Abstract
The activation of macrophages by various stimuli leading to chemotactic migration and phagocytosis is known to be mediated by an increase in intracellular Ca2+ concentration ([Ca2+]i). We measured changes in [Ca2+]i using a Ca2+ imaging method in individual human macrophages differentiated from freshly prepared peripheral blood monocytes during culture of 1–2 days. A transient rise in [Ca2+]i (duration 3–4 min) occurred in 10–15 macrophages in the vicinity of a single tumour cell that was attacked and permeabilized by a natural killer cell in a dish. Similar Ca2+ transients were produced in 90% of macrophages by application of supernatant obtained after inducing the lysis of tumour cells with hypo-osmotic treatment. Ca2+ transients were also evoked by ATP in a dose-dependent manner between 0·1 and 100 μm. The ATP-induced [Ca2+]i rise was reduced to less than one-quarter in Ca2+-free medium, indicating that it is mainly due to Ca2+ entry and partly due to intracellular Ca2+ release. UTP (P2U purinoceptor agonist) was more potent than ATP or 2-chloro-ATP (P2Y agonist). Oxidized ATP (P2Z antagonist) had no inhibitory effect. Both cell lysate- and ATP-induced Ca2+ responses were inhibited by Reactive Blue 2 (P2Y and P2U antagonist) to the same extent, but were not affected by PPADS (P2X antagonist). Sequential stimuli by cell lysate and ATP underwent long-lasting desensitization in the Ca2+ response to the second stimulation. The present study supports the view that macrophages respond to signal messengers discharged from damaged or dying cells to be ingested, and ATP is at least one of the messengers and causes a [Ca2+]i rise via P2U and P2Y receptors.
INTRODUCTION
Monocytes/macrophages are categorized as a cell system that responds to inflammatory stimuli and acquires the abilities of migration, adhesion and phagocytosis. The major intracellular event that couples receptor stimulation to cell activation is an increase in intracellular Ca2+ concentration ([Ca2+]i). In macrophages and related cell lines, a transient rise in [Ca2+]i occurs in response to the factors for induction of chemotaxis1–4 or upon adhesion to opsonized particles by means of cross-linking between immunoglobulin G (IgG) and Fcγ receptors leading to phagocytosis.5,6 A [Ca2+]i rise also occurs in response to extracellular ATP at nanomolar/micromolar concentrations via P2 purinoceptors, as shown in human monocyte-derived macrophages,7 murine peritoneal macrophages,8 human monocyte cell line U937,9–11 mouse macrophage cell line J77412,13 and rat macrophage cell line NR8383.14 ATP can be released from exocytotic vesicles and/or granules of secretory cells and from the cytosol of damaged cells. As phagocytes ingest injured or dying cells as well as extrinsic micro-organisms, it is postulated that ATP could play a role of a signal messenger from damaged cells to phagocytes.15,16 However, no evidence has been provided to date. We have observed the process of necrosis or apoptosis of cultured tumour cells attacked by human natural killer (NK) cells using a Ca2+ imaging method.17–19 The target cells are more or less permeabilized through pores formed by perforin, which is released from NK cells, as detected by leakage of Ca2+-indicator dye from the target cell. In the present study, we investigated whether any [Ca2+]i rise occurs in macrophages in the vicinity of a dying cell that has been attacked by a NK cell. To examine the putative ATP signal, Ca2+ responses to extracellular ATP in single macrophages were analysed in terms of the kinetics of the [Ca2+]i rise and Ca2+ mobilization pathways, and responsible P2 receptor subtypes were identified using P2 purinoceptor agonists and antagonists. Subsequently, Ca2+ responses induced by application of cell lysate and of ATP were compared in those characteristics.
MATERIALS AND METHODS
Monocytes and macrophages
Fresh peripheral blood mononuclear cells (PBMCs) were separated from blood of normal volunteers by Ficoll–Conray centrifugation. PBMCs (3×106/ml) were incubated in glass dishes in RPMI-1640 medium (Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (referred to as RPMI below) at 37° for 1–1·5 hr (5% CO2 in air). For positive cell isolation,2 dishes were washed twice with warm RPMI, and adherent cells were harvested with cold Ca2+- and Mg2+-free phosphate-buffered saline (PBS). For negative selection, cells were treated with anti-human CD3 monoclonal antibody (mAb)-coated magnetic beads (Dynal, Oslo, Norway) (5 μm in diameter; ≈10 beads per cell) at 4° for 30 min, and non-adherent cells were harvested. About 85% of the cells were identified as monocytes by May–Grunwald–Giemsa staining. Cells were stored at −80° in 90% FBS and 10% dimethylsulphoxide (DMSO). When used, cells were rapidly thawed, and DMSO was immediately replaced by RPMI. Cells were cultured in 50% FBS/50% RPMI for 1–2 days before use. Most of monocytes adhered to the bottom of the dish and differentiated to macrophages during the 1–2-day culture.2 Macrophages were recognized as relatively large flattened cells which contained vacuole-like structures and had irregular contour.
