Abstract
CD97 is a newly identified, activation-associated human leucocyte antigen with seven putative transmembrane domains. It has an extended extracellular segment containing several adhesion molecule structure motifs, and has been shown to interact with the human complement regulator, decay-accelerating factor (DAF, CD55). To understand further the interaction between CD97 and DAF, as well as the structure and function of CD97 in general, we have cloned the mouse CD97 cDNA and studied the encoded protein for its membrane association property and ability to interact specifically with the murine decay-accelerating factor. The full-length mouse CD97 cDNA that we have cloned and characterized encodes a protein that is 60% identical to the three epidermal growth factor (EGF) domain-containing form of human CD97 but does not contain the Arg-Gly-Asp (RGD) motif which is present in human CD97. Two other alternatively spliced forms of mouse CD97 were also identified. These forms differ by the number of EGF-like sequence repeats present in the N-terminal region. Northern blot analysis revealed that CD97 is expressed widely in mouse tissues and in resting as well as activated cultured mouse splenocytes. Transient transfection of human embryonic kidney (HEK) 293 cells with the mouse CD97 cDNA in a green-fluorescence protein vector (pEGFP-N1) showed plasma membrane targeting of the expressed protein. Western blot analysis confirmed its membrane association and identified the existence of a processed C-terminal fragment, supporting the notion that CD97 on the cell membrane is composed of post-translationally generated subunits. Adhesion studies demonstrated that normal, but not DAF knockout mouse erythrocytes and splenocytes adhered to mouse CD97-transfected HEK cells. The interaction of CD97 and DAF was found to be species-restrictive in that human erythrocytes were unable to bind to mouse CD97-transfected HEK cells. These results indicate that the general structure, membrane association property and DAF-binding ability of CD97 are conserved and that the adhesive interaction between CD97 and DAF is independent of the RGD motif. The finding that CD97 is distributed widely among various mouse tissues suggests that CD97 may have other roles beyond lymphocyte activation.
INTRODUCTION
CD97 is a leucocyte antigen originally recognized on activated human lymphocyte.1,2 Activation of lymphocytes by a variety of stimuli rapidly induces the surface expression of CD97.1,2 This up-regulation apparently results from a redistribution of preformed CD97 protein as well as from increased mRNA synthesis at the transcriptional level.1–3 Molecular cloning studies of the human CD97 cDNA have revealed some highly unusual structural features of the encoded protein. Human CD97 was predicted to be a seven-transmembrane protein with sequence homology to the secretin receptor superfamily of G-protein-coupled receptors.2,4 However, unlike the previously characterized peptide hormone receptors, human CD97 has an extended extracellular domain containing multiple epidermal growth factor (EGF)-like repeats, calcium-binding sites and an Arg-Gly-Asp (RGD) motif.2,4 Furthermore, biochemical studies have revealed that human CD97 exists on the cell membrane as a processed heterodimer, consisting of an extracellular α-subunit non-covalently linked to a seven-transmembrane β-subunit.4
Since EGF-like domains, calcium-binding sites and the RGD motif are all typical structural features of proteins involved in protein–protein interaction,5,6 a role for human CD97 as an adhesion molecule was initially suspected and later established.2,7 Thus, human decay-accelerating factor (DAF, CD55), a glycosylphosphatidylinositol (GPI)-anchored membrane regulator of complement activation has been identified as a ligand for human CD97.7,8 Besides its potential role in cell adhesion, the fact that human CD97 is a seven-transmembrane protein with sequence homology to G-protein-coupled receptors also suggests that the protein may function as a cell surface receptor to participate in signal transduction.2,4,9 At the present time, however, the significance of its interaction with human DAF and the physiological role of human CD97 in general remain a matter of speculation. A cell-free form of CD97 was found to be present in body fluids from inflamed human tissues,4 raising the possibility that the RGD-motif-containing CD97 α-subunit may be shed from the cell membrane and function as a ligand during inflammatory reactions.4 To facilitate the functional study of CD97 in an animal model, and to extend the structural characterization of human CD97, we have cloned and characterized the mouse CD97 cDNA. Here, we report the deduced amino acid sequences of multiple alternatively spliced forms of mouse CD97, its tissue expression pattern and membrane association characteristics. We also describe our characterization of the specific interaction between mouse CD97 and mouse DAF using erythrocytes and splenocytes from DAF knockout mice recently generated in our laboratory.10
MATERIALS AND METHODS
Cloning of mouse CD97 cDNAs
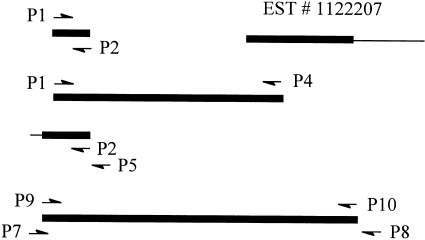
Human CD97 cDNA was used as a probe to screen a murine 129J genomic library to clone the mouse CD97 gene. One genomic fragment was initially isolated and selective sequencing analysis revealed the nucleotide sequence of exons 6 and 7 (Haino et al., unpublished data). Two oligonucleotide primers, 5′-TGTTCCCAGATGTGAATGAGTG-3′ (P1, upstream) and 5′-CTTCCAACCTGGACGGCAGT-3′ (P2, downstream), which correspond to the 5′-end and 3′-end of exon 6 and 7, respectively, were synthesized and used to amplify a 257-base-pair (bp) cDNA fragment by reverse-transcription polymerase chain reaction (RT-PCR). Parallel to the above experiment, the human CD97 cDNA sequence was used as a query sequence to search the European Molecular Biology Laboratory (EMBL) expressed sequence tag (EST) database for related mouse cDNA sequences. One mouse EST clone (ID number: 1122207) was found to contain a cDNA sequence highly homologous to exon 13 of human CD97,4 and the concerned cDNA clone was obtained from Research Genetics, Inc (Huntsville, AL) and analysed. A downstream primer, 5′-TCAGGTCGGTCGTCATACAAC-3′ (P4), based on the EST #1122207 sequence information was synthesized and used in conjunction with primer P1 to amplify by RT-PCR a 1·6-kb cDNA fragment. To obtain the remaining 5′-half of the mouse CD97 cDNA, the 5′-RACE (Rapid Amplification of cDNA Ends) protocol11 was employed with a Marathon cDNA amplification kit from Clontech (Palo Alto, CA). The two nested specific downstream primers used were P2 and P5 (5′-GACCACGGAGGG-ACCCAGAAGTT-3′).
