Abstract
Interferon-γ (IFN-γ) is an important cytokine involved in the regulation of allergen-induced immune responses. We examined the role of IFN-γ in a Brown–Norway rat model of bronchial hyperresponsiveness (BHR) and airway eosinophilia, and its effects on the mRNA expression of T helper type 1 (Th1)/Th2 cytokine. Ovalbumin (OA)-sensitized animals were given either exogenous IFN-γ (105 U/rat over 3 days, intraperitoneally) or anti-IFN-γ blocking antibody (DB-1 0·3 mg/rat, intravenously) prior to exposure to OA aerosol and were studied 18–24 hr later. In sensitized animals, OA induced significant BHR, accumulation of eosinophils, T lymphocytes and neutrophils in bronchoalveolar lavage (BAL) fluid, and also increased eosinophils and CD8+ T cells in the airways. Exogenous IFN-γ attenuated allergen-induced BHR (P<0·02, compared with sham-treated animals) together with a significant reduction in eosinophil and neutrophil numbers in BAL fluid (P<0·005), and eosinophils and CD8+ T cells in airways (P<0·05). By contrast, anti-IFN-γ antibody increased airway CD4+ T cells and BHR. Using reverse transcriptase–polymerase chain reaction, significant increases in Th2 [interleukin-4 (IL-4), IL-5 and IL-10], and IFN-γ cytokine mRNA were found in the lungs of sensitized and OA-exposed animals, while exogenous IFN-γ significantly suppressed IL-4, IL-5 and IL-10 mRNA expression, and anti-IFN-γ antibody increased IL-4 and IL-5 mRNA expression. These results indicate that Th1 effects, such as those mediated by IFN-γ, play a down-regulatory role to suppress the Th2 responses associated with allergen-induced BHR and eosinophilic inflammation.
INTRODUCTION
CD4+ T lymphocytes have been implicated in the chronic eosinophilic airway inflammation of allergic asthma. They have been localized to the airway mucosa of patients with asthma,1 and adoptive transfer of allergen-specific CD4+ T cells can induce bronchial hyperresponsiveness (BHR) and airway eosinophilia in the rat and mouse,2–4 CD4+ T cells in the airways of asthmatic patients express a T helper type 2 (Th2) profile of cytokines, particularly interleukin-4 (IL-4) and IL-5,5 which are important for the regulation of immunoglobulin E (IgE) production and the terminal differentiation and priming of eosinophils, respectively.6,7 On the other hand, the Th1 profile cytokines, as characterized by the expression of interferon-γ (IFN-γ) during allergic inflammation, is not increased, but may be reduced.5,8 IFN-γ is an important cytokine secreted from both CD4+ Th1 cells, and also from CD8+ T cells. IFN-γ inhibits IgE production,9 allergen-stimulated development of Th2 clones,10,11 and allergen-induced eosinophil recruitment in mouse airways.12,13 In addition, allergen-induced airway hyperresponsiveness is also inhibited by IFN-γ administration.14
We have examined further the potential for exogenously administered IFN-γ and for endogenously produced IFN-γ in modulating allergen-induced BHR and airway inflammation in the Brown–Norway rat model of allergic sensitization. This model has been extensively characterized and exhibits several inflammatory and immunological features that resemble those of asthma, including the airway eosinophilic inflammation, the development of BHR and the expression of Th2 cytokines such as IL-4 and IL-5 in the sensitized and challenged lung.2,15,16 We therefore assessed the extent to which IFN-γ could protect against these effects of allergen exposure, and the degree to which endogenously produced IFN-γ participates in the modulation of these effects in this model.
MATERIALS AND METHODS
Animals, sensitization procedures and allergen exposure
Pathogen-free inbred male Brown–Norway rats (Harlan Olac Ltd. Bicester, UK) (200–250 g, 9–13 weeks ld) were cared for following the guidelines in The UFAW Handbook on the Care and Management of Laboratory Animals, published by Universities Federation for Animal Welfare, UK.17 Animals were injected with 1 ml of 1 mg ovalbumin (OA; Grade V, salt-free, Sigma, Dorset, UK) in 100 mg Al(OH)3 (BDH, Dorset, UK) suspension in 0·9% (wt/vol) saline intraperitoneally (i.p.) on 3 consecutive days. OA aerosol exposure (15 min; 1% OA) to rats was performed in a 6·5-l Plexiglas chamber connected to a DeVilbiss PulmonSonic nebulizer (model no. 2512, DeVilbiss Health Care, UK Ltd, Middlesex, UK) that generated an aerosol mist pumped into the exposure chamber by the airflow supplied by a small animal ventilator (Harvard Apparatus Ltd, Kent, UK) set at 60 strokes/min with a pumping volume of 10 ml.
Protocol
Six groups of animals were sensitized as described above and studied as follows.
Saline-exposed and sham-treated animals (group SS, n=6)
Sensitized animals received normal mouse IgG (0·3 mg/rat, Serotec, Oxford, UK) intravenously (i.v.) as a control for anti-IFN-γ antibody on day 20 and 0·9% saline (i.p. 1 ml/rat/day) as a control for recombinant rat IFN-γ from day 19 to day 21, and were exposed on day 21 to saline aerosol.
OA-exposed and sham-treated animals (group SO, N=6)
Sensitized animals received normal mouse IgG (0·3 mg/rat, Serotec) i.v. as a control for anti-IFN-γ antibody on day 20 and 0·9% saline (i.p. 1 ml/rat/day) as a control for recombinant rat IFN-γ from day 19 to day 21, and were exposed to 1% OA aerosol on day 21.
Saline-exposed and IFN-γ-treated animals (group SSifn, N=4)
Sensitized animals received normal mouse IgG (0·3 mg/rat, Serotec) i.v. as a control for anti-IFN-γ antibody on day 20, and recombinant rat IFN-γ (Cambridge Bioscience, Cambridge, UK) 3·3×104 U/rat/day i.p. from day 19 to day 21, and were exposed on day 21 to saline aerosol.