NK cells
PBMCs were transferred to a column of nylon wool (0·5 g in 5 ml RPMI in a syringe) and incubated at 37° for 1 hr. Non-adherent cells were eluted with warm RPMI. NK cell-rich fraction was obtained by centrifugation (650 g for 20 min) in Percoll density gradient solutions (Pharmacia Fine Chemicals, Uppsala, Sweden) layered at every 2·5% concentration gradient between 30 and 40%. T cells were obtained from another fraction. More details have been given previously.17,18 About 95% of harvested cells were identified as NK cells by flow cytometry with an anti-CD16 mAb (Leu-11b; Becton Dickinson, San Jose, CA). NK cells were stored at −80° in 90% FBS and 10% DMSO. Cells were cultured in FBS overnight before use.
Preparation of cell lysate
Cell lysate was obtained by applying hypotonic shock to MOLT-4 cells (T-cell type acute lymphoblastic leukaemia cell line; Riken, Wako, Japan). About 2×106 cells were centrifuged (650 g for 10 min) to form a pellet of about 10 μl volume, resuspended in 100 μl of distilled water, and pipetted for 2 min. Then the osmotic pressure of the medium was recovered by adding 100 μl PBS of the twofold higher concentration. Supernatant was collected after centrifugation. About 10 μl of supernatant was added to a 20-μl drop of RPMI in the experimental dish. Contents of the cytosol were diluted finally to ≈1/70 in these procedures, if cells were completely lysed.
[Ca2+]i measurement by Ca2+ imaging
Details of Ca2+imaging have been described previously.18,19 Briefly, macrophages were detached from the bottom of the culture dish with cold Ca2+- and Mg2+-free PBS and preloaded in a test tube with the Ca2+-sensitive fluorescent dye fura-2 by incubation for 30 min in RPMI containing 2 μm fura-2-AM (acetoxymethyl derivative; Molecular Probes, Eugene, OR). Target cells for NK cells were loaded with fura-2 in the same way. After washing, cells were transferred to a 20-μl drop of RPMI in a 35-mm plastic dish which was mounted on the stage of an inverted microscope (TMD300, Nikon, Tokyo, Japan) and heated at 37°. The drop was covered with paraffin oil to avoid evaporation. Fura-2 fluorescence in the cell was activated by applying 340 nm ultraviolet (UV) light for 0·25 seconds followed 1 second later by 380 nm UV for 0·25 seconds through a ×40 objective lens (Fluor 40; Nikon). Ca2+ images were sampled every 20 seconds. Emission fluorescence (F) was lead to a silicon intensifier target camera through a 510±10-nm bandpass filter. Data were processed to calculate the fluorescence ratio R=F340:F380. A calibration curve between R and [Ca2+] was obtained by measuring R for Ca2+/N-(2-hydroxyethyl)ethylenedinitrilotriacetic acid (EDTAOH) buffer solutions of different Ca2+ concentrations. All these procedures were performed using an image processor (ARGUS-50, Hamamatsu Photonics, Hamamatsu, Japan).
Observation of dying cell–macrophage interaction
Cells from the human cell line K562 (chronic myelogenous leukaemia cells) were mainly used as targets for NK cells. K562 cells are NK cell-sensitive and undergo necrosis when attacked by NK cells.18,19 Fura-2-loaded K562 cells together with macrophages were dispersed in the drop of RPMI in the experimental dish. After the cells were settled on the bottom of the dish, a small amount of suspension of fura-2-unloaded NK cells was gently added to the drop through a pipette. Movement of cells during this procedure was avoided, as the bottom of the dish was coated with poly l-lysin (50 μg/ml; Sigma) to facilitate adhesion of cells. [Ca2+]imeasurement in target cells and macrophages was started, when a NK cell approaching to a target cell was recognized in the optic field (10–20 min after NK cells arrived at the bottom of the dish).
Bright field images were obtained by illumination of red light. Transmitted images were passed through a dichroic mirror at 565 nm and separated from fura-2 fluorescence without mutual interference. Images were detected by a CCD camera (XC-77, Sony, Tokyo) and recorded on a video tape.
Observation of Ca2+ responses to chemicals
Ten microlitre of cell lysate or RPMI containing ATP, UTP, 2-chloro-ATP (RBI, Natick, MA), or 3′-O-(benzoyl)-benzoly ATP (BzATP; Sigma, St Louis, MO) was gently added to the 20-μl drop containing the cells. For examination with P2 receptor inhibitors, cells were dispersed in RPMI conataining Reactive Blue 2 (anthraquinone-sulphonic acid deraivative), pyridoxalphosphate-6-axophenyl-2′,4′-disulphonic acid (PPADS) (both from RBI). Oxidized ATP (Sigma) was administered 10 min prior to application of ATP. The number of cells that showed Ca2+ responses was counted in an optic field (15–30 cells). Experiments in Ca2+-free medium were performed in Ca2+-omitted RPMI containing 0·5 mm ethylenedioxybis(ethylamine)-N,N,N′,N′-tetraacetic acid (EGTA; Dojindo, Kumamoto, Japan).