The complete cDNA sequence of mouse CD97, which contains three EGF-like sequence repeats, was deduced from the overlapping PCR clones and the commercial cDNA clone. Based on the deduced cDNA sequence, new primers were synthesized to amplify the full coding region of mouse CD97 in a two-step nested RT-PCR reaction. The four primers used in the nested RT-PCR reaction were 5′-GTCCCAGACGCTGTCCGTT-3′ (P7) and 5′-GCTGCTGTCCTTCATGGAACT-3′ (P8) for the first round, and 5′-CACCATGAGGGGCGTCAGAT-3′ (P9) and 5′-GCCTTCACATCCCTGATTCTG-3′ (P10) for the second round. In order to increase DNA amplification fidelity, PCR reactions were performed with a proof-reading polymerase mixture (Pfu/Taq, 1/6). All amplified PCR fragments were cloned into the pCR2 vector (TA cloning Kit, Invitrogen, Palo Alto, CA) and sequenced on an automatic DNA sequencer (Cell Center, University of Pennsylvania).
Northern blot analysis of CD97 expression in mouse tissues
Total RNAs from various mouse tissues or from cultured splenocytes of normal and DAF knockout mice10 were isolated using the Trizol Reagent (Gibco/BRL, Grand Island, NY). Splenocytes were prepared by grinding macerated fresh spleen in cold Hanks’ balanced salt solution. The tissue mixture was first passed through a cell strainer and splenocytes were collected by low-speed centrifugation (500 g). Contaminating erythrocytes were removed by lysis in ACK lysing buffer [150 mm NH4Cl, 1 mm KHCO3, 0·1 mm ethylenediaminetetraacetic acid (EDTA), pH 7·3]. Cells (106/ml) were then cultured in Xvivo 20 (Biowhitka, Walkersville, MD) for various lengths of time in the presence of anti-CD3 (0·5 μg/ml) as a T-lymphocyte activator. RNA samples (10 μg each lane) were separated on a 1·0% formaldehyde–agarose gel and transferred onto a nylon membrane (Hybond-N, Amersham, Arlington Heights, IL) via capillary action overnight in 5×sodium–sodium citrate buffer (SSC). Membranes were cross-linked under ultraviolet (UV) light and hybridized with a 32P-labelled full-length mouse CD97 cDNA probe synthesized with random primers. RNA hybridizations were carried out in QuikHyb solution (Stratagene, La Jolla, CA) at 68° for 1 hr. The membranes were washed, first in 2×SSC–0·1% sodium dodecyl sulphate (SDS) at 55° for 15 min and then in 0·1×SSC–0·1% SDS at 55°, and exposed to X-ray film.
Construction of mouse CD97 expression plasmids
The form of mouse CD97 containing three EGF domains was expressed in HEK 293 cells for cell adhesion and membrane association studies. For cell adhesion studies, the full-coding cDNA was subcloned into the mammalian expression vector pCDNA3 (Invitrogen) at the EcoRI site. Plasmid DNA of pCDNA3 containing mouse CD97 in the sense or antisense orientation was prepared using a Qiagen (Chatsworth, CA) plasmid purification kit (CD97 in the antisense orientation was used as a negative control in transfection experiments). For membrane localization studies, the mouse CD97 was expressed as a fusion protein with the green fluorescence protein (GFP) attached to its C-terminal. For this purpose, PCR reaction was performed to remove the stop codon in the mouse CD97 cDNA and to add the sequence GGATCC which corresponds to a BamHI site to facilitate subcloning. The modified coding sequence of mouse CD97 cDNA was first cloned into the pCR2 vector and then subcloned into the GFP-fusion protein expression vector pEGFP-N1 (Clontech, Palo Alto, CA) at EcoRI and BamHI sites.
