Abstract
We previously described the processing of antibodies to CD74 (the major histocompatibility complex class II-associated invariant chain, Ii), by B-cell lymphoma cell lines. These cells expressed relatively low levels of Ii on the surface, but the molecules were rapidly internalized and replaced by new molecules, so that ≈8×106 antibody molecules per cell were taken up per day. We herein report the results of similar studies with other cell types, namely a melanoma, a colon carcinoma, a T-cell lymphoma and B-lymphoblastoid cell lines. The melanoma and the carcinoma were treated with interferon-γ to induce high levels of the antigen. The T-cell lymphoma, HUT 78, was selected specifically because it was previously reported to lack cell surface Ii, while expressing the molecule intracellularly. However, HUT 78 displayed Ii on the cell surface, as did the other cell lines tested, and catabolism of the antibody was very fast on all of the cell lines. The capacity of four of the cell lines for cumulative antibody uptake was evaluated, using ‘residualizing’ radiolabels, which are trapped within the cell after catabolism of the antibody to which they were conjugated. A high level of uptake was observed in all cases, although there was significant variation between the cell lines. With melanoma SK-MEL-37, the total LL1 uptake in 24 hr was nearly 107 molecules per cell and the average turnover time for Ii on the cell surface was 4 min; with carcinoma HT-29, the total LL1 uptake in 24 hr was ≈106 molecules per cell, and the average turnover time for Ii on the cell surface was 27 min. Based on the cell content of mature class II antigens (αβ), these data suggest that a large fraction, or all, of immature class II molecules (αβIi) reach the cell surface before entering the peptide-loading compartment, independent of the particular cell type.
INTRODUCTION
The invariant chain associated with the major histocompatibility complex (MHC) class II antigen, Ii, plays a key role in the presentation of peptide antigens to T-cell antigen receptors.1,2 This subunit is present on the immature class II antigen, blocking the peptide-binding groove. Ii is proteolytically cleaved and removed at an intracellular site, which allows antigenic peptides to bind, and the resulting mature class II antigen then is transported to the cell surface. The route followed by the immature class II molecule before arriving at the peptide-loading compartment is uncertain and controversial. It has been known for many years that some Ii is present on the cell surface, and constitutes the CD74 antigen.3 However, this cell surface route has been considered to be a minor pathway, with most of the Ii being transported directly from the trans-Golgi to the peptide-loading compartment.1,2,4–7 Experiments to investigate this issue are complicated by the fact that Ii remains on the cell surface for a very short time.8,9 Roche et al.9 calculated the half-life to be <10 min, and recognized that, due to this rapid internalization and replenishment of cell surface Ii with newly synthesized molecules, the total number of Ii molecules appearing on the cell surface per day was large, ≈4·3×106 molecules per cell on a B-cell lymphoblastoid line. If each Ii molecule is associated with αβ subunits of the class II antigen, then this calculation suggests that a large fraction of immature class II molecules appears briefly on the cell surface, assuming that the half-life of mature class II antigens is greater than 1 day, and that maturation of class II antigens is an efficient process (that the majority of immature molecules become mature molecules). Both of these assumptions have experimental justification.1,5,6,10
We investigated previously the processing of a new anti-Ii monoclonal antibody (mAb), LL1, by B-cell lymphomas, using ‘residualizing’ radiolabels on the antibody.11 Residualizing labels are trapped inside the cell after catabolism of the antibody to which they were conjugated, and are helpful in analysing these events because Ii, and the antibody bound to it, are rapidly catabolized after internalization. We reported that LL1 was internalized and catabolized very rapidly by B-cell lymphomas, and that the total capacity of the process, determined by using near-saturating antibody concentrations, was nearly 107 antibody molecules per cell per day. The internalization was not dependent on cross-linking, since it occurred at similar rates with the Fab′ antibody fragment. Several lines of evidence suggested that an Ii molecule transported only a single antibody molecule into the cell, and that both were then rapidly catabolized. It was important to extend these observations to cell lines of other histological types, to determine the generality of the results obtained. Although some expression of CD74 on cells other than B cells is known,3,12,13 it has also been suggested that some cells producing Ii might not express it on the cell surface,7,8,14 a suggestion that can be considered compatible with the idea that, even on B cells, only a minority of Ii reaches the cell surface. Therefore, we have herein analysed the processing of anti-CD74 antibodies bound to the melanoma SK-MEL-37, the colon carcinoma HT-29, two B-lymphoblastoid cell lines, and the T-cell lymphoma HUT-78. Since HT-29 expresses no detectable MHC class II antigen, and SK-MEL-37 expresses a low level of MHC class II antigen, in the absence of treatment with interferon-γ (IFN-γ),15,16 most of the experiments with these two cell lines were with IFN-γ-treated cells. The data presented demonstrate that the processing of anti-Ii antibodies by all of these cell lines is very similar to that on B-cell lymphomas.