Statistics
Experimental values were presented by mean±standard error of the mean (n=number of cells examined). A Student’s t-test was used for analysis between groups. A P-value of 0·05 or less was considered significant.
RESULTS
Signalling from a dying cell to macrophages
We found that a transient rise in [Ca2+]i was generated in macrophages in the vicinity of a dying cell that was attacked by a NK cell (Fig. 1). K562 cells used as target cells have been shown to be permeabilized by NK cells and to undergo necrosis.18,19 Figure 1(a) is a fluorescence image taken before addition of NK cells. Smaller cells of dense fura-2 fluorescence (activated by 380 nm UV light; F380) are macrophages and larger cells of lesser F380 are K562 cells. Figure 1(b) is the digital Ca2+ image obtained from the image of Fig. 1(a) and the image taken 1 second ahead (F340), by calculating the ratio R=F340:F380. [Ca2+]i was expressed by pseudocolours (see the scale bar). NK cells were added immediately after the image of Fig. 1(a) was taken. Figure 1(c) is a Ca2+ image at about 20 min after the addition of NK cells. NK cells are not seen in this image, as they were not loaded with fura-2. A K562 cell was attacked by a NK cell (recognized in bright field; see the Materials and Methods) and displayed an abrupt, large [Ca2+]i rise (arrow in Fig. 1c). Figure 1(d), which was taken 1 min after the time for Fig. 1(c), shows that F380 of the attacked cell was remarkably reduced (see ref. 19 for the precise NK cell-to-target cell interaction). The large [Ca2+]i rise and subsequent loss of fluorescence in the attacked cell indicate Ca2+ entry into the cell and leakage of fura-2 out of the cell due to permeabilization through perforin pores.18,19 Subsequently, macrophages near the attacked K562 cell exhibited a transient [Ca2+]i rise (14 cells in Fig. 1e, f). These phenomena were observed in seven experiments (in 10–15 macrophages in each experiment).
Figure 1.
Increases in [Ca2+]i in the macrophages near a K562 cell that was attacked and permeabilized by a NK cell. The thick arrow indicates the attacked K562 cell. (a) Fluorescence image of K562 cells and macrophages before addition of NK cells. (b) Digital Ca2+ image obtained from the image of (a); scale bar is 20 μm. (c) Ca2+ image about 20 min after addition of NK cells. (d) Fluorescence image 1 min after the time for (c). (e, f) Ca2+ images 1 and 2 min after the time for (c), respectively. Arrows 1, 2 and 3 indicate the three macrophages of which the Ca2+ responses are illustrated in Fig. 2(b).
Figure 2(a) presents changes in F340 and F380 of the attacked cell shown in Fig. 1, and Fig. 2(b) illustrates changes in [Ca2+]i obtained from F340:F380 in the attacked cell and three macrophages near the cell. In the attacked K562 cell, F340 increased while F380 decreased, resulting in an increase in the ratio; that is, a [Ca2+]i rise. After [Ca2+]i declined nearly to the basal level, both F340 and F380 reached a level lower than the original level, indicating leakage of fura-2 out of the cell. [Ca2+]i in macrophages began to increase 30–60 seconds after the onset of the [Ca2+]i rise in the attacked cell and reached the peak of 200–600 nm. The [Ca2+]i rise was a little more delayed and attained a lower peak in the cells located farther from the attacked cell (macrophages 1–3 in Fig. 2b). All of these Ca2+ responses were transient, with duration of ≈3 min. These results indicated that a certain factor that was discharged from the permeabilized cell diffused towards macrophages and induced a Ca2+ response.
Figure 2.
(a) Changes in fluorescence intensity (F340 and F380) in the NK cell-attacked K562 cell indicated by the thick arrow in Fig. 1. (b) Changes in [Ca2+]i calculated from the ratio F340:F380 in the NK cell-attacked K562 cell and three macrophages indicated by arrows 1, 2 and 3 in Fig. 1(f). Fluorescence intensity was averaged over a cell.
Using 10 cell lines that can be the target of NK cells,17 we examined whether any Ca2+ response occurs in the cells surrounding a killed cell of the same cell line. The Ca2+ response was observed only in the monocyte cell line U937, but not in K562, MOLT-4 (see the Materials and Methods), Daudi, Namalwa, Raji (originated from Burkitt’s lymphoma), Reh, KM3 (from non-T, non-B acute lymphoblastic leukaemia), Peer (γδT-cell acute lymphoblastic leukaemia), and FMO (Epstein–Barr virus-induced B-cell line) (data not shown).