Membrane targeting studies
For transient transfection of HEK 293 cells with the mouse CD97-GFP fusion protein construct, 1×105 cells were seeded onto a two-well chamber slide (Lab-Tek, Scotts Valley, CA) in complete Dulbecco’s modified Eagle’s minimal essential medium (DMEM) containing 10% fetal bovine serum (FBS). Cells were transfected using Lipofectamine Plus (Life Technologies, Grand Island, NY) 24 hr later. Briefly, 0·75 μg of pEGFP-N1-CD97 plasmid or pEGFP-N1 vector control DNA was mixed with 5 μl PLUS reagent and 3 μl Lipofectamine and added to the cells in 0·6 ml Opti-Mem (Life Technologies). After 3 hr incubation, an equal volume (0·6 ml) of DMEM containing 20% FBS was added. After 48 hr, the cells were fixed with 4% paraformadehyde in situ and the slide was mounted with Aqua Polymount (Biomedia Corp., Foster City, CA). GFP protein (vector transfection) or GFP-CD97 fusion protein was visualized under a confocal fluorescence microscope. In separate experiments, HEK 293 cells were grown in 100-mm plates and 4 μg of plasmid DNA (either pEGFP-N1 or pEGFP-N1-CD97) was used for transient transfection as described above. After 48 hr, membrane and cytosolic proteins were prepared from the transfected cells for Western blot analysis using a monoclonal antibody against GFP (Berkeley Antibody Co., Richmond, CA). Cells were first sonicated in ice-cold buffer (20 mm Tris, pH 8·0, 1 mm EDTA, 1 mm dithiothreitol, 1 mm phenylmethylsulphonyl fluoride). The cell lysate was then centrifuged at 10 000 g for 15 min to collect the cytosolic fraction. The pellet containing membrane fragments was re-suspended and taken as the membrane fraction. Denatured membrane and cytosolic proteins were electrophoresed on 10% SDS–polyacrylamide gels (10 μg per lane), transferred onto Hybond enhanced chemiluminescence (ECL) nitrocellulose membranes (Amersham, Life Science) and probed with the GFP monoclonal antibody. Immunodetection was performed with the ECL Western blotting detection system from Amersham.
Cell adhesion assays
HEK cells grown in 35-mm plates were transiently transfected as described above with pCDNA3 containing mouse CD97 in the sense or antisense (negative control). After 48 hr, 1×108 mouse or human erythrocytes or 1×107 mouse splenocytes were overlaid onto the transfected cells. Mouse erythrocytes were obtained by tail vein bleeding from normal and DAF knockout mice (C57B6/129J mixed background).10 Human erythrocytes were obtained from a healthy volunteer. The plates were left undisturbed for 30 min at room temperature (22°) and non-adhering erythrocytes or splenocytes were removed afterwards by gentle washing with PBS (three times). The degree of erythrocyte binding was quantified by measuring haemoglobin release after hypotonic lysis of the bound erythrocytes (optical density measurement at λ415). A similar strategy was used for determining the degree of splenocyte binding. For this purpose, splenocytes (freshly prepared or treated with anti-CD3 for 18 hr) were first loaded with a fluorescent dye, calcein-AM (Molecular probes Inc., Eugene, OR; 1 mg/ml in DMEM), for 1 hr at 37°. They were then washed three times in PBS and used in the binding assay. Bound splenocytes were lysed with 0·1% Triton X-100 and the amount of fluorescent dye released was determined by a fluorospectrophotometer (excitation: λ485, emission: λ525) and used as measure of splenocyte binding.
RESULTS
To clone the mouse CD97 cDNA, RT-PCR reactions were performed to amplify overlapping cDNA sequences. Based on a partial genomic sequence (Haino et al., unpublished results), two primers, P1 and P2, were used to generate a 257-bp fragment by RT-PCR using total RNA isolated from the mouse testis (Fig. 1). Simultaneously, scanning of the EMBL expressed sequence tag (EST) data bank using human CD97 cDNA sequence as a reference identified a mouse cDNA clone (ID #1122207) whose 5′-sequence is highly homologous to exon 13 of human CD97.4 The cDNA clone was obtained from Research Genetics Inc and its insert was analysed by sequence analysis. This confirmed that EST #1122207 represented a 1·2-kb partial cDNA clone of mouse CD97.
Figure 1.
Schematic representation of overlapping mouse CD97 cDNA clones. Coding regions are shown as black horizontal bars and non-coding regions as thin lines. The position and direction of PCR primers used are indicated by arrows. The 5′-ends of the cDNAs are to the left and the 3′-ends are to the right.
