Abstract
Peroxidation of polyunsaturated fatty acids in lipoproteins and cell membrane phospholipids occurs in many situations in the body, both under normal and pathological conditions. Low-density lipoprotein is particularly prone to oxidation and is believed to be a pathogenetic component in atherogenesis. Both antibody responses and T-cell responses to oxidatively modified lipoproteins have been demonstrated in humans as well as in animal models. However, little is known about how these responses arise or how T cells recognize these antigens. In the present study, mice were immunized with homologous albumin covalently modified with a series of defined aldehydes which are known to be generated during lipid peroxidation. T-cell hybridomas from immunized animals demonstrated major histocompatibility complex-restricted and protein sequence-dependent responses to modified albumin, but not to native albumin. In addition to the response to modified epitopes, some aldehyde modifications resulted in strong antibody responses also to the non-modified protein. This T-cell-dependent break of tolerance constitutes a novel pathway for induction of autoimmunity by lipid peroxidation. The findings have implications in many situations where lipid peroxidation products are generated, including atherosclerosis and inflammatory and infectious diseases.
INTRODUCTION
Lipid peroxidation results from the attack by reactive free radicals on the polyunsaturated fatty acids of membrane phospholipids and lipoproteins.1 It occurs in vivo, both under normal and pathological conditions.2–8 Through the fragmentation of lipid hydroperoxides, a number of reactive aldehydes and other metabolites are formed. Malondialdehyde (MDA) and 4-hydroxynonenal (HNE) have been extensively studied,1,9 and have been shown to form covalent adducts on different amino acid side chains.10 Such adducts are targets of B-cell-mediated immune responses as antibodies to MDA-modified low-density lipoprotein (LDL) can be detected in the plasma of humans and hypercholesterolemic animals.11–13 Large numbers of T cells are also present in atherosclerotic plaques, in humans as well as in animal models,14,15 and we have previously demonstrated that T-cell clones isolated from human plaques recognize oxidized LDL.16 Several recent studies in gene targeted knockout mice provide evidence that immune function affects plaque development.17
However, much remains to be clarified regarding the specific immune responses to oxidatively modified antigens in atherosclerosis and other inflammatory conditions. Firstly, in contrast to the antibody epitopes, which have been studied in detail,13,18–20 no T-cell epitopes have been described at a molecular level. The presence of immunoglobulin G (IgG) antibodies to modified lipoproteins suggests T-cell-induced switch from production of IgM-type to IgG-type antibodies.21 However, the possibility that T-cell-independent antibody responses occur has not been formally excluded. Antigens with repetitive epitopes can induce an antibody response of IgG type in the absence of specific T-cell help.22 This type of epitope might arise during aldehyde modification of lipoproteins where substantial amounts of several reactive aldehydes are formed and can react with many lysine groups in the same protein.1 Finally, introduction of aldehyde adducts on self proteins may disturb tolerance also to the non-modified self protein by, for example, altered antigen processing,23 and thus potentially induce autoimmune disease.24
In the present study, mice were immunized with homologous albumin modified with a series of defined aldehydes and the antibody and T-cell responses were monitored. We could show that the antibody response to aldehydes generated during lipid peroxidation is T-cell-dependent. Responsive T-cell hybridomas showed major histocompatibility complex (MHC)-restricted and protein sequence-dependent responses to modified proteins. Furthermore, we could demonstrate that some aldehyde modifications lead to a break of tolerance to the self protein.
MATERIALS AND METHODS
Mice
Female C57BL/6J and BALB/c mice, 6 weeks of age, were obtained from Charles River (Uppsala, Sweden) and kept in the animal facility at Karolinska Hospital. Female T-cell-deficient nude mice, back-crossed on C57BL/6J, were from Bomholtgård, Ry, Denmark. The absence of T cells in nude mice was certified by flow cytometry analysis of spleen cells (data not shown).
Haptens
MDA was prepared by acid hydrolysis of tetramethoxypropane (Sigma, St Louis, MO) as previously described.18 Nonanal and 2,4-heptadienal were from Aldrich (Gillingham, Kent, UK). HNE was obtained in ethanol from Cayman Chemical Company (Ann Arbor, MI). Butanal was from Acros Chimica (Geel, Belgium).
Protein modification
Murine serum albumin (MSA, essentially fatty acid-free, Sigma) was used as carrier protein for hapten antigens. MSA was modified at 2 mg/ml by incubation with 50 mm aldehyde at 37° over night in phosphate-buffered saline (PBS), pH 7·4. HNE was used at 6 mm. Reduction was carried out by addition of 50 mm NaCNBH3 (Sigma) during incubation. For nonanal and heptadienal 12·5 mm NaCNBH3 was used. Protein was separated from unreacted free aldehyde by two rounds of PD-10 Sephadex chromatography (Pharmacia Biotec, Uppsala, Sweden) followed by dialysis against RPMI-1640 (Life Technologies, Palo Alto, CA) culture medium over night. Normal mouse, rat, bovine and human serum was similarly modified.
To monitor modification of albumin, native polyacrylamide gel electrophoresis was performed at pH 8·6 and determination of remaining free lysine groups was performed by the trinitrobenzenesulphonic acid assay as previously described,25 using trinitrobenzenesulphonic acid from Sigma. Protein concentration was determined with bicinchoninic acid protein assay (Pierce, Rockford, IL). Fresh IgG-depleted mouse albumin was prepared by passing diluted mouse serum three times through a Hi-Trap protein G column (Pharmacia Biotec).