Ca2+ responses to ATP
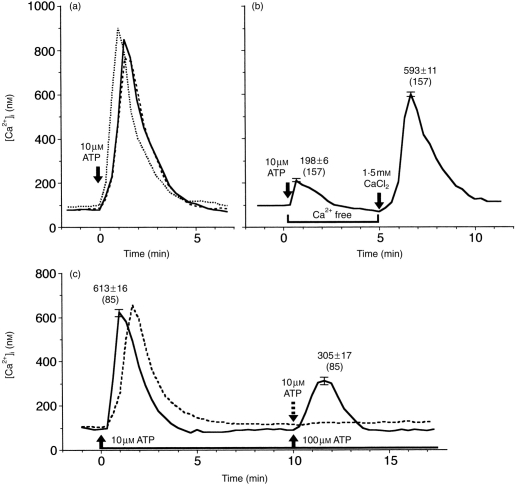
A transient [Ca2+]i rise occurred in more than 70% of macrophages in response to 10 μm ATP, as shown in Fig. 3(a) by three representative records obtained from Ca2+ images. The [Ca2+]i before stimulation was 96±2 nm (n=245 cells). [Ca2+]i increased immediately after application of ATP and reached a peak of 840±12 nm (n=277) in about 1 min. The total duration of the Ca2+ transient was 4–5 min. Additional Ca2+ transients (Ca2+ oscillations) were seldom observed during [Ca2+]i measurement for 20 min. Figure 4 shows the dose-dependence of the percentage of responding cells and the peak [Ca2+]i. The minimal effective ATP concentration was 0·1 μm or less and the efficiency was almost saturated at 100 μm. No ATP-induced Ca2+ response was observed in NK cells and T cells (data not shown).
Figure 3.
Ca2+ responses to ATP in macrophages. (a) Three representative responses to 10 μm ATP. (b) Ca2+ responses in Ca2+-free medium and upon addition of 1·5 mm CaCl2. A representative record in which the peak [Ca2+]i was close to the averaged value is shown. Numbers in parentheses are the numbers of cells examined. (c) Ca2+ responses to repeated application of 10 μm ATP (broken line) or 100 μm (solid line) 5 min after the first application of 10 μm ATP.
Figure 4.
Dose–response relationship of ATP-induced Ca2+ responses in macrophages. Responses are presented in terms of the percentage of cells that responded and the average of the peak [Ca2+]i in those cells.
One of the characteristic properties of ATP-induced Ca2+ responses in macrophages was desensitization. No substantial [Ca2+]i rise was induced by the second application of 10 μm ATP 5 min after the previous Ca2+ response had ceased (Fig. 3c, dotted line) (n=30). Even when 100 μm ATP was used for the second stimulation, the peak [Ca2+]i was 305±17 nm (n=85), about one-half of the first response to 10 μm ATP (Fig. 3c, solid line). It should be noted that the desensitization persisted for a fairly long time. For example, complete recovery of the Ca2+ response was not obtained, when 100 μm ATP was applied twice, after an interval of 20 min.
Ca2+ mobilization pathway for ATP-induced [Ca2+]i rise
The peak of Ca2+ responses to 10 μm ATP in Ca2+-free medium (Ca2+-omitted RPMI plus 0·5 mm EGTA) was 198±6 nm (n=157) (Fig. 3b, left), which was 23% of that in normal medium. A large Ca2+ response was restored (Fig. 3b), when 10 μl of RPMI containing 5 mm CaCl2 (without ATP) was added to Ca2+-free medium after the small Ca2+ response ceased (5 min after the application of 10 μm ATP). The estimated final concentration of free Ca2+ is ≈1 mm in the presence of 0·5 mm EGTA. The peak [Ca2+]i was 593±11 nm (n=157), 70% of that in normal medium. Thus, the [Ca2+]i rise induced by ATP is mainly due to Ca2+ influx from outside the cell, and the minor component that remained in Ca2+-free medium is thought to be due to intracellular Ca2+ release from Ca2+ stores. Interestingly, the fact that a fairly large [Ca2+]i rise is generated by delayed addition of Ca2+ implies thatthe Ca2+ influx pathway is not inactivated even in the continuous presence of ATP, unless Ca2+ influx has occurred previously.
Ca2+ response to UTP and other P2 receptor agonists
Ca2+ responses to UTP and other agonists were recorded in another series of experiments using macrophages from another donor. In this series, the percentage of cells that responded to 10 μm ATP was 87% (n=61), and the peak [Ca2+]i was 556±24 nm (n=52). Ca2+ responses were induced by 10 μm UTP in 82% of cells (n=85). The peak [Ca2+]i was 623±20 nm (n=69), which was significantly larger than that for 10 μm ATP (P<0·05). The time–course was similar to that of ATP-induced responses (Fig. 5a). Thus, ATP-induced [Ca2+]i rise is, at least, mediated by the P2U receptor.
Figure 5.