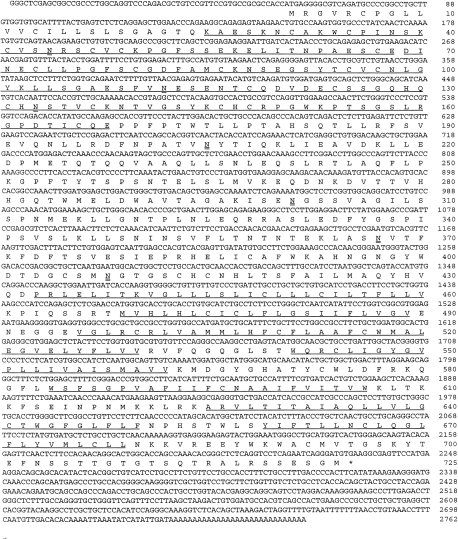
Using the sequence information of EST #1122207, a downstream primer P4 was synthesized and used with P1 to generate a 1·6-kb cDNA fragment by RT-PCR (Fig. 1). The fragment was cloned into the pCR2 vector and its sequence was determined. Subsequently, a new primer P5 (3′ to P2) was synthesized and used with P2 in a nested PCR reaction to amplify the remaining 5′-end of the cDNA by the 5′-RACE method.11 This produced a 500-bp cDNA fragment which after cloning and sequence analysis was found to contain the expected translation initiation codon (ATG) and a short (58 bp) 5′-untranslated region (Fig. 1). Subsequently, a 2·2-kb full-coding region containing mouse CD97 cDNA was obtained after nested RT-PCR reactions using primers P7, P8, P9 and P10 (Fig. 1). From these overlapping cDNA clones (EST #1122207 and PCR fragments), a full-length 2762-bp mouse CD97 cDNA was derived (Fig. 2). The complete sequence of this cDNA contains a single open reading frame and is predicted to encode a protein of 724 amino acids with a calculated molecular weight of 80 298. Hydrophobicity analysis indicated seven putative transmembrane domains12 at the C-terminal part of the molecule (Fig. 2, underlined). At its N-terminal end, three EGF-like domains (underlined),5 with two of them containing a calcium-binding site,13,14 were recognized. Thus, this cDNA sequence is homologous to the three EGF-like sequence repeat-containing form of human CD97 cDNA.2,4
Figure 2.
Nucleotide and deduced amino acid sequences of the cloned mouse CD97. The three EGF-like repeats at the N-terminal region and the seven-transmembrane domains at the C-terminal region are underlined. Putative N-glycosylation sites in the extracellular domain of the protein are double underlined.
There are seven putative N-glycosylation sites (Fig. 2, double underlined) in the extracellular region, suggesting that the mouse CD97 may exist as a highly glycosylated protein in vivo. Comparison of the mouse sequence with the three EGF-domain-containing form of human CD97 shows a 60% overall sequence identity (Fig. 3). The highest sequence identity is found in the seven transmembrane and the cytoplasmic tail region (75%), followed by the EGF-like sequence repeats at the N-terminus (62%). The regions between the EGF-like repeats and the putative transmembrane domains only share 46% sequence identity. It is of interest that the Arg-Gly-Asp (RGD) motif present in human CD972,4 is not found in the mouse sequence.
Figure 3.
Alignment of the amino acid sequences of the three EGF domain-containing forms of mouse and human CD97. Identical amino acid residues in the two sequences are indicated. Note that the degree of sequence identity is higher in the N-terminal and C-terminal regions and much lower in the middle part of the sequence.
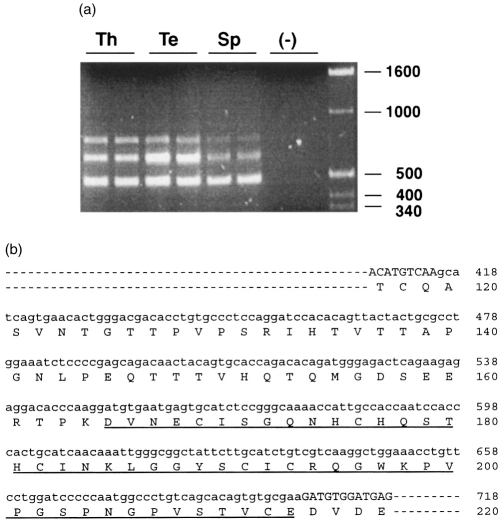
Multiple alternatively spliced forms of human CD97 are known to exist which differ by the number of EGF-like sequence repeats they contain at their N-terminus.4 To determine if the same is true with the mouse CD97, primers (P5 and P9) that flank the region containing the three EGF-like sequence repeats in the initially cloned full-length cDNA (Fig. 2) were used in further RT-PCR experiments. This amplified in several mouse tissues the expected 488-bp fragment (containing the three EGF-like sequence repeats) as well as two larger fragments (Fig. 4a). All three PCR products were cloned and sequenced. The sequences of the three fragments were found to be identical at the 5′- and the 3′-end, but each of the two larger fragments contained an insertion (of different length) between EGF domain 2 and 3 of the full-length sequence shown in Fig. 2. The shorter insertion in the medium-sized fragment clearly corresponds to a fourth EGF domain (Fig. 4b). The longer insertion in the largest fragment contains the same fourth EGF domain plus a 45-amino acid sequence which lacks the critical cysteine residues present in the four EGF-like sequence modules (Fig. 4b).
Figure 4.
Identification of alternatively spliced forms of mouse CD97. RT-PCR of the N-terminal region of mouse CD97 isoforms was performed with primers P5 and P9 (see the Materials and Methods) and with total RNAs from thymus (Th), testis (Te) and spleen (Sp). (a) Three fragments were amplified from all the tissues examined but not from the negative control reaction (−, no first-strand cDNA added). The smallest fragment corresponds to the three EGF domain-containing form of mouse CD97 shown in Fig. 2. Positions of molecular weight markers (in base pairs) are shown on the right. (b) Nucleotide and amino acid sequences of the insertions in the two larger fragments shown in (a). Nucleotide sequence of the insertion is shown in lower case letters. The medium-sized fragment in (a) contains a 147-bp insertion which encodes a fourth EGF-like sequence repeat (underlined). The largest fragment in (a) contains a 282-bp insertion which encodes the same extra EGF-like sequence repeat plus a 45 amino acid sequence. In both fragments, the insertion occurs between EGF-like sequence repeat 2 and 3 of the cloned full-length mouse CD97 shown in Fig. 2. The numbering of nucleotide and amino acid sequences is the same as that shown in Fig. 2.