MATERIALS AND METHODS
Cell lines, antibodies and radiolabelling
SK-MEL-37 was obtained from Dr K. O. Lloyd (Memorial Sloan-Kettering Cancer Center, New York, NY); HT-29 and HUT-78 were obtained from the American Type Culture Collection (ATCC, Rockville, MD); and the B-lymphoblastoid cell lines PREISS and 8.1.6, transformed by Epstein–Barr virus, were provided by Dr E. Mellins (Stanford University School of Medicine, Stanford, CA). The adherent cells SK-MEL-37 and HT-29 were cultured as previously described.17 Before most experiments, the cells were grown for 2–4 days in medium supplemented with 250 U/ml IFN-γ (IFNγ-1b, Actimmune, Genentech, South San Francisco, CA). Methods for culturing the non-adherent cell lines were also described.11 Cell lines were tested routinely for mycoplasma contamination using the Mycotect Assay (Life Technologies, Grand Island, NY), and were negative. Antibody LL1, anti-CD74, a mouse IgG1, was described previously.11 L243, anti-mature class II, a mouse IgG2a, was produced by hybridoma cells obtained from the ATCC. It has been demonstrated that L243 does not react with the immature class II antigen (containing Ii), presumably due to a conformational difference.18 The antibodies MA103 (anti-CD147) and RS11 (anti-epithelial glycoprotein-2) were previously described.19,20 Antibodies were purified by affinity chromatography on protein A–Sepharose.17 Conventional 125I-labelling used chloramine-T, as described.17 Labelling with dilactitol-tyramine (DLT) was also described previously.11 Briefly, the DLT was first incubated with 125I in the presence of Iodogen, then treated with galactose oxidase to activate the sugar, followed by reaction with the antibody.
Determination of antigenic sites per cell
Near-confluent cells in 96-well plates were washed and then incubated with varying concentrations of antibody. Cells in control wells were trypsinized and counted. Plates and media were carefully kept cold throughout the assay. Control wells contained large amounts of unlabelled antibody, as an indication of non-specific binding. The concentration of unlabelled antibody added was 250 μg/ml for LL1 and a 1:10 dilution of hybridoma ascites for L243, and this was added to the plate in 50 μl 15 min before addition of the labelled antibody, which was in 100 μl. After a 2-hr incubation, the cells were washed, solubilized with 2·0 m NaOH, and harvested on cotton swabs. The specific activity of the antibody was corrected for the counts per minute (c.p.m.) that were not precipitable by trichloroacetic acid (always <10%), and the antibody molecules bound per cell was calculated.
Antibody processing experiments
The initial processing experiments followed methods previously described in detail.11,17 Briefly, adherent cells near-confluence in 96-well plates were washed and then incubated for 2 hr at room temperature with 5×105 c.p.m. of a conventional radioiodinated antibody. The cells were then washed, tissue culture medium was added, and the fate of the bound antibody was followed for 2–3 days. At each time-point, the c.p.m. in the supernatant and cell bound was determined, and the supernatant c.p.m. was analysed by precipitation with 10% trichloroacetic acid. Non-adherent cells were handled in a generally similar procedure.11
To determine uptake capacity, the antibody was kept in the medium for the duration of the experiment, 2–3 days, and the iodo-DLT label was used. For adherent target cells, the total volume was 0·2 ml/well. Again, control wells contained large amounts of unlabelled antibody, to ascertain the antigen-specificity of uptake. In these experiments, unlabelled LL1 was used at 62·5 μg/ml final concentration. The specific activity of the labelled antibody was varied by adding appropriate amounts of unlabelled antibody. At various times, the cell bound c.p.m. was determined by aspirating the medium, washing the cells four times with 250 μl of medium, and then solubilizing and harvesting the cells as described above. Non-adherent cells were handled by a generally similar procedure, as described.11
RESULTS
Processing of anti-Ii antibodies bound to a melanoma, a carcinoma, a T-cell lymphoma, and two B-cell lymphoblastoid cell lines
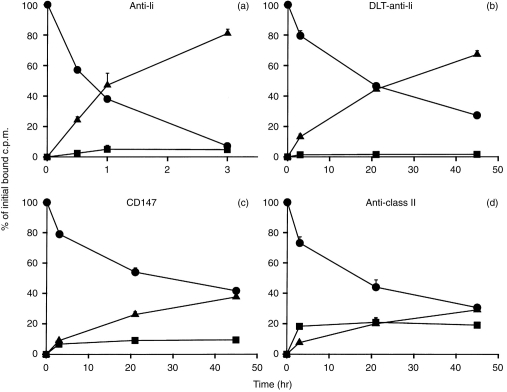
The fate of the antibody LL1 bound to the surface of SK-MEL-37 cells, using a conventional 125I label, is shown in Fig. 1(a). The antibody was catabolized at a rapid rate, and released into the supernatant as a low molecular weight catabolite, undoubtedly iodotyrosine.21,22 This catabolism was virtually complete in 2–3 hr. When a residualizing 125I label, I-DLT, was used in the same experiment, retention of the radioactivity by the cells was markedly prolonged (Fig. 1b). This result is important with regard to the uptake experiments described below, and also confirms that the antibody is catabolized in an intracellular compartment. It should be noted that the DLT is not permanently retained intracellularly, but rather is released from the cells at a slow rate, ≈50% in 1 day. (With the conventional iodine label, in comparison, 81% of the bound c.p.m. was released in 3 hr.) Similar gradual release of DLT was described previously, with other cell lines and antibodies.11,20,23 The processing of other antibodies by SK-MEL-37 was also tested, to confirm that the rapid uptake of LL1 was unique. One of the antibodies tested was MA103 (anti-CD147), which is useful for this purpose because it reacts with every human cell line tested (>150), and many processing experiments with this antibody, using a variety of cell lines, have been described.17,19,24 MA103 has always been processed as slowly as any other antibody tested on the same cell line, so we have suggested that MA103 catabolism reflects the basal rate of membrane turnover of the cell. As shown in Fig. 1(c), MA103 bound to SK-MEL-37 behaved ‘typically’, with very gradual uptake and catabolism, having a half-life of ≈2 days. Results with L243, anti-mature class II antigen, are shown in Fig. 1(d). This antibody was internalized slowly, similarly to MA103, and very differently from LL1.
