [DOI] [PubMed] [Google Scholar]
- 32.Maeda K, Sone S, Ohmoto Y, Ogura T. A novel differentiation antigen on human monocytes that is specifically induced by granulocyte–macrophage colony-stimulating factor or IL-3. J Immunol. 1991;146:3779. [PubMed] [Google Scholar]
- 33.Kaplan G, Gaudernack G. In vitro differentiation of human monocytes. Differences in monocyte phenotypes induced by cultivation on glass or on collagen. J Exp Med. 1982;156:1101. doi: 10.1084/jem.156.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker. S. Interferons as modulators of human monocyte-macrophage differentiation. I. Interferon-gamma increases HLA-DR expression and inhibits phagocytosis of zymosan. J Immunol. 1984;132:1249. [PubMed] [Google Scholar]
- 35.Becker S, Warren MK, Haskill S. Colony stimulating factor-induced monocyte survival and differentiation into macrophages in serum-free culture. J Immunol. 1987;139:3703. [PubMed] [Google Scholar]
- 36.Zhang DE, Hetherington CJ, Gonzalez DA, Chen HM, Tenen DG. Regulation of CD14 expression during monocytic differentiation induced with 1 alpha,25-dihydroxyvitamin D3. J Immunol. 1994;153:3276. [PubMed] [Google Scholar]
- 37.Lemaire I, Yang H, Lauzon W, Gendron N. M-CSF and GM-CSF promote alveolar macrophage differentiation into multinucleated giant cells with distinct phenotypes. J Leukocyte Biol. 1996;60:509. doi: 10.1002/jlb.60.4.509. [DOI] [PubMed] [Google Scholar]
- 38.Ammon C, Kreutz M, Rehli M, Krause SW, Andreesen R. Platelets induce monocyte differentiation in serum-free coculture. J Leukocyte Biol. 1998;63:469. doi: 10.1002/jlb.63.4.469. [DOI] [PubMed] [Google Scholar]
- 39.Denham S, Brookes SM, Hutchings GH, Parkhouse RME. Granulocyte–macrophage colony stimulating factor promotes prolonged survival and the support of virulent infection by African swine fever virus of macrophages generated from porcine bone marrow and blood. J Gen Virol. 1996;77:2625. doi: 10.1099/0022-1317-77-10-2625. [DOI] [PubMed] [Google Scholar]