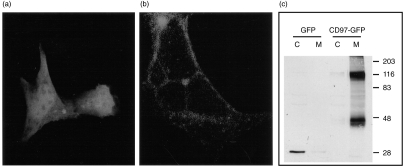
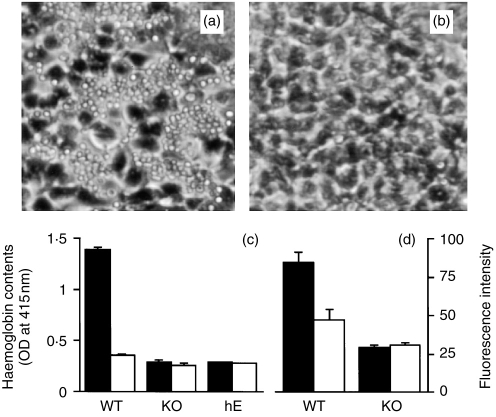
To provide direct evidence that CD97 is a plasma membrane-associated protein, mouse CD97 was expressed in HEK 293 cells as a fusion protein with the green fluorescence protein attached to its C-terminus. The subcellular localization of the expressed CD97-GFP fusion protein was evaluated by direct observation under a confocal fluorescence microscope and by Western blot analysis using a GFP-specific antibody. When HEK 293 cells were transfected with the control GFP vector, GFP was found to be localized to the cytoplasm as expected (Fig. 5a). In contrast, the CD97-GFP fusion protein was found to be targeted almost exclusively to the plasma membrane (Fig. 5b). Thus, the sequence information contained in mouse CD97 was sufficient to convert GFP from a cytosolic protein to a protein anchored on the plasma membrane. On Western blot analysis, the free GFP protein appeared as a narrow band found only in the cytosolic fraction (Fig. 5c). In contrast, the mouse CD97-GFP fusion protein was recovered from the membrane fraction and the protein bands appeared to be broad, suggesting that the fusion protein was glycosylated (Fig. 5c). Additionally, two distinct CD97-GFP fusion protein bands, estimated to be 116 000 MW and 47 000 MW, respectively, were observed on Western blot analysis (Fig. 5c). Since the antibody used in the Western blot analysis was GFP-specific and the GFP protein was localized C-terminal to CD97, the 47 000 MW band must have represented a fusion protein with its N-terminal portion removed.
Figure 5.
Membrane localization of mouse CD97. HEK 293 cells were transfected either with the GFP vector as a control or with the mouse CD97-GFP fusion protein vector. (a) Under a confocal fluorescence microscope, simple GFP protein was shown to be present in the cytoplasm of the cells. (b) In contrast to simple GFP, CD97-GFP fusion protein was shown to be localized to the plasma membrane. (c) Western blot analysis of cytosolic (C) and membrane (M) fractions of GFP or CD97-GFP vector transfected HEK 293 cell lysates. Simple GFP was a 27 000 MW protein which was detected only in the cytosolic fraction. Mouse CD97-GFP fusion proteins were detected only in the membrane fraction. Two distinct fusion protein bands, with apparent molecular weights of 116 000 and 47 000, respectively, were recognizable. Sizes and positions of protein molecular weight markers (in kilodaltons) are shown on the right side.
We next examined the specific interaction between mouse CD97 and mouse DAF. HEK 293 cells were transfected with a pCDNA3 plasmid containing the full-coding sequence of mouse CD97 cDNA either in the sense or antisense orientation. When normal mouse erythrocytes were added to HEK 293 cells, they were found to bind to cells tranfected with the mouse CD97 cDNA in the sense orientation only (Fig. 6a). No binding was observed between erythrocytes and HEK cells which had been transfected with the cDNA in the antisense orientation (Fig. 6b). When erythrocytes from DAF knockout mice10 were used in similar experiments, no binding was detected to HEK 293 cells which had been transfected with mouse CD97 cDNA either in the sense or antisense orientation (Fig. 6c). These results established that mouse CD97 and DAF can interact specifically with each other. To evaluate possible cross-species interaction between the two molecules, human erythrocytes were tested in a similar way for their ability to bind to mouse CD97 cDNA-transfected HEK 293 cells. Surprisingly, no binding of human erythrocytes was detected (Fig. 6c). Finally, we investigated if DAF expressed on mouse splenocytes was also able to function as a cellular ligand for mouse CD97. Normal and DAF knockout mouse splenocytes were loaded with a fluorescent dye and tested for their adhesion to mouse CD97 cDNA transfected HEK 293 cells. Figure 6(d) shows that normal but not DAF-deficient mouse splenocytes showed increased binding to CD97- expressing HEK 293 cells.
Figure 6.