Figure 1.
Processing of 125I-labelled antibodies bound to melanoma SK-MEL-37, treated with IFN-γ. After antibody binding, the cells were washed and the fate of the bound antibody was followed for up to 45 hr: (•), cell-bound; (▪), intact in supernatant (TCA precipitable); (▴), degraded in supernatant. (a) Anti-Ii (antibody LL1); (b) anti-Ii labelled with [125I]DLT, a residualizing label, that is trapped in lysosomes after catabolism of the antibody to which it was conjugated; (c) anti-CD147; (d) anti-MHC class II (antibody L243). Values shown are means±standard deviations of triplicates; any standard deviations not seen are too small to be visible above the symbols. Results are representative of two to four experiments with each antibody. The total c.p.m. binding was 4292 c.p.m. for LL1, 6224 c.p.m. for DLT-LL1, 33 077 c.p.m. for CD147, and 40 748 c.p.m. for L243. Note the difference in the x-axis scale: (a) extends for only 3 hr, at which time essentially all of the bound antibody had been catabolized.
A similar series of experiments was performed with the human colon carcinoma HT-29, treated with IFN-γ. In addition to MA103, an additional control antibody, anti-epithelial glycoprotein-2 (RS11) was also tested. The results obtained were very similar to those described for SK-MEL-37, with rapid catabolism of LL1 and slow catabolism of the other antibodies tested (data not shown).
The experiments shown used target cells pretreated for 2–3 days with IFN-γ, but similar experiments were also attempted in the absence of IFN-γ. SK-MEL-37, but not HT-29, was reported to have significant expression of MHC class II antigen in the absence of IFN-γ.15,25 There was significant binding of LL1 to SK-MEL-37 in the absence of IFN-γ, but the c.p.m. bound were too low to provide entirely convincing data. More specifically, in an experiment in which cells with and without IFN-γ were tested at the same time, 5896±245 c.p.m. of [125I]LL1 were specifically bound per well of IFN-γ-treated cells, while only 341±48 c.p.m. were specifically bound per well in the absence of IFN-γ. Despite this low level of c.p.m. bound, it was of interest to perform the antibody processing experiments in the absence of IFN-γ. The results demonstrated clearly that the processing pattern in the absence of IFN-γ was the same as after IFN-γ treatment, with similar rapid catabolism (data not shown). The effect of IFN-γ on mature class II antigen expression by SK-MEL-37 cells was also monitored, with antibody L243. Binding on IFN-γ-treated cells was 52 318±1,104, while binding in the absence of IFN-γ was 10 391±458, a fivefold difference. Thus, the increase in mature class II, due to IFN-γ treatment, appears to be proportional to the increase in Ii on the cell surface, although, as noted, the low c.p.m. bound in the absence of IFN-γ makes this conclusion tentative. To determine whether IFN-γ treatment affects the processing of other, unrelated, antibodies, the processing of antibodies MA103 and RS11 was also tested on HT-29 in the absence of IFN-γ: IFN-γ had no significant effect on the processing results with these antibodies, and also did not affect the total antibody binding.
Experiments (in the absence of IFN-γ) with two B-lymphoblastoid cell lines (data not shown) and with the T-cell lymphoma HUT 78 (Fig. 2) also displayed very rapid catabolism of LL1, similar to the results described above. The HUT 78 cell line was selected specifically because it was previously reported to be negative for cell surface Ii, although expressing it intracellularly.14 However, HUT 78 had readily detectable levels of surface Ii. Under our standard assay conditions, with 106 cells and 5×105 c.p.m. of [125I]LL1, with a specific activity of 5–10 mCi/mg, there were 90 000–130 000 c.p.m. specifically bound to HUT 78 cells, which is comparable to, or greater than, the binding obtained with other cell lines.
Figure 2.