OA-exposed and IFN-γ-treated animals (group SOifn, n=6)
Sensitized animals received normal mouse IgG (0·3 mg/rat, Serotec) i.v. as a control for anti-IFN-γ antibody on day 20, and recombinant rat IFN-γ (Cambridge Bioscience) 3·3×104 U/rat/day i.p. from day 19 to day 21, and were exposed on day 21 to 1% OA aerosol.
Saline-exposed and DB-1-treated animals (group SSdb, N=4)
Sensitized animals received the mouse anti-rat IFN-γ neutralizing IgG1 monoclonal antibody DB-1 (0·3 mg/rat i.v. via a caudal vein) on day 20, and 0·9% saline (i.p. 1 ml/rat/day) as a control for recombinant rat IFN-γ from day 19 to day 21, and were exposed on day 21 to saline aerosol. The antibody was produced from hybridoma cells as previously described, and effectively neutralizes the antiviral activity of IFN-γ in rats and mice.18
OA-exposed and DB-1-treated animals (group SOdb, N=6)
Sensitized animals received mouse anti-rat IFN-γ neutralizing IgG1 monoclonal antibody DB-1 (0·3 mg/rat i.v. via a caudal vein) on day 20, and 0·9% saline (i.p. 1 ml/rat/day) as a control for recombinant rat IFN-γ from day 19 to day 21, and were exposed on day 21 to OA aerosol.
All rats were studied 18–24 hr after exposure to either 1% OA or 0·9% NaCl aerosol. One control experimental group of non-sensitized rats exposed to OA was not included since in a previous study, this procedure did not provoke airway inflammation or BHR.15
Measurement of airway responsiveness to acetylcholine
Airway responsiveness was measured as previously described.15 In brief, anaesthetized, tracheostomized, paralysed and ventilated rats were monitored for airflow with a pneumotachograph (model F1L, Mercury Electronics Ltd, Glasgow, UK) connected to a transducer (model FCO40; ±20 mmH2O, Furness Controls Ltd, Sussex, UK), transpulmonary pressure via a transpleural catheter connected to a transducer (model FCO40; ±1000 mmH2O, Furness Controls) and blood pressure via carotid artery catheterization. Lung resistance (RL) was simultaneously calculated using a software (LabView, National Instruments, Austin, TX) on a Macintosh II computer (Apple Computer Inc., Cupertino, CA). Aerosol generated from increasing half log10 concentrations of acetylcholine chloride (ACh) (Sigma) was administered by inhalation (45 breaths of 10 ml/kg stroke volume) with an initial concentration of 10−3·5 mol/l and the maximal concentration of 0·1 mol/l, through a different circuit from the one for RL measurements. The concentration of ACh needed to increase RL 200% above baseline (PC200) was calculated by interpolation of the log concentration-lung resistance curve.
Bronchoalveolar lavage (BAL) and cell counting
This is also described in detail elsewhere.16 Briefly, after an overdose of anaesthetic, rats were lavaged with 20 ml 0·9% sterile saline via the endotracheal tube. Total cell counts, viability and differential cell counts from cytospin preparations stained by May–Grünwald–Giemsa stain were determined under an optical microscope (Olympus BH2, Olympus Optical Company Ltd, Tokyo, Japan). At least 500 cells were counted and identified as macrophages, eosinophils, lymphocytes and neutrophils according to standard morphology under ×400 magnification.
Collection of lung tissues
After opening of the thoracic cavity and removal of the lungs, the right lung without major vascular and connective tissues was cut into pieces and snap-frozen in liquid nitrogen (BOC, Luton, UK), and then stored at −80° for later assays for mRNA expression. The left lung was inflated with 3 ml saline/optimum cutting temperature (O.C.T.) tissue embedding medium (1:1). Two blocks of 0.5 cm3 were cut from the left lung around the major bronchus, embedded in O.C.T. medium (Raymond A. Lamb, London, UK), and snap-frozen in melting isopentane (BDH) and liquid nitrogen. Cryostat sections (6 μm) of the tissues were cut, air-dried, fixed in acetone, and then air-dried again, wrapped in aluminium foil and stored at −80° for later immunohistochemical studies.
Immunohistochemistry
For detection of eosinophils, we used a mouse IgG1 monoclonal antibody against human major basic protein (MBP), clone BMK-13 (Monosan®, Bradsure Biologicals Ltd, Leicestershire, UK), which has been shown to be both sensitive and specific for staining rat eosinophils in frozen sections.19 The cryostat sections were incubated with BMK-13 at a dilution of 1:50 for 30 min at room temperature. After labelling with the second antibody, rabbit anti-mouse IgG, positively stained cells were visualized by the alkaline phosphatase–anti-alkaline phosphatase method.
For staining of CD2+, CD4+ and CD8+ T lymphocytes in tissues, sections were incubated with mouse anti-rat monoclonal antibodies (Pharmingen, Cambridge Bioscience, Cambridge, UK), anti-rat CD2 (pan T-cell marker), anti-rat CD4 and anti-CD8 antibodies at a dilution of 1:500 for 1 hr. Biotin goat anti-mouse antibody (Pharmingen) and avidin phosphatase (Dako Ltd, High Wycome, UK) at a dilution of 1:200 were applied for 30 min in turn.
For all tissue sections, alkaline phosphatase was developed as a red stain after incubation with Naphthol AS-MX phosphate in 0·1 m trismethylamine–HCl buffer (pH 8·2) containing levamisole to inhibit endogenous alkaline phosphatase and 1 mg/ml Fast Red-TR salt (Sigma). Then, sections were counterstained with Harris Haematoxylin (BDH) and mounted in Glycergel (Dako). System and specificity controls were carried out for all staining. Slides were read in a coded randomized blind fashion, under a microscope. Cells within 175 μm beneath the basement membrane were counted in all airways. Submucosal area was quantified with the aid of a computer-assisted graphic tablet visualized by a sidearm attached to the microscope. Counts were expressed as cells per mm2 of cross-sectional subepithelial area.
Reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA from lung tissue was extracted according to the method of Chomczynski and Sacchi.20 The yield of RNA was measured by optical density at 260 nm in a spectrophotometer. The RNA was analysed on a 1·5% agarose/formaldehyde gel in order to check for degradation, and stored at −80° until later use. After denaturing at 70° for 5 min, 1 μg of total RNA was used for reverse transcription in a 20-μl reaction volume containing 1×AMV buffer [50 mm Tris–HCl, pH 8·3, 50 mm KCl, 10 mm MgCl2, 10 mm dithiothreitol (DTT), 0·5 mm spermidine], 1 mm of 4 deoxynucleotide triphosphates (dNTP), including deoxyadenosine triphosphate (dATP), deoxycytidine triphosphate (dCTP), deoxyguanosine triphosphate (dGTP) and thymidine 5′-triphosphate (dTTP), ribonuclease inhibitor 32 U, 0·2 μg random primer pd(N)6 sodium salt (Pharmacia, Milton Keynes, UK), 8 U AMV reverse transcriptase (all apart from the random primer from Promega, Southampton, UK) at 42° for 60 min. Complementary DNA (cDNA) product was diluted to 100 μl in water. PCR was performed on 5 μl of diluted cDNA product in a total volume of 25 μl with a final concentration of 1×KCl or NH4 buffer with 1·5 mm MgCl2, 0·2 mm dNTP, 0·2 μg each of sense and antisense primers, and 1 U Taq polymerase (Bioline, London, UK) in a thermal cycler. The primers were designed according to published sequences.21 The PCR reagents were overlaid with mineral oil and amplification was carried out using a multiwell thermal cycler through 20–40 cycles of denaturation at 94° for 30 seconds, annealing at individual temperature for 30 seconds and extension at 72° for 30 seconds, followed by final extension at 72° for 10 min. The optimal PCR conditions, in terms of suitable buffer, annealing temperature and number of cycles, were determined by PCR with pooled cDNA from all samples. Annealing temperatures were 62° for GAPDH, IL-4 and IFN-γ, 58° for IL-5, and 65° for IL-2 and IL-10. Serial sampling every two cycles through 20–42 cycles was used to determine the exponential phase of the product amplification curve. The cycle numbers we used for PCR were 26 for GAPDH, 36 for IL-2, 38 for IL-4 and IL-5, and 34 for IL-10 and IFN-γ.
Southern blotting and Cerenkov counting
Each PCR product (10 μl) was size-fractionated and visualized with ethidium bromide (Sigma) on 1·5% agarose gel electrophoresis, followed by Southern blotting to Hybond-N membrane (Amersham, Bucks, UK) and hybridization to the appropriate cloned cDNA in order to confirm the identity of the product and, because all primer pairs cross at least one intron, to check for possible genomic contamination. Hybridizations were carried out at 65° overnight with the appropriate cloned cDNA, which had been 32P-labelled, in 6×standard saline citrate (SSC), 10×Denhardt’s solution (0·2% w/v each of bovine serum albumin, Ficoll and polyvinylpyrolidone), 5 mm ethylenediamine tetraacetic acid (EDTA), 0·5% sodium dodecyl sulphate (SDS) and 0·2% sodium pyrophosphate, 100 μg/ml sonicated salmon sperm DNA. In addition, 5 μl of each PCR reaction was dot-blotted on to Hybond-N membrane and also hybridized to cDNA probe. Dot blots were excised and radioactivity was measured by Cerenkov counting. All measurements were made below the saturation level of a Packard 1900CA liquid scintillation analyser (Packard Instrumentation BV, Groningen, the Netherlands). Results were generated from the counting of dot blots and expressed as a ratio of cytokine to GAPDH count, the latter used as an internal control.
Data analysis
Data were presented as mean±SEM. For multiple comparison of different groups, one-way analysis of variance (anova) was used on logarithmic transformed data, followed by Duncan’s multiple range test as post hoc correction. If the anova test was significant, we then used t-test for comparison between two individual groups. Data analysis was performed using SPSS for Windows statistical software package. A P-value of < 0·05 was considered to be significant.
RESULTS
Bronchial responsiveness to ACh
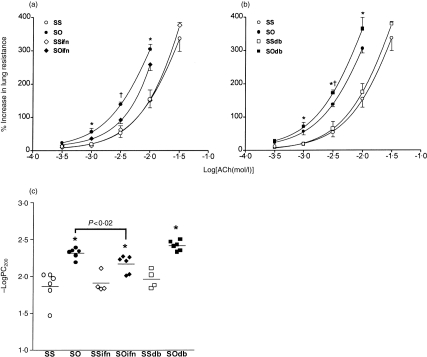
There was a significant increase in baseline RL after DB-1 treatment and OA exposure of sensitized animals (P<0·05, data not shown), but no significant difference was noted for the other five groups. Exogenous IFN-γ had no effect on the baseline responsiveness to ACh. Sensitized OA-exposed rats showed a significant increase in mean -logPC200 compared to sensitized saline-exposed rats (P<0·005; Fig. 1). Exogenous IFN-γ inhibited the induced BHR (P<0·02, compared to sensitized OA-exposed rats treated with IgG control antibody), while DB-1 antibody did not significantly increase bronchial responsiveness to ACh in sensitized OA-exposed rats (Fig. 1). DB-1 antibody had no effect on bronchial responsiveness in sensitized and saline-exposed rats.
Figure 1.