Ca2+ responses to 10 μm UTP (a), 2-chloro-ATP (b), or BzATP (c), and responses to 10 μm ATP which was added 10 min after the previous application of 100 μm oxidized ATP (d). Three representative records are shown for each stimulation.
A Ca2+ transient was induced by 10 μm 2-chloro-ATP as well, a potent agonist of P2Y receptor,20 in 70% of macrophages (n=63) (Fig. 5b). The peak [Ca2+]i was 362±20 nm (n=42), which was significantly smaller than that for 10 μm ATP (P<0·001). BzATP (10 μm), a potent agonist of P2Z receptor,21 caused a [Ca2+]i rise in more than 80% of cells, although responses were variable (Fig. 5c). [Ca2+]i increased to a peak (358±29 μm, n=30) more slowly than the Ca2+ transients described above. Then [Ca2+]i was held at 200–300 nm in approximately two-thirds of the cells or continued to increase over 800 nm during the 10-min recording in other cells (broken line in Fig. 5c). These results suggest that both P2Y and P2Z receptors exist in macrophages.
Effects of P2 receptor inhibitors
Macrophages were preincubated with P2 receptor inhibitors for 1–2 hr in the experimental dish and then 10 μm ATP was gently added to the medium. In the presence of 30 μm Reactive Blue 2, an inhibitor of P2Y receptor,22,23 the percentage of responding cells to 10 μm ATP was reduced to 37% (Fig. 6a), and the peak [Ca2+]i was significantly smaller (57%; P<0·001) than the control value (Fig. 6b). ATP-induced Ca2+ responses were not affected by 30 μm PPADS, the P2X receptor inhibitor24,25 (Fig. 6). Oxidized ATP, the P2Z receptor inhibitor,26 at 100 μm did not affect Ca2+ responses to 10 μm ATP (Fig. 5d).
Figure 6.

Effects of Reactive Blue 2 and PPADS on the percentage of responding cells (a) and the peak [Ca2+]i of Ca2+ responses to 10 μm ATP or cell lysate. The concentration of chemicals was 30 μm. Control was obtained in the same series of experiments. The inhibitory effect of Reactive Blue 2 was significant (P<0·001).
Ca2+ response induced by cell lysate
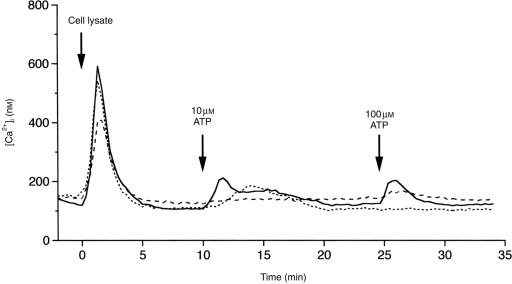
As expected, a transient [Ca2+]i rise was induced by cell lysate in 88% of macrophages. The peak was 692±10 nm (n=120), and the duration was 4–5 min. The time–course was similar to that of the ATP-induced response (Fig. 7). Once a large [Ca2+]i rise was elicited by cell lysate, responses to the subsequent application of 10 or 100 μm ATP were much smaller (Fig. 7), compared with those evoked by the first ATP stimulation with the same concentrations (see Fig. 4). This desensitization was produced by the reverse order of stimuli as well (ATP followed by cell lysate; not shown). Thus, cell lysate-induced and ATP-induced Ca2+ responses interfered with each other, indicating a convergence of the signalling pathway activated by the two stimuli. The Ca2+ response induced by cell lysate was inhibited by Reactive Blue 2 (Fig. 6), but not by PPADS, to the similar extent observed in ATP-induced Ca2+ responses. It was very likely that the Ca2+ response was induced by ATP contained in the cell lysate.
Figure 7.
Three representative Ca2+ responses to cell lysate and to additional application of 10 and 100 μm ATP.