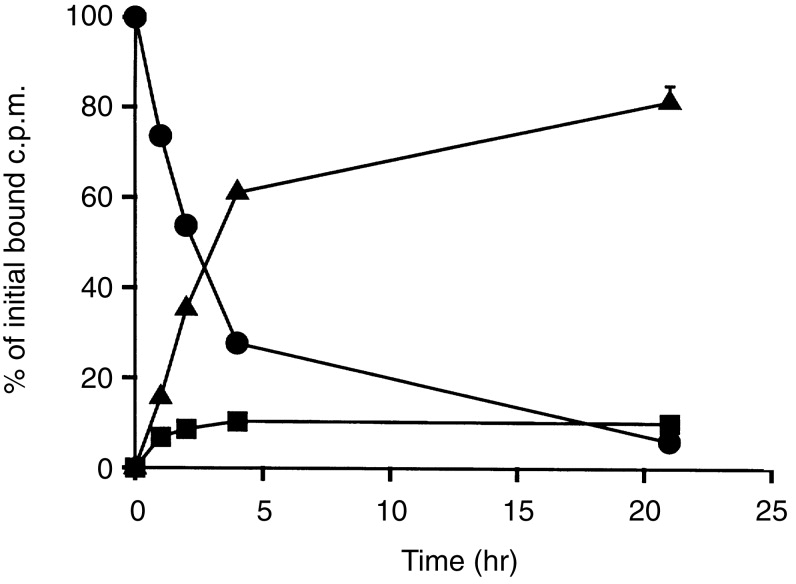
Processing of [125I]LL1 bound to T-cell lymphoma HUT 78. After antibody binding, the cells were washed and the fate of the bound antibody was followed for up to 21 hr: (•), cell-bound; (▪), intact in supernatant (TCA precipitable); (▴), degraded in supernatant. Values shown are means±standard deviations of duplicates; any standard deviations not seen (which are almost all) are too small to be visible above the symbols. Results shown are representative of two experiments. The total c.p.m. binding was 127 755 c.p.m., of which 99·3% was specifically inhibitable by excess cold LL1.
Cumulative uptake of anti-Ii antibodies by cells incubated continuously with the antibody
To determine the capacity of the uptake process, experiments were performed using a prolonged antibody incubation, meaning that the antibody was kept in the medium continuously. Increasing concentrations of antibody were tested, up to 8·1 μg/ml, in an attempt to saturate the antigen on the cell surface. By using a residualizing label, antibody uptake could be estimated by monitoring the bound radioactivity per cell. The total number of antibody-equivalents that were bound per cell at various times, for up to 2 days, was calculated. At later time-points, the antibody that had been internalized was largely degraded, and only the residualizing label remained in the cells; for this reason, the bound c.p.m. is referred to in terms of antibody-equivalents. Figure 3(a) shows the time course of antibody uptake, for SK-MEL-37 and HT-29, and demonstrates that nearly all antibody binding was antigen-specific, since it was inhibited by a large excess of unlabelled antibody. The uptake at 21 hr was much higher than that at 4 hr. This is characteristic of LL1, due to the high turnover rate of the antigen, and does not occur with most other antibodies, for which 21-hr binding is similar to 4-hr binding, in similar experiments.20,26 Figure 3(b) shows antibody uptake in terms of antibody molecule-equivalents bound per cell. Both SK-MEL-37 and HT-29 had a high uptake capacity, although the uptake was considerably higher, ≈ninefold, for SK-MEL-37 than for HT-29. In two experiments (one of which is shown in Fig. 3a), the mean number of antibody-equivalents bound per cell in 21 hr was 5·9±0·8×106 for SK-MEL-37, and 6·5±0·4×105 for HT-29. There are several reasons why the marked difference between SK-MEL-37 and HT-29, seen in Fig. 3(b), is much less apparent in Fig. 3(a). First, there were more cells/well of HT-29 than SK-MEL-37, by a factor of ≈2·3-fold. Second, the specific activity of the labelled antibody was ≈twofold higher in the HT-29 experiments than in the SK-MEL-37 experiments. Similar uptake experiments were performed with the two B-lymphoblastoid cell lines, PREISS and 8.1.6. The number of antibody molecule-equivalents bound per cell at 24 hr was 1·9±1·0×106 and 2·6±1·3×106, respectively. These values are ≈three- to fourfold lower than those for the Raji B-cell lymphoma, but this can be attributed to differences in the quantity of Ii expressed on the cell surface, as described below.
Figure 3.