(a,b) Mean percentage increase in lung resistance to increasing concentrations of acetylcholine (ACh) for six different groups of sensitized rats: SS, normal mouse IgG-treated and saline-exposed, n=6; SO, normal mouse IgG-treated and 1% OA-challenged, n=6; SSifn, exogenous IFN-γ-treated and saline-exposed, n=4; SOifn, exogenous IFN-γ treated and OA-exposed, n=6; SSdb, anti-IFN-γ antibody DB-1-treated and saline-exposed, n=4; and SOdb, DB-1-treated and OA-exposed, n=6. The effects of exogenous IFN-γ (a) and DB-1 (b) are shown separately. The concentration–response curves are shifted leftward for groups SO, SOifn and SOdb, compared to groups SS, SSifn and SSdb. There was no effect of exogenous IFN-γ or DB-1 antibody on sensitized saline-exposed animals, but IFN-γ reduced, and the DB-1 antibody further increased bronchial responsiveness of sensitized allergen-exposed rats. *P<0·05 as group SO and SOifn, or SOdb compared to groups SS and SSifn, or SSdb; †P<0·04 as SO compared to other groups. Data shown as mean±SEM. (c) Mean –logPC200, which is the negative logarithm of the provocative concentration of ACh needed to increase baseline lung resistance by 200%, for the six groups of rats detailed above. Exogenous IFN-γ reduced, and anti-IFN-γ antibody DB-1-enhanced allergen-induced increase in −logPC200 (P<0·04). *P<0·05 as compared to groups SS, SSifn and SSdb. Data shown as mean±SEM.
Inflammatory cell response
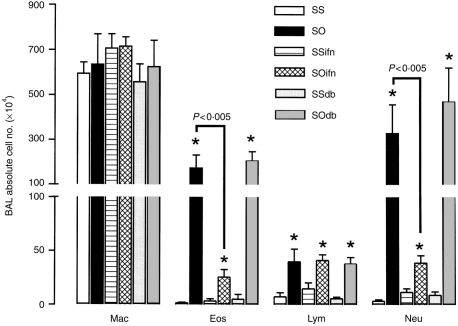
There was a significant increase in the numbers of eosinophils, lymphocytes and neutrophils recovered in BAL fluid of sensitized rats exposed to OA compared to sensitized rats exposed to saline (P<0·01). IFN-γ and DB-1 antibody did not alter the cell profile of sensitized rats exposed to saline, and had no effect on the lymphocyte counts in sensitized, sham-treated rats exposed to OA. However, IFN-γ-treated sensitized OA-exposed rats showed a significant reduction in eosinophil and neutrophil counts in BAL fluid (P<0·005), while DB-1 antibody had no significant effect (Fig. 2). These results indicate that endogenously released IFN-γ did not modulate allergen-induced BAL eosinophilia, while exogenously administered IFN-γ has the capacity to inhibit allergen-induced BAL eosinophilia.
Figure 2.
Mean numbers of total cell, macrophage (Mac), eosinophil (Eos), lymphocyte (Lym) and neutrophil (Neu) in BAL fluid from six different groups of rats as for Fig. 1. Exogenous IFN-γ significantly reduced the increase in eosinophils and neutrophils induced by allergen exposure of sensitized rats in BAL fluid (P<0·005), while the DB-1 antibody caused a further non-significant increase of these cells. Neither treatment had any effect on influx of lymphocytes. Groups as in Fig. 1. *P<0·05 as compared to groups SS, SSifn and SSdb. Data shown as mean±SEM.
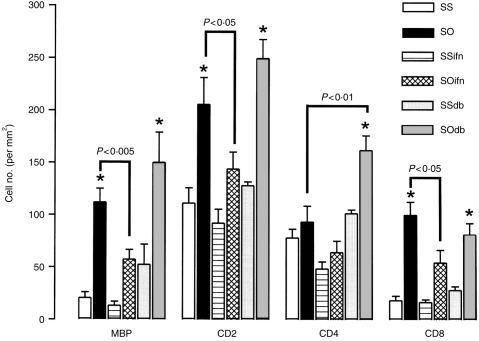
In the airways, allergen exposure of sensitized rats resulted in a significant increase in eosinophil counts, CD2+ T cells, and CD8+ T cells. Eosinophil, CD2+, and CD8+ T cell counts induced by allergen exposure were significantly attenuated from 112·0±13·1 to 57·4±9·4, 205·8±25·6 to 143·9± 16·3/mm2, and from 99·8±12·5 to 54·2±12·2/mm2, respectively, by the exogenous IFN-γ (P<0·05), while reduction in CD4+ T-cell counts from 93·1±15·4 to 64·3±10·9/mm2 was not significant. By contrast, DB-1 antibody enhanced the increase in CD4+ T-cell infiltration in airways from 93·1±15·4 to 161·9±13·9/mm2 (P<0·01), while the eosinophil and CD2+ T-cell counts were not significantly increased (Fig. 3). IFN-γ and the DB-1 antibody had no significant effect on tissue eosinophils and lymphocytes in sensitized and saline-exposed rats.
Figure 3.
Mean eosinophil (MBP-positive) and T-lymphocyte subsets (CD2+, CD4+ and CD8+) counts in airway submucosa expressed as per mm2 of the six groups of rats as detailed in Fig. 1. Allergen exposure of sensitized rats increased the infiltration of eosinophils, as well as T-cell subsets CD2+ and CD8+ T cells. Exogenous IFN-γ treatment reduced the increase of eosinophil, CD2+, and CD8+ cell counts induced by OA exposure of sensitized animals, but had a non-significant inhibitory effect on CD4+ cell infiltration. In contrast, DB-1 antibody further increased CD4+ T cells in tissue (P<0·01). CD8+ T-cell counts were not significantly affected, and eosinophil and CD2+ T-cell counts were not significantly increased after DB-1 antibody treatment of sensitized and OA-exposed rats. Legends as in Fig. 1. *P<0·05 as compared to SS, SSifn and SSdb groups. Data shown as mean±SEM.