Specific interaction of mouse CD97 with mouse DAF. HEK 293 cells were transfected with a pCDNA3 plasmid containing mouse CD97 cDNA either in the sense or antisence orientation. Erythrocyte and splenocyte-binding assays were performed 48 hr later as described in the Materials and Methods. (a) Normal mouse erythrocytes bound to HEK 293 cells transfected with mouse CD97 cDNA in the sense orientation. (b) Normal mouse erythrocytes failed to bind to HEK 293 cells transfected with mouse CD97 cDNA in the antisense orientation. (c) In contrast to wild-type mouse erythrocytes (WT), DAF knockout mouse erythroctes (KO) and human erythrocytes (hE) did not bind to HEK 293 cells transfected with mouse CD97 cDNA either in the sense (filled bar) or antisense (open bar) orientation). (d) Normal but not DAF knock-out mouse splenocytes had higher binding to HEK 293 cells transfected with mouse CD97 cDNA in the sense orientation (filled bar) than to cells transfected with the cDNA in the antisense orientation (open bar).
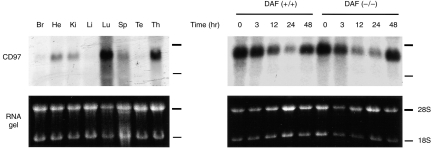
To evaluate the tissue distribution pattern of CD97, Northern blot analysis was performed on total RNAs isolated from various mouse tissues. Mouse CD97 was found to be expressed widely, with particularly high levels of expression in the lung and the thymus gland (Fig. 7). To compare the steady-state level of CD97 mRNA in resting and activated mouse lymphocytes, splenocytes isolated from normal and DAF knockout mice were cultured in the presence of an anti-CD3 antibody. Total splenocyte RNAs were prepared at various time-points after anti-CD3 stimulation and were subjected to Northern blot analysis for CD97 mRNA. Anti-CD3-dependent T-cell activation in the cultured cells was confirmed by markedly increased thymidine incorporation into cellular DNA (3H-labelled thymidine added at time 0, incorporation assay performed at 48 hr, data not shown). No increase in the steady-state CD97 mRNA level was observed during anti-CD3-induced T-cell activation (Fig. 7). On the contrary, there was a significant time-dependent decrease in the CD97 mRNA level up to 24 hr post stimulation. This decline in CD97 mRNA level was reversible and by 48 hr, CD97 expression returned almost to control level (Fig. 7). No difference between wild-type and DAF knockout mice was observed in their splenocyte expression of CD97, either before or after anti-CD3 stimulated T-cell activation (Fig. 7).
Figure 7.
Northern blot analysis of mouse CD97 expression in various mouse tissues (a) and in cultured splenocytes of normal and DAF knock-out mice (b). (a) Total RNAs from brain (Br), heart (He), kidney (Ki), liver (Li), lung (Lu), spleen (Sp), testis (Te) and thymus (Th) were analysed. (b) Splenocytes from normal (DAF (+/+)) and DAF knock-out (DAF (−/−)) mice were cultured in the presence of 10 μg/ml anti-CD3 for various lengths of time and total RNAs were prepared and analysed for CD97 expression.
DISCUSSION
CD97 belongs to a group of proteins with a hybrid structure of EGF-like repeat-containing proteins and seven-transmembrane G-protein-coupled receptors. These proteins have been referred to as the EGF-TM7 family of leucocyte surface antigens9 which, in addition to CD97, also include F4/80,15 a mouse macrophage-restricted antigen, and the human EGF module-containing mucin-like hormone receptor 1 (EMR1).16 The biological function(s) of these molecules are not yet known at the present time. The structural characteristics of their extracellular domains suggest that they may function as cell adhesion molecules.9 Additionally, the presence of seven transmembrane domains with homology to G-protein-coupled peptide receptors has led to the speculation that EGF-TM7 proteins may also act as cell surface receptors to transduce signals across the cell membrane.9 Evidence was obtained from previous studies which suggested that human CD97 exists on the cell membrane as a processed heterodimer, consisting of an extracellular α-subunit non-covalently linked to a seven-transmembrane β-subunit.4 In a separate line of investigation, human DAF, a GPI-linked membrane regulator of complement activation,17,18 has been identified as a cellular ligand for human CD97.7,8
In this study, we have cloned and characterized the mouse CD97 cDNA. We found that the overall structure of mouse CD97 is remarkably similar to that of human CD97. Both possess a seven-transmembrane region and an extended extracellular domain which contains multiple EGF-like sequence repeats and calcium-binding sites. Like human CD97, several alternatively spliced forms of mouse CD97, differing in the number of EGF-like sequence modules, exist in the mouse (Fig. 4). This indicates that alternative splicing of CD97 is not a species-specific phenomenon and suggests that different forms of CD97 may have distinct biological functions. It is of interest to note that the seven-transmembrane and the EGF-like repeat domains of CD97 from the two species are conserved to a higher degree (75% and 62%) than the regions in between (46%) (Fig. 3). This suggests that these domains may be more important for the biological function(s) of CD97, which is consistent with the two suspected roles of CD97 in cellular adhesion and signal transduction, respectively. One significant difference between the mouse and human CD97 proteins is that an RGD motif present in the human protein2,4 is not found in the mouse sequence. Thus, interaction of CD97 with other cellular integrins via the RGD motif probably is not a central mode of action for its physiological function(s). The same may be true for other EGF-TM7 proteins. An RGD motif is present in the mouse macrophage-restricted antigen F4/80 but is not present in its human homologue EMR1.15,16
Our expression studies of mouse CD97-GFP fusion protein have provided direct evidence for its plasma membrane anchoring property. Both by direct observation under a fluorescence microscope and by Western blot analysis, CD97-GFP fusion protein was found to be targeted to the plasma membrane (Fig. 5). Earlier characterization of endogenous and expressed human CD97 using a panel of specific antibodies revealed that the protein was processed to an extracellular α- and a transmembrane β-subunit.4 Our finding from Western blot analysis of mouse CD97-GFP fusion protein expressed in HEK 293 cells supports the conclusion of post-translational processing of CD97. Thus, a distinct C-terminal fragment of mouse CD97-GFP fusion protein, presumably representing the β-subunit, was detected from membrane preparations of the transfected cells (Fig. 5).