DISCUSSION
The present study first demonstrated in single cells that human macrophages differentiated from monocytes in vitro exhibit a transient [Ca2+]i rise in response to extracellular ATP. NK and T cells displayed no response to ATP. Neutrophils are known to show Ca2+ responses to ATP.15,27 The responsiveness to ATP appears to be characteristic in phagocytes. The major component of the [Ca2+]i rise was Ca2+ influx and the minor component was intracellular Ca2+ release, similar to platelet-activating factor-induced [Ca2+]i rise in macrophages3 and adhesion-induced [Ca2+]i rise in monocytes.6
As to P2 purinoreceptors, several subtypes are distinguished by the order of agonist potency for responses.15,16 The ATP-induced [Ca2+]i rise in macrophages is thought to be mediated by P2U, since UTP produced greater Ca2+ responses than ATP. In murine peritoneal macrophages, the [Ca2+]i rise, which is due to Ca2+ release followed by Ca2+ influx, is mediated by P2U.8 On the other hand, a P2Y-mediated [Ca2+]i rise has been reported in human monocyte cell line U937.10 The participation of P2Y is likely in macrophages, as 2-chloro-ATP elicited a Ca2+ transient and ATP-induced Ca2+ responses were inhibited by Reactive Blue 2. It has been shown that Reactive Blue 2 inhibits the inward membrane current for which the sequence of nucleotides is consistent with P2Y,22,23 but that the dye can inhibit not only P2Y- but also P2U-mediated endothelium-dependent vasodilatation, although to the lesser extent.28,29 Reactive Blue 2 therefore probably acted on both P2U and P2Y in macrophages. The involvement of P2X is negligible, as 30 μm PPADS had no significant effect. P2Z, which is coupled to plasma membrane pores,30 is expressed in macrophages and participates in the ATP-induced [Ca2+]i rise31 or Ca2+ inward current.32 In our study, BzATP generated Ca2+ responses, but the [Ca2+]i rise was sustained for a long time or augmented with time, unlike ATP-induced Ca2+ transient. Oxidised ATP did not affect responses to 10 μm ATP. The P2Z-mediated Ca2+ influx, if any, is probably minor in the Ca2+ response on which the present study focused, since the half maximal effective concentration of ATP was ≈1 μm (Fig. 4) while it is over 100 μm for activation of P2Z in various cells.30 In summary, it is reasonable to consider that the ATP-induced Ca2+ response in human macrophages differntiated in vitro is mediated by P2U and P2Y receptors.
Both P2Y and P2U receptors are coupled to G protein which activates phospholipase C, leading to production of inositol 1,4,5-triphosphate (InsP3) and InsP3-induced Ca2+ release from the endoplasmic reticulum through InsP3 receptors.15,33 On the other hand, a P2Y- or P2U-mediated Ca2+ influx pathway has not been clearly identified. Candidates are Ca2+ channels or cation channels activated by intracellular Ca2+ or InsP3, or store-operated Ca2+ channels.33–35 The present study showed that Ca2+ influx can be induced by addition of Ca2+ to Ca2+-free medium even after the [Ca2+]i rise due to Ca2+ release has ceased (Fig. 3b); that is, Ca2+ influx is not directly activated by intracellular Ca2+ or by Ca2+ release. Activation of Ca2+ influx by depletion of Ca2+ stores would be possible, if Ca2+ channels could be continuously opened for a fairly long time (at least 5 min to explain the result of Fig. 3b). Another interesting candidate is the TRP5 Ca2+ channel, a homologue of Drosophila TRP, which mediates ATP-induced Ca2+ influx in the same manner as shown in Fig. 3(b) without activation via store depletion.36 A characteristic property of the ATP-induced Ca2+ response in macrophages was desensitization, which took place after Ca2+ influx was induced (Fig. 3c) but did not occur without previous Ca2+ influx (Fig. 3b). The desensitization persisted for as long as 20 min or more. It is conceivable that Ca2+ influx itself or a large [Ca2+]i rise causes desensitization in Ca2+ channels and/or signal transduction from the P2Y or P2U receptor to Ca2+ channels. These properties provide useful information for future studies on identification of the receptor-mediated Ca2+ influx.
Cell lysate induced a [Ca2+]i rise in macrophages with a time–course similar to the ATP-induced rise. The response was inhibited by Reactive Blue 2 and was interfered with by the ATP-induced rise. These results indicate that ATP, which is contained in cell lysate, induces the [Ca2+]i rise. Since cell contents were diluted to roughly 1/70 (see the Materials and Methods) and cytosolic ATP is usually 3–5 mm,15 ATP in the applied cell lysate was estimated to be 40–70 μm, if cells were completely lysed by hypo-osmotic treatment. ATP in the cell lysate is enough to produce a Ca2+ response even if it is diluted, as the minimal effective concentration of ATP was 0·1 μm or less. ATP that is discharged from a damaged or dying cell must be capable of inducing Ca2+ responses in neighbouring macrophages, as demonstrated using NK cell-attacked and permeabilized K562 cells. Among 10 cell lines, only the monocye cell line U937 showed a similar Ca2+ response. This experimental system, however, depended on appropriate cell arrangement in the dish and on the chance of attack by the NK cell on the target, so it was difficult to examine possible inhibitory effects of Reactive Blue 2. ATP is known to activate the function of macrophages, such as phagocytosis37 and respiratory burst (superoxide production).38 It is interesting to examine morphological changes, such as migration or phagocytosis of the macrophages, in which [Ca2+]i rise was recorded. Unfortunately, such changes could not be observed in the present experimental conditions. Despite these limitations, the present study provided evidence for ATP as a signal messenger from dying cells to macrophages.
Acknowledgments
We thank Drs S. Mitani and H. Shirakawa for their advice in the experiments, and Mr Y. Konuma for his help in processing data and preparing figures. This work was supported by a Grant-in-Aid for General Scientific Research (C) from the Japan Ministry of Education, Science, Sports, and Culture.