Cytokine expression in lungs
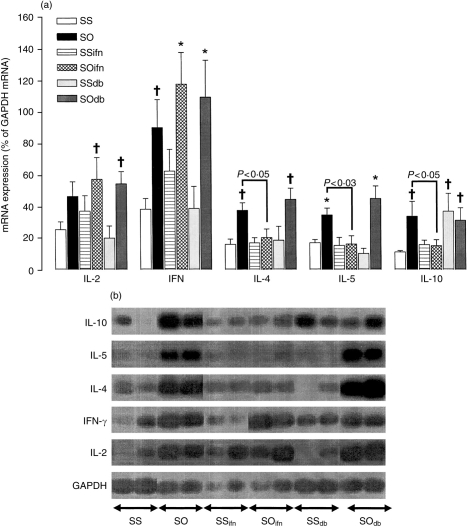
A significant increase in mRNA expression for IFN-γ (P<0·02), as well as for IL-4, IL-5 and IL-10 (P<0·005,<0·005, and <0·02, respectively, as compared to saline-exposed and sham-treated animals), was observed in the lungs of sensitized rats exposed to OA aerosol. Exogenous IFN-γ treatment in OA-exposed rats significantly suppressed the allergen-induced increase in IL-4, IL-5 and IL-10 mRNA expression (P<0·05, 0·03, and 0·05, respectively, as compared to OA-exposed animals). DB-1 antibody did not increase IL-4 and IL-5 mRNA expression in OA-exposed animals, but increased IL-10 mRNA expression in sensitized and saline-exposed animals (P<0·002, Fig. 4). IFN-γ had no significant effect on the cytokine expression profile of sensitized and saline-exposed rats.
Figure 4.
Mean IL-2, IL-4, IL-5, IL-10 and IFN-γ mRNA expression in rat lung expressed as a ratio to GAPDH mRNA (a), as determined by RT-PCR, followed by Southern blot analysis. The expression was obtained on a radioactive probe-hybridized dot-blot of the PCR products. Representative bands from gel electrophoresis of PCR products are shown in (b). OA exposure of sensitized and sham-treated rats increased mRNA expression for all five cytokines examined except for a non-significant increase in IL-2 mRNA. IFN-γ treatment suppressed the increase in IL-4, IL-5 and IL-10 mRNA expression. In contrast, neutralization of IFN-γ with DB-1 antibody resulted in a non-significant increase in IL-4 and IL-5 mRNA expression in OA-exposed rats, and also a significant increase in the expression of IL-10 mRNA in sham-treated and saline-exposed animals. (b) Shows representative hybridized dot-blots of PCR products for these cytokines (two for each group). Legends as in Fig. 1. *P<0·05 as compared to group SS, SSifn and SSdb; †P<0·05 as compared to group SS.
DISCUSSION
We used two different approaches to investigate the potential for IFN-γ in modulating allergen-induced BHR and airway inflammation. Exogenous IFN-γ significantly reduced allergen-induced airway eosinophilia and neutrophilia, CD8+ T-cell recruitment, BHR, along with suppression of IL-4, IL-5 and IL-10 mRNA expression. By contrast, blocking the effects of endogenous IFN-γ with an anti-IFN-γ DB-1 antibody increased CD4+ T cells, while the increases in bronchial responsiveness, eosinophil recruitment to airways and in IL-4 and IL-5 mRNA expression were not significant. Therefore, endogenously produced IFN-γ contributes to some extent to protecting against allergen-induced BHR, and exogenous IFN-γ does not completely inhibit BHR and eosinophilia induced by allergen exposure.
The major sources of IFN-γ are CD4+ Th1 lymphocytes, CD8+ T cells, and natural killer cells. IFN-γ inhibits IgE production by murine and human lymphocytes induced by IL-4 in vitro,10 and in mice in vivo,9 and suppresses the development of Th2 clones by allergen stimulation.11,12 In the Brown–Norway rat model, IFN-γ administration clearly inhibited the mRNA expression of the Th2-derived cytokines, IL-4, IL-5 and IL-10. This effect of IFN-γ could underlie the mechanisms by which it inhibits airway eosinophilia since IL-5 is important in the terminal differentiation and activation of eosinophils.7 Our results are similar to those obtained in mice where IFN-γ administration inhibits allergen-induced airway eosinophilia and hyperresponsiveness.9,13,14,22 Liposome-mediated gene transfer of IFN-γ to the pulmonary epithelium in sensitized mice before secondary allergen exposure also reduced the pulmonary allergic response.23 Although it has been suggested that IFN-γ may be inhibitory by inducing the formation of IL-10,24 our studies on the contrary indicate that IFN-γ inhibits IL-10 mRNA expression. This is in agreement with the in vitro observation that an anti-IFN-γ antibody enhances IL-10 mRNA expression in rat CD8+ T cells.25 It is unlikely that the effects we have observed could have resulted from an inhibition of IgE synthesis through a suppression of IL-4 expression.10 Both IFN-γ and the anti-IFN-γ antibody were administered ≈19 days after sensitisation and within a short period prior to allergen exposure during a period when the IgE levels are known to be well-established after sensitization.26 Indeed, the inhibitory effect of IFN-γ observed in a previous study in mice may have involved IgE suppression since the IFN-γ was administered during the period of sensitization.9
The mechanism(s) by which IFN-γ improves or protects against allergen-induced BHR are unknown. IFN-γ did not completely inhibit BHR induced by allergen exposure. Because we used only one dose of IFN-γ, it is possible that larger doses could have provided a larger inhibitory effect. In the present study, we used exogenous IFN-γ at a dose of 105 U/rat over 3 days, comparable to the dose of 4×102–4×104 U/ animal in the study of Nagai et al. in mice with weights around one-tenth those of rats and who showed complete inhibition of allergen-induced BHR and eosinophilia.14 In rats, a single dose of 105 U/kg of IFN-γ treatment is enough to change tissue antigenicity, in terms of increase of tissue dendritic cells and induction of class II major histocompatibility complex (MHC) antigens in capillary endothelial cells in rats, for at least 5 days,27 and one single dose of 5×104 U/rat of IFN-γ altered the severity of antigen-induced juvenile arthritis.28 Another possibility is that other mediators are involved in BHR, particularly the Th2 cytokines IL-4 and IL-5 which have been implicated in BHR,29–31 and that IFN-γ inhibited their expression and effects on BHR. It is tempting to speculate that the inhibitory effect of IFN-γ on BHR may be mediated by its action in inhibiting eosinophil recruitment. However, we observed no significant increase in airway and BAL eosinophilia with the anti-IFN-γ antibody treatment, despite a significant enhancement of BHR. No clear relationship between eosinophilia and BHR has been demonstrated in other studies in the Brown–Norway rat. For example, inhibition of allergen-induced eosinophilia with the immunosuppressant cyclosporin A was not accompanied by a reduction in BHR,32 while inhibition of BHR has been observed in the absence of any reduction in airway eosinophilia following anti-intracellular adhesion molecule type 1 (ICAM-1) antibody treatment.33 Therefore, the relationship between eosinophil recruitment and BHR is not so mutually important as previously thought, which brings into question the essential role of the eosinophil in allergen-induced BHR.