Our cell adhesion studies using normal and DAF knockout mouse erythrocytes have established the interaction between mouse DAF and mouse CD97. In the mouse, there is a second DAF gene which encodes a non-conventional transmembrane form of DAF (TM-DAF).10,19 Although the TM-DAF gene is still intact in our DAF knockout mice,10 the fact that erythrocytes from these mice were unable to bind to mouse CD97-transfected HEK cells suggests that either TM-DAF is not expressed on erythrocytes or that it does not interact with CD97. It is of interest to note the species specificity of DAF as a ligand for mouse CD97. In contrast to mouse DAF, human DAF apparently was unable to interact with mouse CD97 (Fig. 6c). This probably is not very surprising considering the relatively low sequence identity between the mouse and human DAF (50% at the amino acid level).19 Thus, although the structures of mouse and human DAF have diverged substantially during the course of evolution, their ability to interact with CD97 within a given species has been conserved. This would again argue for a specific biological function for the interaction between CD97 and DAF. We also demonstrated increased binding of normal but not DAF knockout mouse splenocytes to mouse CD97-transfected HEK 293 cells (Fig. 6d). Thus, the interaction between CD97 and DAF may play a role in leucocyte migration in vivo. This hypothesis can now be tested in animal models of inflammation using the DAF knockout mice. A further conclusion derived from our results is that the interaction between CD97 and DAF is independent of an RGD sequence motif since it is not present in the mouse protein.
Human CD97 was initially identified as a lymphocyte activation-associated antigen which was also expressed constitutively on monocytes and neutrophils.1,2 Its mRNA level in peripheral blood mononucleated cells was transiently up-regulated after in vitro activation of T cells within the population.1,2 We found in this study that CD97 mRNA was relatively abundant in isolated resting mouse splenocytes (Fig. 7). Interestingly, treatment of cultured mouse splenocytes with soluble anti-CD3 as a T-cell activator transiently decreased CD97 mRNA levels (Fig. 7). No apparent difference was observed in the kinetics of the steady-state CD97 mRNA levels between splenocytes of normal and DAF knockout mice (Fig. 7). This suggested that CD97 mRNA transcription was not regulated in a feedback mechanism by its interaction with DAF (the splenocytes were cultured in a density that was high enough for CD97 and DAF interaction to occur between neighbouring cells). Although human CD97 was initially identified as a leucocyte antigen, it was later found to be expressed on a variety of non-haematopoietic cell types as well.3 In our Northern blot analysis, we have similarly found that mouse CD97 was expressed in many non-haematopoietic mouse tissues. These findings together suggest that CD97 may have other biological roles beyond leucocyte activation and trafficking. At the present time, the pattern of CD97 protein expression is unknown, and evaluation of this question awaits antibody reagents that detect mouse CD97. Human CD97 has been shown recently to be a dedifferentiation marker in thyroid carcinoma cells.20 It is conceivable that CD97–DAF interaction, either between tumour cells and leucocytes or between tumour cells and the surrounding tissues, may play a role in tumour progression and metastasis.20 In this regard, it is relevant to note the similarly wide tissue distribution pattern of human and mouse DAF19,21,22 and the well-described observation that human DAF is up-regulated on many types of human cancer cells.23–26
References
- 1.Eichler W, Aust G, Hamann D. Characterization of an early activation-dependent antigen on lymphocytes defined by the monoclonal antibody BL-Ac (F2) Scand J Immunol. 1994;39:111. doi: 10.1111/j.1365-3083.1994.tb03348.x. [DOI] [PubMed] [Google Scholar]
- 2.Hamann J, Eichler W, Hamann D, et al. Expression cloning and chromosomal mapping of the leukocyte activation antigen CD97, a new seven-span transmembrane molecule of the secretin receptor superfamily with an unusual extracellular domain. J Immunol. 1995;155:1942. [PubMed] [Google Scholar]
- 3.Eichler W, Hamann J, Aust G. Expression characteristics of the human CD97 antigen. Tissue Antigens. 1997;50:429. doi: 10.1111/j.1399-0039.1997.tb02897.x. [DOI] [PubMed] [Google Scholar]
- 4.Gray JX, Haino M, Roth MJ, et al. CD97 is a processed, seven-transmembrane, heterodimeric receptor associated with inflammation. J Immunol. 1996;157:5438. [PubMed] [Google Scholar]