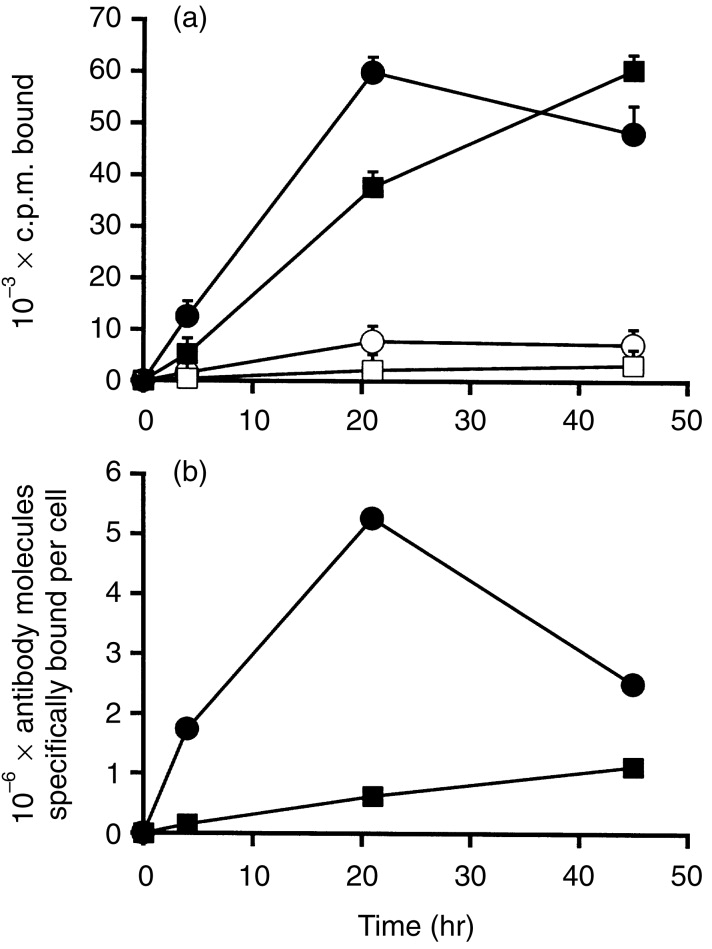
Uptake of [125I]DLT-LL1 by the cell lines SK-MEL-37 (circles) or HT-29 (squares). The antibody was used at a near-saturating concentration, 6·0 μg/ml for SK-MEL-37 and 2·7 μg/ml for HT-29, and was present in the medium for the duration of the experiment. (a) c.p.m. bound per well of a 96-well plate. Filled symbols show total bound c.p.m., and the open symbols show the c.p.m. bound in the presence of a large excess of unlabelled antibody (non-specific c.p.m. bound). Values shown are means±standard deviations of triplicates, and are representative of two separate experiments. (b) The antibody molecules bound per cell, calculated from the data in panel (a).
It should be appreciated that our assay of cell-bound c.p.m. provides an underestimate of the antibody molecules that are internalized per cell, since iodo-DLT, or other residualizing labels, are not absolutely trapped within the cell. The relatively slow, but significant, escape of [125I]DLT from SK-MEL-37 cells into the medium is shown in Fig. 1(b). For our present purpose this difference is not a major factor, but we can estimate the impact of this effect. From the rate of loss of iodo-DLT-LL1 from SK-MEL-37 cells, shown in Fig. 1(b), the half-life for loss is ≈26 hr. Therefore, in a prolonged incubation, in which antibody is continuously binding to newly synthesized antigen, we calculate that at 21 hr after the start of the incubation ≈76% of the antibody that has bound during the incubation remains cell-bound, while 24% is degraded and released into the supernatant. Thus, the antibody molecules handled per cell would be ≈32% greater that the antibody molecules cell-bound at that time-point. With this correction, we calculate that the number of anti-Ii antibody molecules handled per cell per day (based on our 21-hr experiments) is 8·9×106 for SK-MEK-37 and 9·8×105 for HT-29. Assuming that internalized Ii molecules do not recycle to the cell surface, this provides a minimum estimate of the number of Ii molecules synthesized per cell per day (which does not include any Ii molecules synthesized that do not reach the cell surface). This will be an underestimate if some Ii molecules present on the cell surface are internalized before they bind an antibody molecule.
Steady-state levels of CD74 and HLA-DR on the cell surface, and correlation with the rate of Ii internalization
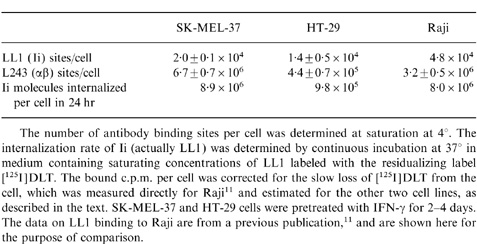
The level of Ii on the cell surface was determined using saturating concentrations of LL1. These experiments were performed at 0–4°, so cell surface antigen at a single time-point was detected. The mean number, ±standard deviation, of LL1 binding sites per cell was as follows: SK-MEL-37 (induced by IFN-γ), 2·0±0·1×104; HT-29 (induced by IFN-γ), 1·4±0·5×104; lymphoblastoid cell line 8·1.6, 9·9±4·0×103; and Raji (determined previously, 4·6±2·8×104. From these values and the Ii synthetic rate, calculated as described above, the average residence time of Ii on the cell surface was calculated to be 4·3 min for SK-MEL-37, 27·2 min for HT-29, and 4·2 min for 8·1.6. The value for Raji, determined previously,11 was 8·6 min. Thus, the large difference in 24-hr antibody uptake between SK-MEL-37 and HT-29, shown in Fig. 3(b), appears to be due primarily to the 6·3-fold difference in turnover time, although there is also a 1·4-fold difference in the number of antigenic sites per cell.
An alternative approach for analysis of these data is to calculate the theoretical half-life for Ii internalization, as has been done in similar experiments,8,9 rather than the average replacement time. However, it is uncertain if internalization is a stochastic process or an assembly line process. If Ii expressed on the cell surface is delivered to coated pits by a non-random process, as suggested for other receptors that enter coated pits constitutively,27 then calculation of a half-life is inappropriate. We prefer the simple calculation of the average turnover rate of Ii, which requires fewer assumptions regarding the mechanism involved.