In order to suppress the effects of endogenously produced IFN-γ, we used an anti-IFN-γ antibody (DB-1 antibody) which possesses efficient neutralizing effects against rat and mouse IFN-γ in terms of antiviral activity.18 An antibody to IFN-γ increased the antigen-induced increase in eosinophil infiltration in mouse trachea.12 Our data are consistent with these findings in that we found a further, though not statistically significant, increase in eosinophil counts in allergen-exposed rats, accompanied by a significant increase in airway CD4+ T-cell recruitment, most of which are likely to be Th2 cells, as indicated by the profile of mRNA expression. A similar increase in Th2 lymphocytes was observed, together with a prolonged lung eosinophilia, when mice lacking the IFN-γ receptor were sensitized and exposed to allergen.34 Another inflammatory cell of interest was the neutrophil, which is recruited after allergen exposure. Neutrophils have been implicated in the induction of BHR,35,36 but the relationship between neutrophilia and BHR is still not well established. Neutrophil infiltration is not a constant finding in subjects with asthma, while in the Brown–Norway rat model, neutrophil influx induced by allergen is usually prominent.15,16,21,32 Exogenous IFN-γ treatment significantly reduced BAL neutrophilia; while anti-IFN-γ treatment had no significant effect. The mechanisms by which IFN-γ affects neutrophil recruitment is not clear. Nevertheless, the effect of IFN-γ on neutrophils may not be through suppression of Th2 responses or eosinophilia, since in a previous study, cyclosporin A did not inhibit neutrophilia despite a suppression of eosinophilia and IL-4 and IL-5 mRNA expression in this model.37 Whether there is another pathway between neutrophilia and BHR, independent of Th2 responses and eosinophilia, is not known.
By contrast to our results and to those of others,12–14 one study reported that anti-IFN-γ antibody reduces BHR in a murine model of allergic asthma without affecting eosinophilia, indicating that IFN-γ induced BHR.38 However, in a subsequent study, the same group found that aerosolized or parenteral IFN-γ inhibited BHR.22 These studies indicate that IFN-γ may have both inhibitory and potentiating effects on BHR. While a beneficial effect of IFN-γ may occur through the inhibition of Th2 cytokines, IL-4 and IL-5, IFN-γ has also pro-inflammatory effects. Thus, IFN-γ can also activate alveolar macrophages to produce tumour necrosis factor-γ,39,40 and epithelial cells to induce the expression of ICAM-1,41 stimulate the production of IL-1, platelet activating factor and hydrogen peroxide from monocytes,42,43 and the proinflammatory cytokine IL-6 from eosinophils.44 Indeed, many of these cytokines have been implicated in the pathogenesis of BHR.44–46 This dual effect of IFN-γ on BHR may also underlie the partial inhibitory effect of anti-IFN-γ antibody observed in the current study.
CD8+ T lymphocytes were increased in the airway submucosa following exposure to allergen, and these numbers were attenuated following administration of IFN-γ and increased after anti-IFN-γ antibody. A reduction of CD8+ T cells using an anti-CD8+ antibody led to a reduction in the expression of IFN-γ mRNA in the lungs of Brown–Norway rats,21 which is possible since antigen-specific CD8+ T cells produce IFN-γ, and circulating CD8+ T cells are a major source of IFN-γ in the rat.47,48 CD8+ T cells appear to be protective against bronchial hyperresponsiveness and the induction of airway eosinophilia,21,49 probably through the expression of the Th1 cytokine, IFN-γ. Because IFN-γ in the present study reduced the number of CD8+ T lymphocytes, this could represent another factor that may enhance BHR. Alternatively, this reduction may reflect a feedback inhibition of CD8+ T-cell activation or may result from an inhibition of Th2-derived growth factors, such as IL-4, necessary for CD8+ T cells.25
We conclude that IFN-γ plays a protective role in the pathogenesis of allergen-induced BHR and airway inflammation. The protective function via IFN-γ might be conducted by Th1 T lymphocytes or CD8+ T-cells, to suppress Th2 cells, eosinophils and neutrophils, and reduce the Th2 response-orchestrated airway inflammation and BHR. The inhibition of BHR by IFN-γ is unlikely to be mediated through the action of eosinophils, and the underlying mechanism of action remains unclear but deserves further study.
Abbreviations
- ACh
acetylcholine
- BAL
bronchoalveolar lavage
- BHR
bronchial hyperresponsiveness
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IFN-γ
interferon-γ, IL, interleukin
- i.p.
intraperitoneal
- i.v.