Abbreviations
- BzATP
3′-O-(benzoyl)-benzoyl-ATP
- [Ca2+]i
intracellular Ca2+ concentration
- DMSO
dimethylsulphoxide
- EDTAOH
N-(2-hydroxyethyl)ethylenedinitrilotriacetic acid
- EGTA
ethylenedioxybis(ethylamine)-N,N,N′,N′-tetraacetic acid
- FBS
fetal bovine serum
- InsP3
inositol 1,4,5-trisphosphate
- mAb
monoclonal antibody
- NK cell
natural killer cell
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate-buffered saline
- PPADS
pyridoxal-phosphate-6-axophenyl-2′,4′-disulphonic acid, UV light, ultraviolet light
References
- 1.Bueb J-L, Gallois A, Schneider J-C, Parini J-P, Tschirhart E. A double-labelling fluorescent assay for concomitant measurements of [Ca2+]i and O2 production in human macrophages. Biochim Biophys Acta. 1995;1244:79. doi: 10.1016/0304-4165(94)00198-7. [DOI] [PubMed] [Google Scholar]
- 2.Bernardo J, Billingslea AM, Ortiz MF, et al. Adherence-dependent calcium signaling in monocytes: induction of a CD14-high phenotype, stimulus-responsive subpopulation. J Imm Meth. 1997;209:165. doi: 10.1016/s0022-1759(97)00157-9. [DOI] [PubMed] [Google Scholar]
- 3.Katnik C, Nelson DJ. Platelet activating factor-induced increase in cytosolic calcium and transmembrane current in human macrophages. J Membr Biol. 1993;134:213. doi: 10.1007/BF00234502. [DOI] [PubMed] [Google Scholar]
- 4.Badolato R, Johnston JA, Wang JM, et al. Serum amyloid A induces calcium mobilization and chemotaxis of human monocytes by activating a pertussis toxin-sensitive signaling pathway. J Immunol. 1995;155:4004. [PubMed] [Google Scholar]
- 5.Hishikawa T, Cheung JY, Yelamarty RV, Knutson DW. Calcium transients during Fc receptor-mediated and nonspecific phagocytosis by murine peritoneal macrophages. J Cell Biol. 1991;115:59. doi: 10.1083/jcb.115.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim E, Enelow RI, Sullivan GW, Mandell GL. Regional and generalized changes in cytosolic free calcium in monocytes during phagocytosis. Infect Immunity. 1992;60:1244. doi: 10.1128/iai.60.3.1244-1248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmid-Antomarchi H, Schmid-Alliana A, Romey G, et al. Extracellular ATP and UTP control the generation of reactive oxygen intermediates in human macrophages through the opening of a charybdotoxin-sensitive Ca2+-dependent K+ channel. J Immunol. 1997;159:6209. [PubMed] [Google Scholar]
- 8.Alonso-Torre SR, Trautmann A. Calcium responses elicited by nucleotides in macrophages. Interaction between two receptor subtypes. J Biol Chem. 1993;268:18640. [PubMed] [Google Scholar]
- 9.Maudsley DJ, Morris AG. Rapid intracellular calcium changes in U937 monocyte cell line: transient increases in response to platelet-activating factor and chemotactic peptide but not interferon-γ or lipopolysaccharide. Immunology. 1987;61:189. [PMC free article] [PubMed] [Google Scholar]
- 10.Pleass R, Cusack NJ, Westwick J. An ATP receptor that mediates increase in intracellular calcium in U937 cells is a P2y purinoceptor. Eur J Pharmacol. 1990;183:1605. [Google Scholar]
- 11.Ventura MA, Thomopoulos P. ADP and ATP activate distinct signaling pathways in human promonocytic U-937 cells differentiated with 1,25-dihydroxy-vitamin D3. Mol Pharmacol. 1995;47:104. [PubMed] [Google Scholar]
- 12.Greenberg S, Virgilio FD, Steinberg TH, Silverstein SC. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J Biol Chem. 1988;263:10337. [PubMed] [Google Scholar]
- 13.Darbha S, Marchase RB. Regulation of intracellular calcium is closely linked to glucose metabolism in J774 macrophages. Cell Calcium. 1996;20:361. doi: 10.1016/s0143-4160(96)90042-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhang GH, Helmke RJ, Mork AC, Martinez JR. Regulation of cytosolic free Ca2+ in cultured rat alveolar macrophages (NR8383) J Leukocyte Biol. 1997;62:341. doi: 10.1002/jlb.62.3.341. [DOI] [PubMed] [Google Scholar]
- 15.Dubyak GR, El-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:C577. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 16.Illes P, Nieber K, Frohlich R, Norenberg W. P2 purinoceptors and pyrimidinoceptors of catecholamine-producing cells and immunocytes. Ciba Found Symp. 1996;198:110. doi: 10.1002/9780470514900.ch6. [DOI] [PubMed] [Google Scholar]
- 17.Oshimi Y, Oda S, Honda Y, Nagata S, Miyazaki S. Involvement of Fas ligand and Fas-mediated pathway in the cytotoxicity of human natural killer cells. J Immunol. 1996;157:2909. [PubMed] [Google Scholar]