These experiments were not intended to directly determine the fraction of total Ii that appears on the cell surface before transport to the peptide-loading compartment, since we have not attempted to measure Ii that does not appear on the cell surface. However, a related question that can be addressed is whether the number of Ii molecules appearing on the cell surface are sufficient to account for the entire cell content of mature class II molecules. To approach this question, the number of mature class II molecules present on the surface of these cells was determined, using saturating concentrations of the anticlass II DR antibody L243. L243 reacts only with the mature form of DR, and not with the immature form (complexed with Ii),18 which is convenient for this purpose (although the amount of αβIi on the cell surface is very small relative to the amount of αβ in any case). The great majority of class II molecules present on human cells are DR molecules, with DP and DQ molecules being minor fractions.1 There were 6·7±0·7×106 L243 binding sites per cell on SK-MEL-37, and 4·4±0·7×105 sites per cell on HT-29. Thus, there was 15·2-fold more class II antigen per cell on MEL-37 than on HT-29.
Table 1 lists the cell surface DR content and the Ii internalization rate for the three cell lines that have been examined, and demonstrates a correlation between these two, independently derived, parameters. Based on the assumption that each Ii molecule present on the cell surface carries one αβ complex (which is further discussed below), the correlation between the DR content and the Ii internalization rate supports the idea that all immature class II molecules appear briefly on the cell surface, although these data do not conclusively rule out other possibilities. More specifically, if all else is equal, if all immature class II antigen reaches the cell surface, and if one cell has an Ii internalization rate that is ninefold higher than another cell (the difference between SK-MEL-37 and HT-29), then the first cell should have a ninefold higher steady-state level of class II antigen. This predicted difference in class II expression, based on the rate of Ii internalization, is reasonably close to the actual difference measured.
Table Expression of Ii and mature MHC class II antigen on the cell surface
 |
DISCUSSION
These results, together with previous data, strongly support two suppositions. First, that all cells producing class II antigens express Ii on the surface; and second, that Ii rapidly internalizes, while being replaced by newly synthesized molecules, so that the cumulative expression of Ii on the cell surface is high, even though the steady-state level is relatively low. While cells of many histological types were known to synthesize Ii, particularly after IFN-γ treatment,28 its expression on the cell surface, as CD74, has been reported for relatively few cell types, among them Langerhans’ cells12 and IFN-γ-induced HT-29 cells.13 We are not aware of previous reports of CD74 on melanoma cells. In addition, it has been suggested that some cells producing Ii do not express it on the cell surface.7,8,14 In this study, we purposely included HUT 78, which was reported previously to lack cell surface Ii while producing intracellular Ii.14 We found strong expression of Ii on the surface of HUT 78, comparable to the levels expressed on B-cell lymphomas. Similarly, although monocytes have been considered to have low expression of CD74,3 LL1 was found to react strongly with monocytes.29 The cause of these discrepancies is not known, but several possibilities can be suggested. First, it is clear that LL1 is a considerably stronger antibody than many other CD74 antibodies.11 Second, the same cell line tested in different laboratories, with an interval of years, may display some variation. Third, we suggest that lack of detection of surface Ii, in some experiments, may be a consequence of the very rapid internalization. That is, if a sandwich immunoassay is used, and if cells are not carefully kept cold during all procedures, then most of the first antibody may be already internalized before the second antibody has an opportunity to bind. We have observed a strong effect of this type when a B-cell lymphoma was stained for surface Ii by indirect immunofluorescence (data not shown). Pieters et al.30 found that Ii was not detectable on the surface of the human melanoma cell line Mel JuSo that produced both Ii and αβ chains. Although there is no direct or indirect evidence that this particular cell line has cell surface Ii, it should be noted that the cells were examined without IFN-γ treatment, so the expected level of surface Ii is very low; and that the anti-Ii antibody used was LN2. While this is a classical CD74 antibody,3 it reacts much more weakly with the cell surface than LL1, although it appears to react with the same epitope.11 In our opinion, the extant data strongly suggest that all cells producing Ii express it on the cell surface.
The continuous, rapid, high-level internalization of CD74 has now been demonstrated for B-lymphoblastoid cell lines,9 B-cell lymphomas,11 carcinomas and melanomas. In addition, Saudrais et al.18 recently demonstrated that on dendritic cells a large fraction of the Ii synthesized appears briefly on the cell surface, in a complex with αβ. It should be noted that these authors also reported that similar high cell surface expression of Ii did not occur on B-cell lines or monocytes. However, the methods used might not have detected a very brief appearance of Ii on the cell surface, and the difference between cell lines may be in the average length of time that Ii stays on the surface before internalization. The results presented above indicate that different cell lines may vary by at least sixfold in the turnover time of Ii on the cell surface.