- 5.Davis CG. The many faces of epidermal growth factor repeats. New Biol. 1990;2:410. [PubMed] [Google Scholar]
- 6.D’Souza SE, Ginsberg MH, Plow EF. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends Biochem Sci. 1991;16:246. doi: 10.1016/0968-0004(91)90096-e. [DOI] [PubMed] [Google Scholar]
- 7.Hamann J, Vogel B, Van Schijindel GMW, Van Lier RAW. The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF) J Exp Med. 1996;184:1185. doi: 10.1084/jem.184.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamann J, Stortelers C, Kiss-Toth E, Vogel B, Eichler W, Van Lier RAW. Characterization of the CD55 (DAF)-binding site on the seven-span transmembrane receptor CD97. Eur J Immunol. 1998;28:1701. doi: 10.1002/(SICI)1521-4141(199805)28:05<1701::AID-IMMU1701>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.McKnight AJ, Gordon S. The EGF-TM7 family: unusual structures at the leukocyte surface. J Leukoc Biol. 1998;63:271. doi: 10.1002/jlb.63.3.271. [DOI] [PubMed] [Google Scholar]
- 10.Sun X, Funk CD, Deng C, Sahu A, Lambris JD, Song W-C. Role of decay-accelerating factor in regulating complement activation on the erythrocyte surface as revealed by gene targeting. Proc Natl Acad Sci USA. 1999;96:628. doi: 10.1073/pnas.96.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CC, Caskey CT. Rapid amplification od cDNA ends. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols, a Guide to Methods and Applications. NY: Academic Press; 1990. p. 46. [Google Scholar]
- 12.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 13.Handford PA, Mayhew M, Baron M, Winship PR, Campbell ID, Brownlee GG. Key residues involved in calcium-binding motifs in EGF-like domains. Nature. 1991;351:164. doi: 10.1038/351164a0. [DOI] [PubMed] [Google Scholar]
- 14.Selander-Sunnershagen MMM, Ullner E, Persson O, Teleman J, Stenflo, Drakenberg T. How an epidermal growth factor (EGF) -like domain binds calcium. J Biol Chem. 1992;267:19642. doi: 10.2210/pdb1ccf/pdb. [DOI] [PubMed] [Google Scholar]
- 15.McKnight A, MacFarlane AJ, Turley P, Dri L, Willis AG, Gordon S. Molecular cloning of F4/80, a murine macrophage-restricted cell surface glycoprotein with homology to the G-protein-linked transmembrane 7 hormone receptor family. J Biol Chem. 1996;271:486. doi: 10.1074/jbc.271.1.486. [DOI] [PubMed] [Google Scholar]
- 16.Baud V, Chissoe SL, Viegas-Pequignot E, et al. EMR1, an unusual member in the family of hormone receptors with seven transmembrane segments. Genomics. 1995;26:334. doi: 10.1016/0888-7543(95)80218-b. [DOI] [PubMed] [Google Scholar]
- 17.Lublin D, Atkinson JP. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson-Weller A, Wang CE. Structure and function of decay accelerating factor. J Lab Clin Med. 1994;123:485. [PubMed] [Google Scholar]
- 19.Song W-C, Deng C, Raszmann K, et al. Mouse decay-accelerating factor: selective and tissue-specific induction by estrogen of the gene encoding the glycosylphosphatidylinositol-anchored form. J Immunol. 1996;157:4166. [PubMed] [Google Scholar]
- 20.Aust GW, Eichler S, Laue I, et al. CD97: a dedifferentiation marker in human thyroid carcinomas. Cancer Res. 1997;57:1798. [PubMed] [Google Scholar]
- 21.Medof ME, Walter EI, Rutgers JL, Knowles DM, Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987;165:848. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hindmarsh EJ, Marks RM. Decay-accelerating factor is a component of subendothelial extracellular matrix in vitro, and is augmented by activation of endothelial protein kinase C. Eur J Immunol. 1998;28:1052. doi: 10.1002/(SICI)1521-4141(199803)28:03<1052::AID-IMMU1052>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 23.Koretz K, Bruderlein S, Henne C, Moller P. Decay-accelerating factor (DAF, CD55) in normal colorectal mucosa, adenomas and carcinomas. Br J Cancer. 1992;66:810. doi: 10.1038/bjc.1992.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernet-Camard M-F, Coconnier M-H, Hudault S, Servin ALL. Differential expression of complement proteins and regulatory decay-accelerating factor in relation to differentiation of cultured human colon adenocarcinoma cell lines. Gut. 1995;38:248. doi: 10.1136/gut.38.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjorge L, Hakulinen J, Wahlstrom T, Matre R, Meri S. Complement-regulatory proteins in ovarian malignancies. Int J Cancer. 1997;70:14. doi: 10.1002/(sici)1097-0215(19970106)70:1<14::aid-ijc3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Terachi T, Stanescu G, Pontes JE, Medof ME, Caulfield M. Coexistence of autologous antibodies and decay-accelerating factor, an inhibitor of complement, on human renal tumor cells. Cancer Res. 1991;51:2521. [PubMed] [Google Scholar]