intravenous
- MBP
major basic protein
- OA
ovalbumin
- PBS
phosphate-buffered saline
- PC200
provocative concentration of acetylcholine needed to increase lung resistance by 200% above baseline
- RL
lung resistance
- RT-PCR
reverse transcription–polymerase chain reaction
- Th
T-helper
References
- 1.Azzawi M, Bradley B, Jeffery PK, et al. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990;142:1407. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- 2.Haczku A, MacAry P, Huang T-J, et al. Adoptive transfer of allergen-specific CD4+ T-cells induces airway inflammation and hyperresponsiveness in Brown-Norway rats. Immunology. 1997;91:176. doi: 10.1046/j.1365-2567.1997.d01-2221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe A, Mishima H, Renzi PM, Xu L-J, Hamid Q, Martin JG. Transfer of allergic airway responses with antigen-primed CD4+ but not CD8+ T-cells in Brown-Norway rats. J Clin Invest. 1995;96:1303. doi: 10.1172/JCI118165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X-M, Schofield BH, Wang Q-F, Kim K-H, Huang S-K. Induction of pulmonary allergic responses by antigen-specific Th2 cells. J Immunol. 1998;160:1378. [PubMed] [Google Scholar]
- 5.Hamid Q, Azzawi M, Ying S, et al. Expression of mRNA for interleukins in mucosal bronchial biopsies from asthma. J Clin Invest. 1991;87:1541. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romagniani S. Regulation and deregulation of human IgE synthesis. Immunol Today. 1990;11:316. doi: 10.1016/s0167-5699(10)80004-0. [DOI] [PubMed] [Google Scholar]
- 7.Sanderson CJ. Interleukin-5, eosinophils and disease. Blood. 1992;79:3101. [PubMed] [Google Scholar]
- 8.Nurse B, Haus M, Puterman AS, Meinberg EG, Potter PC. Reduced interferon-γ but normal IL-4 and IL-5 release by peripheral blood mononuclear cells from Xhosa children with atopic asthma. J Allergy Clin Immunol. 1997;100:662. doi: 10.1016/s0091-6749(97)70171-4. [DOI] [PubMed] [Google Scholar]
- 9.Lack G, Bradley KL, Hamelmann E, et al. Nebulized IFN-γ inhibits the development of secondary allergic responses in mice. J Immunol. 1996;157:1432. [PubMed] [Google Scholar]
- 10.Maggi E, Parronchi P, Manetti R, et al. Reciprocal regulatory effects of IFN-γ and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992;148:2142. [PubMed] [Google Scholar]
- 11.Noble A, Staynov DZ, Kemeny DM. Generation of rat Th2-like cells in vitro is interleukin-4-dependent and inhibited by interferon-γ. Immunology. 1993;79:562. [PMC free article] [PubMed] [Google Scholar]
- 12.Iwamoto I, Nakajima H, Endo H, Yoshida S. Interferon γ regulates allergen-induced eosinophil recruitment into the mouse airways by inhibiting the infiltration of CD4+ T cells. J Exp Med. 1993;177:573. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakajima H, Iwamoto I, Yushida S. Aerosolized recombinant interferon-gamma prevents antigen-induced eosinophil recruitment in mouse trachea. Am Rev Respir Dis. 1993;148:1102. doi: 10.1164/ajrccm/148.4_Pt_1.1102. [DOI] [PubMed] [Google Scholar]
- 14.Nagai H, Maeda Y, Tanaka H. The effect of anti-IL-4 monoclonal antibody, rapamycin and interferon-γ on airway hyperreactivity to acetylcholine in mice. Clin Exp Allergy. 1997;27:218. [PubMed] [Google Scholar]
- 15.Elwood W, Lotvall JO, Barnes PJ, Chung KF. Characterisation of allergen-induced inflammation and bronchial hyperresponsiveness in sensitised Brown-Norway rats. J Allergy Clin Immunol. 1991;88:951. doi: 10.1016/0091-6749(91)90253-k. [DOI] [PubMed] [Google Scholar]
- 16.Haczku A, MacAry P, Haddad E-B, et al. Expression of Th-2 cytokines interleukin-4 and -5 and of Th-1 cytokine interferon-γ in ovalbumin-exposed sensitised Brown-Norway rats. Immunology. 1996;88:247. doi: 10.1111/j.1365-2567.1996.tb00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole TB, Robinson R. The UFAW Handbook on the Care and Management of Laboratory Animals. 6. London: Longman Scientific and Technical; 1987. [Google Scholar]
- 18.Van Der Meide PH, Dubbeld M, Vijverberg K, Kos T, Schellekens H. The purification and characterization of rat gamma interferon by use of two monoclonal antibodies. J Gen Virol. 1986;67:1059. doi: 10.1099/0022-1317-67-6-1059. [DOI] [PubMed] [Google Scholar]
- 19.Haczku A, Moqbel R, Jacobson M, Kay AB, Barnes PJ, Chung KF. T-cells subsets and activation in bronchial mucosa of sensitised Brown-Norway rats after single allergen exposure. Immunology. 1995;85:591. [PMC free article] [PubMed] [Google Scholar]
- 20.Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidium thiocyanate-phenol chloroform extraction. Anal Biochem. 1987;162:156. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 21.Huang T-J, MacAry PA, Kemeny DM, Chung KF. Effect of CD8+ T-cell depletion on bronchial hyperresponsiveness and inflammation in sensitised and allergen-exposed Brown-Norway rats. Immunology. 1999;96:416. doi: 10.1046/j.1365-2567.1999.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofstra CL, Van Ark I, Hofman G, Nijkamp FP, Jardieu PM, Van Oosterhout AJ. Differential effects of endogenous and exogenous interferon-gamma on immunoglobulin E, cellular infiltration, and airway responsiveness in a murine model of allergic asthma. Am J Respir Cell Mol Biol. 1998;19:826. doi: 10.1165/ajrcmb.19.5.3027. [DOI] [PubMed] [Google Scholar]
- 23.Li X-M, Chopra RK, Chou T-Y, Schofield BH, Wills-Karp M, Huang S-K. Mucosal IFN-γ gene transfer inhibits pulmonary allergic responses in mice. J Immunol. 1996;157:3216. [PubMed] [Google Scholar]
- 24.Chomarat P, Rissoan MC, Banchereau J, Miossec P. Interferon gamma inhibits interleukin 10 production by monocytes. J Exp Med. 1993;177:523. doi: 10.1084/jem.177.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noble A, MacAry PA, Kemeny DM. IFN-γ and IL-4 regulate the growth and differentiation of CD8+ T-cells into subpopulations with distinct cytokine profiles. J Immunol. 1995;155:2928. [PubMed] [Google Scholar]