Henne et al.31 recently suggested that free Ii reaches the cell surface, and can associate with mature class II, thereby providing a recycling pathway for class II molecules into the de novo peptide-loading compartment. This possibility is distinct from the recycling of mature class II antigens into a different peptide-loading compartment that allows exchange or replacement of bound peptides and does not involve Ii.2 The suggestion that free Ii is transported in large amounts to the cell surface would, in a sense, reconcile some of the apparent inconsistencies in our understanding, since it would readily explain high-level uptake of anti-Ii antibodies, while allowing most of the immature αβIi complexes to be directed to the peptide-loading compartment without appearing at the cell surface. However, the data currently available do not support this possibility. First, there is no evidence for free Ii at the surface of cells that produce normal levels of αβ chains (although in some mutant or transfected cells that express Ii but not αβ, free Ii is present on the cell surface31). Roche et al.9 investigated this point by immunoprecipitation after cell-surface labelling, and concluded that most or all cell surface Ii was associated with αβ, although it remains possible that free Ii binds to αβ chains instantly upon reaching the cell surface. While Ii is normally synthesized in excess of αβ, the excess normally appears to be directed from the endoplasmic reticulum to the lysosomes for degradation.7 Secondly, this model implies that, after cell surface iodination, pre-existing mature class II antigens would become associated with newly synthesized Ii. Such an association was not detected in the experiments of Roche et al. who performed immunoprecipitation with anti-Ii and anti-αβ chains at various times after surface iodination. Immunoprecipitation experiments intended to detect free Ii on the surface of non-B-cell lines, including the lines used in the present study, have not yet been performed, but since the general CD74 processing pathway is very similar in all cell types examined, there is no reason to suspect that these other cell types would be different from B-cell lymphomas in cell surface expression of free Ii.
It is experimentally difficult to demonstrate that all αβIi appears on the cell surface, and equally difficult to prove that only a fraction does so, but our data support the first possibility, since knowledge of the synthetic rate and the catabolic half-life allows prediction of the steady-state level of mature class II antigen, which is twice the amount synthesized in one catabolic half-life. As discussed above, the rate of Ii internalization provides an estimate, which can be considered a minimum estimate, of the rate of synthesis of class II antigens. While the half-life of mature class II antigen on these cell lines is unknown, it seems reasonable, for the purpose of these calculations, to assume a 2-day half-life, which is the value for a B-cell lymphoblastoid cell line.10 Considering the function of the molecule, a much shorter half-life would not be expected. On this basis, the predicted steady-state level of mature class II antigen is 2·8×107 molecules per cell for SK-MEL-37 and 2·8×106 molecules per cell for HT-29. Since these value are four- to sixfold higher than the measured values of DR molecules per cell, it can be concluded that it is at least possible that all αβIi briefly appears on the cell surface. Moreover, if it is argued that only a minority of immature class II molecules reach the cell surface, then it is necessary to explain what becomes of the large amount of αβIi that is internalized from the cell surface.
Acknowledgments
We thank Philip Andrews for assistance with radiolabelling, and Paul A. Roche for a critical review of the manuscript. This work was supported in part by NIH grant CA39841 (DMG).
References
- 1.Cresswell P. Assembly, transport and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 2.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 3.Dorken B, Möller P, Pezzutto A, Schwartz-Albiez R, Moldenhauer G. B-cell antigens: CD74. In: Knapp W, Dörken B, Gilks WR, et al., editors. Leukocyte Typing IV. Oxford: Oxford Univ Press; 1989. p. 106. [Google Scholar]
- 4.Odorizzi CG, Trowbridge IS, Xue L, Hopkins CR, Davis CD, Collawn JF. Sorting signals in the MHC class II invariant chain cytoplasmic tail and transmembrane region determine trafficking to an endocytic processing compartment. J Cell Biol. 1994;126:317. doi: 10.1083/jcb.126.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bénaroch P, Yilla M, Raposo G, et al. How MHC class II molecules reach the endocytic pathway. EMBO J. 1995;14:37. doi: 10.1002/j.1460-2075.1995.tb06973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morton PA, Zacheis ML, Giacoletto KS, Manning JA, Schwartz BD. Delivery of nascent MHC Class II-invariant chain complexes to lysosomal compartments and proteolysis of invariant chain by cysteine proteases precedes peptide binding in B-lymphoblastoid cells. J Immunol. 1995;154:137. [PubMed] [Google Scholar]
- 7.Bertolino P, Rabourdin-Combe C. The MHC class II-associated invariant chain: a molecule with multiple roles in MHC class II biosynthesis and antigen presentation to CD4+ T cells. Critical Rev Immunol. 1996;16:359. [PubMed] [Google Scholar]
- 8.Koch N, Moldenhauer G, Hofmann WJ, Möller P. Rapid intracellular pathway gives rise to cell surface expression of the MHC class II-associated invariant chain (CD74) J Immunol. 1991;147:2643. [PubMed] [Google Scholar]