- 18.Oshimi Y, Oshimi. K, Miyazaki S. Necrosis and apoptosis associated with distinct Ca2+ response patterns in target cells attacked by human natural killer cells. J Physiol. 1996;495:319. doi: 10.1113/jphysiol.1996.sp021596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honda Y, Miyazaki S. Distinct Ca2+ response patterns in human natural killer cells during induction of necrosis or apoptosis of target cells. Call Calium. 1996;19:297. doi: 10.1016/s0143-4160(96)90070-6. [DOI] [PubMed] [Google Scholar]
- 20.Pacaud P, Feolde E, Frelin C, Loirand G. Characterization of the P2Y-purinoceptor involved in the ATP-induced rise in cytosolic Ca2+ concentration in rat ileal myocytes. Br J Pharmacol. 1996;118:2213. doi: 10.1111/j.1476-5381.1996.tb15665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erb L, Lustig KD, Ahmed AH, et al. Covalent incorporation of 3′-O-(4-benzoyl) benzoyl-ATP into a P2 purinoceptor in transformed mouse fibroblasts. J Biol Chem. 1990;265:7424. [PubMed] [Google Scholar]
- 22.Inoue K, Nakazawa K, Ohara-Imaizumi M, et al. Antagonism by reactive blue 2 but not by brilliant blue G of extracellular ATP-evoked responses in PC12 phaeochromo-cytoma cells. Br J Pharmacol. 1991;102:851. doi: 10.1111/j.1476-5381.1991.tb12265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fieber LA, Adams D. Adenosine triphosphate-evoked current in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991;434:239. doi: 10.1113/jphysiol.1991.sp018467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziganshin AU, Hoyle CHV, Bo X, et al. PPADS selectively antagonizes P2X-purinoceptor-mediated responses in the rabbit urinary bladder. Br J Pharmacol. 1993;110:1491. doi: 10.1111/j.1476-5381.1993.tb13990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valera S, Hussy N, Evans RJ, et al. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 26.Margia M, Hanau S, Pizzo P, et al. Oxydized ATP: an irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem. 1993;268:8199. [PubMed] [Google Scholar]
- 27.Cowen DS, Lazarus HM, Shurin SB, et al. Extracellular adenosine triphosphate activates calcium mobilization in human phagocytic leukocytes and neutrophil/monocyte progenitor cells. J Clin Invest. 1989;83:1651. doi: 10.1172/JCI114064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ralevic V, Burnstock G. Relative contribution of P2U- and P2Y-purinoceptors to endothelium-dependent vasodilatation in the hamster isolated mesenteric arterial bed. Br J Pharmacol. 1996;117:1797. doi: 10.1111/j.1476-5381.1996.tb15357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansmamm G, Bultmann R, Tuluc F. Characterization by antagonists of P2-receptors mediating endothelium-dependent relaxation in the rat aorta. Naunyn-Schmiedeberg’s Arch Pharmacol. 1997;356:641. doi: 10.1007/pl00005101. [DOI] [PubMed] [Google Scholar]
- 30.Di Virgilio F. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995;16:524. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 31.Falzoni S, Munerati M, Ferrari D, et al. The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J Clin Invest. 1995;95:1207. doi: 10.1172/JCI117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naumov AP, Kaznacheyeva EV, Kiselyov KI, et al. ATP-activated inward current and calcium-permeable channels in rat macrophage plasma membranes. J Physiol. 1995;486:323. doi: 10.1113/jphysiol.1995.sp020815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnerd EA, Burnstock G, Webb TA. G protein-coupled receptors for ATP and other nucleotides: a new receptor family. Trends Pharmacol Sci. 1994;15:67. doi: 10.1016/0165-6147(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 34.Fasolato C, Innocenti B, Pozzan T. Receptor-activated influx: how many mechanisms for how many channels. Trends Pharmacol. 1994;15:77. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 35.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 36.Okada T, Shimizu S, Wakamori M, et al. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J Biol Chem. 1998;273:10279. doi: 10.1074/jbc.273.17.10279. [DOI] [PubMed] [Google Scholar]
- 37.Ichinose M. Modulation of phagocytosis by P2-purinergic receptors in mouse peritoneal macrophages. Jpn J Physiol. 1995;45:707. doi: 10.2170/jjphysiol.45.707. [DOI] [PubMed] [Google Scholar]
- 38.Murphy JK, Livingston FR, Gozal E, Torres M, Forman HJ. Stimulation of the rat alveolar macrophage respiratory burst by extracellular adenine nucleotides. Am J Resp Cell and Mol Biol. 1993;9:505. doi: 10.1165/ajrcmb/9.5.505. [DOI] [PubMed] [Google Scholar]