- 26.Pauwels R, Bazin H, Platteau B, Van Der Straeten M. Effect of antigen dose on the secondary IgE response in Brown-Norway rats. Int Arch Allergy Appl Immunol. 1978;57:472. doi: 10.1159/000232139. [DOI] [PubMed] [Google Scholar]
- 27.Leszczynski D, Ferry B, Schellekens H, Van Der Meide PH, Hayry P. Antagonistic effects of γ interferon and steroids on tissue antigenicity. J Exp Med. 1986;164:1470. doi: 10.1084/jem.164.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacob CO, Holoshitz J, Van Der Meide P, Strober S, McDevitt HO. Heterogeneous effects of IFN-γ in adjuvant arthritis. J Immunol. 1989;142:1500. [PubMed] [Google Scholar]
- 29.Hogan SP, Koskinen A, Matthaei KI, Young IG. Interleukin-5-producing CD4+ T-cells play a pivotal role in aeroallergen-induced eosinophilia, bronchial hyperreactivity, and lung damage in mice. Am J Respir Crit Care Med. 1998;157:210. doi: 10.1164/ajrccm.157.6.mar-1. [DOI] [PubMed] [Google Scholar]
- 30.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and and lung damage in a mouse asthma model. J Exp Med. 1996;183:195. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corry DB, Folkesson HG, Warnock ML, et al. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183:109. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elwood W, Lotvall JO, Barnes PJ, Chung KF. Effect of dexamethasone and cyclosporin A on allergen-induced airway hyperresponsiveness and inflammatory cell response in sensitized Brown-Norway rats. Am Rev Respir Dis. 1992;145:1289. doi: 10.1164/ajrccm/145.6.1289. [DOI] [PubMed] [Google Scholar]
- 33.Sun J, Elwood W, Haczku A, Barnes PJ, Hellewell PG, Chung KF. Contribution of intercellular-adhesion molecule-1 in allergen-induced airway hyperresponsiveness and inflammation in sensitised Brown-Norway rats. Int Arch Allergy Immunol. 1994;104:291. doi: 10.1159/000236679. [DOI] [PubMed] [Google Scholar]
- 34.Coyle AJ, Tsuyuki S, Bertrand C, et al. Mice lacking the IFN-γ receptor have an impaired ability to resolve a lung eosinophilic inflammatory response associated with a prolonged capacity of T-cells to exhibit a Th2 cytokine profile. J Immunol. 1996;156:2680. [PubMed] [Google Scholar]
- 35.O’Byrne PH, Walters EH, Gold BD, et al. Neutrophil depletion inhibits airway hyperresponsiveness induced by ozone exposure. Am Rev Respir Dis. 1984;130:214. doi: 10.1164/arrd.1984.130.2.214. [DOI] [PubMed] [Google Scholar]
- 36.Murphy KR, Wilson MC, Irvin CG, et al. The requirement for polymorphonuclear leukocytes in the late asthmatic response and heightened airway reactivity in an animal model. Am Rev Respir Dis. 1986;134:62. doi: 10.1164/arrd.1986.134.1.62. [DOI] [PubMed] [Google Scholar]
- 37.Huang T-J, Newton R, Haddad E-B, Chung KF. Differential regulation of cytokine expression after allergen exposure of sensitized rats by cyclosporin A and corticosteroids: Relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol. 1999. in press. [DOI] [PubMed]
- 38.Hessel FM, Van Oosterhout AJ, Van Ark I, et al. Development of airway hyperresponsiveness is dependent on interferon-gamma and independent of eosinophil infiltration. Am J Respir Cell Mol Biol. 1997;16:325. doi: 10.1165/ajrcmb.16.3.9070618. [DOI] [PubMed] [Google Scholar]
- 39.Jaffe HA, Buhl R, Mastrangeli A, et al. Organ specific cytokine therapy: local activation of mononuclear phagocytes by delivery of an aerosol of recombinant interferon-γ to the human lung. J Clin Invest. 1991;88:397. doi: 10.1172/JCI115291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gifford GE, Lohmann-Matthess ML. Gamma interferon priming of mouse and human macrophages for induction of tumor necrosis factor production by bacterial lipopolysaccharide. J Natl Cancer Inst. 1987;78:121. doi: 10.1093/jnci/78.1.121. [DOI] [PubMed] [Google Scholar]
- 41.Look DC, Rapp SR, Keller BT, Holzman MJ. Selective induction of intracellular adhesion molecule-1 by interferon-gamma in human airway epithelial cells. Am J Physiol. 1992;263:L79. doi: 10.1152/ajplung.1992.263.1.L79. [DOI] [PubMed] [Google Scholar]
- 42.Billiau A, Dijkmans R. Interferon-gamma: mechanism of action and therapeutic potential. Biochem Pharmacol. 1990;40:1433. doi: 10.1016/0006-2952(90)90437-p. [DOI] [PubMed] [Google Scholar]
- 43.Sen GC, Lenggel P. The interferon system: a bird’s view of its biochemistry. J Biol Chem. 1992;267:5017. [PubMed] [Google Scholar]
- 44.Valerius T, Repp P, Kalden JR, Platzer E. Effects of IFN on human eosinophils in comparison with other cytokines: a novel class of eosinophil activators with delayed onset of action. J Immunol. 1990;145:2950. [PubMed] [Google Scholar]
- 45.Pennings HJ, Kramer K, Bast A, Buurman WA, Wouters EF. Tumor necrosis factor-alpha induces hyperreactivity in tracheal smooth muscle of the guinea pig in vitro. Eur Respir J. 1998;12:45. doi: 10.1183/09031936.98.12010045. [DOI] [PubMed] [Google Scholar]
- 46.Dicosmo BF, Geba GP, Picarella D, et al. Airway epithelial cell expression of interleukin-6 in transgenic mice. Uncoupling of airway inflammation and bronchial hyperreactivity. J Clin Invest. 1994;94:2028. doi: 10.1172/JCI117556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salgame P, Abrams JS, Clayberger C, et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 48.McMenamin C, Holt PG. The natural immune response to inhaled soluble protein antigen involves major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but MHC class II-restricted CD4+ T cell-dependent immune deviation resulting in selective suppression of immunoglobulin E production. J Exp Med. 1993;178:889. doi: 10.1084/jem.178.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renz H, Lack G, Saloga J, et al. Inhibition of IgE production and normalisation of airways responsiveness by sensitised CD8 T-cells in a mouse model of allergen-induced sensitisation. J Immunol. 1994;152:351. [PubMed] [Google Scholar]