- 9.Roche PA, Teletski CL, Stang E, Bakke O, Long EO. Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc Natl Acad Sci USA. 1993;90:8581. doi: 10.1073/pnas.90.18.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis JE, Cresswell P. Lack of detectable endocytosis of B lymphocyte MHC class II antigens using an antibody-independent technique. J Immunol. 1990;144:990. [PubMed] [Google Scholar]
- 11.Hansen HJ, Ong GL, Diril H, et al. Internalization and catabolism of radiolabeled antibodies to the MHC class II invariant chain by B-cell lymphomas. Biochem J. 1996;320:293. doi: 10.1042/bj3200293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claesson-Welsh L, Scheynius A, Tjernlund U, Peterson PA. Cell surface expression of invariant γ-chain of class II histocompatibility antigens in human skin. J Immunol. 1986;136:484. [PubMed] [Google Scholar]
- 13.Henne C, Moldenhauer G, Möller P. CD74 expression precedes IFN-γ-driven induction of MHC class II antigens on HT-29 colon carcinoma cells and is independent of concomitant class II expression in a mutant cell line. In: Schlossman SF, Boumsell L, Gilks W, et al., editors. Leukocyte Typing V. Vol. 1. Oxford: Oxford University Press; 1995. p. 573. [Google Scholar]
- 14.Wraight CJ, van Endert P, Möller P, et al. Human major histocompatibility complex class II invariant chain is expressed on the cell surface. J Biol Chem. 1990;265:5787. [PubMed] [Google Scholar]
- 15.Houghton AN, Thompson TM, Gross D, Oettgen HF, Old LJ. Surface antigens of melanoma and melanocytes. Specificity of induction of Ia antigens by human γ-interferon. J Exp Med. 1984;160:225. doi: 10.1084/jem.160.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sollid LM, Gaudernack G, Markussen G, Kvale D, Brandtzaeg P, Thorsby E. Induction of various HLA class II molecules in a human colonic adenocarcinoma cell line. Scand J Immunol. 1987;25:175. doi: 10.1111/j.1365-3083.1987.tb01061.x. [DOI] [PubMed] [Google Scholar]
- 17.Kyriakos RJ, Shih LB, Ong GL, Patel K, Goldenberg DM, Mattes MJ. The fate of antibodies bound to the surface of tumor cells in vitro. Cancer Res. 1992;52:835. [PubMed] [Google Scholar]
- 18.Saudrais C, de Spehner D, la Salle H, et al. Intracellular pathway for the generation of functional MHC class II peptide complexes in immature human dendritic cells. J Immunol. 1998;160:2597. [PubMed] [Google Scholar]
- 19.Vangeepuram N, Ong GL, Mattes MJ. Processing of antibodies bound to B-cell lymphomas and lymphoblastoid cell lines. Cancer Suppl. 1997;80:2425. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2425::aid-cncr14>3.3.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Stein R, Goldenberg DM, Thorpe SR, Basu A, Mattes MJ. Effects of radiolabeling monoclonal antibodies with a residualizing iodine radiolabel on the accretion of radioistope in tumors. Cancer Res. 1995;55:3132. [PubMed] [Google Scholar]
- 21.Vaes G. Digestive capacity of lysosomes. In: Hers HG, Van Hoof F, editors. Lysosomes and Storage Diseases. New York: Academic Press; 1973. p. 43. [Google Scholar]
- 22.Geissler F, Anderson SK, Press O. Intracellular catabolism of radiolabeled anti-CD3 antibodies by leukemic T cells. Cell Immunol. 1991;137:96. doi: 10.1016/0008-8749(91)90060-o. [DOI] [PubMed] [Google Scholar]
- 23.Shih LB, Thorpe SR, Griffiths GL, et al. The processing and fate of antibodies and their radiolabels bound to the surface of tumor cells in vitro: a comparison of nine radiolabels. J Nucl Med. 1994;35:899. [PubMed] [Google Scholar]
- 24.Mattes MJ, Griffiths GL, Diril H, Goldenberg DM, Ong GL, Shih LB. Processing of antibody-radioisotope conjugates after binding to the surface of tumor cells. Cancer Suppl. 1994;73:787. doi: 10.1002/1097-0142(19940201)73:3+<787::aid-cncr2820731307>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Winchester RJ, Wang C-Y, Gibofsky A, Kunkel HG, Lloyd KO, Old LJ. Expression of Ia-like antigens on cultured human malignant melanoma cell lines. Proc Natl Acad Sci USA. 1978;75:6235. doi: 10.1073/pnas.75.12.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattes MJ, Shih LB, Govindan SV, et al. The advantage of residualizing radiolabels for targeting B-cell lymphomas with a radiolabeled anti-CD22 monoclonal antibody. Int J Cancer. 1997;71:429. doi: 10.1002/(sici)1097-0215(19970502)71:3<429::aid-ijc21>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Bretscher MS. Endocytosis: relation to capping and cell locomotion. Science. 1984;224:681. doi: 10.1126/science.6719108. [DOI] [PubMed] [Google Scholar]
- 28.Momberg F, Koch N, Möller P, Moldenhauer G, Butcher GW, Hammerling GJ. Differential expression of Ia and Ia-associated invariant chain in mouse tissues after in vivo treatment with IFN-γ. J Immunol. 1986;136:940. [PubMed] [Google Scholar]
- 29.Pawlak-Byczkowska EJ, Hansen HJ, Dion AS, Goldenberg DM. Two new monoclonal antibodies, EPB-1 and EPB-2, reactive with human lymphomas. Cancer Res. 1898;49:4569. [PubMed] [Google Scholar]
- 30.Pieters J, Horstmann H, Bakke O, Griffiths G, Lipp J. Intracellular transport and localization of major histocompatibility complex class II molecules and associated invariant chain. J Cell Biol. 1991;115:1213. doi: 10.1083/jcb.115.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henne C, Schwenk F, Koch N, Möller P. Surface expression of the invariant chain (CD74) is independant of concomitant expression of major histocompatibility complex class II antigen. Immunology. 1995;84:177. [PMC free article] [PubMed] [Google Scholar]