Immunization with modified proteins
Mice were immunized subcutaneously in the hind foot pads and tail base with a total of 50 μg antigen in 200 μl complete Freund’s adjuvant (Pierce). Immunization was repeated with the same amount of antigen in incomplete Freund’s adjuvant (Pierce) after 2 and 4 weeks. Mice were killed 7 days after the second booster immunization. Serum and spleen cells were collected.
Antibody enzyme-linked immunosorbent assay (ELISA)
ELISA plates (Costar, Cambridge, MA) were coated with 50 μl antigen at 10 μg/ml in PBS at 4° overnight. They were then rinsed with tap water and residual binding was blocked with 50 μl blocking buffer (0·05% Tween-20, 0·25% bovine serum albumin in PBS, pH 7·4) for 30 min at room temperature. They were rinsed again as above and incubated 2 hr at room temperature with mouse serum at indicated dilutions in blocking buffer. After rinses they were incubated 40 min at 37° with 50 μl alkaline phosphatase-conjugated secondary antibody (goat anti-mouse IgM, anti-mouse IgG were from Southern Biotechnology, Birmingham AL, while rat anti-IgG1, anti-IgG2a and anti-IgG2b were from Pharmingen, San Diego, CA) diluted 1:1000 with blocking buffer. After final rinses, the plates were developed for 10 min at room temperature with 50 μl p-nitrophenyl phosphate (p-NPP) substrate (Southern Biotechnlogy) in substrate solution (240 μm MgCl2, 10% dithioethanolamine in water). Absorbance was read at 405 nm.
SDS–PAGE and immunoblot
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis, (SDS–PAGE), was performed according to standard protocols and the proteins were electroblotted to nitrocellulose membranes (Hybond-P, Amersham, Buckinghamshire, UK) in transfer buffer (25 mm Tris, 192 mm glycine, pH 8·2 with 20% methanol). The membranes were incubated in blocking buffer consisting of Tris buffer (20 mm Tris, 500 mm NaCl, pH 8·0; TBS) with 3% gelatin. They were then washed in TTBS (TBS with 0·05% Tween-20) and incubated with mouse serum diluted 1:50 with antibody buffer (TTBS with 1% gelatin). After washings in TTBS they were incubated with alkaline phosphatase-conjugated secondary antibody (goat anti-mouse IgG, as above) diluted 1:1000 in antibody buffer. After final washes in TTBS and TBS they were developed together in substrate solution (Bio-Rad, Hercules, CA) for 20 min. All incubations were performed at room temperature.
T-cell culture and generation of T-cell hybridomas
Cell culture was performed in complete Dulbecco’s minimal essential medium (DMEM), containing 5% fetal calf serum (FCS, Myoclone super plus), 20 mm l-glutamine, 10 mm sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, 0·05 mm 2β-mercaptoethanol, all obtained from GibcoBRL (Life Technologies, Palo Alto, CA). Spleen cells were seeded at 2×106 cells/well in 24-well plates (Costar) and stimulated with antigen at 50 μg/ml. After 10 days the cells were restimulated with antigen and recombinant mouse interleukin-2 (IL-2) was added at 7·5 U/ml (Genzyme, Cambridge, MA). The cultures were further expanded for 5 weeks with antigen restimulation every 7–10 days. T-cell hybridomas were then generated by fusion with the BW5147 lymphoma cell line 3 days after the last stimulation as described.26
IL-2 assay
Hybridoma cells (5×104–10×104/well) were cultured together with irradiated (1·6 Gy) mouse spleen cells as antigen-presenting cells (2×105/well) in the presence of the antigen at indicated concentrations. After 18 hr the supernatants were harvested and frozen at −20°. Then, 50 μl of supernatant was incubated with CTLL-2 cells (5×103 cells/well) in a total volume of 100 μl for 48 hr. One microcurie [3H]thymidine (New England Nuclear, Boston, MA) was added for the last 20 hr and incorporation of radioactivity was measured using a Microbeta Plus β-counter (Wallac, Turku, Finland).
RESULTS
Aldehydes generated during lipid peroxidation induce strong T-cell-dependent antibody responses
Copper-induced oxidation of human LDL is the most extensively studied model of lipid peroxidation. During this process a number of reactive aldehydes such as malondialdehyde, hexanal, hydroxynonenal and other, saturated and unsaturated aldehydes are formed.1 For immunization studies we used a panel of five aldehydes to modify the homologous protein, murine serum albumin, selectively. Three unsaturated aldehydes; MDA, HNE and heptadienal, and two saturated aldehydes; butanal and nonanal, were used.
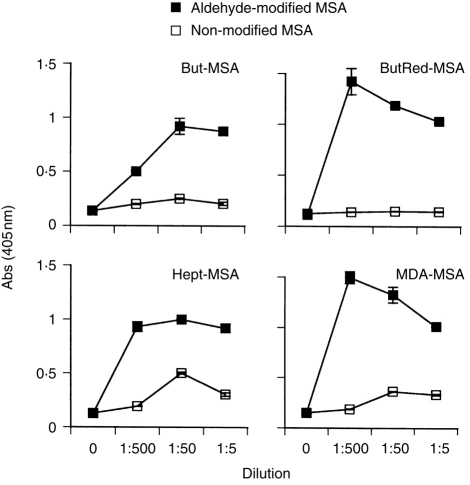
In agreement with previous studies,18,19 all haptenizing antigens induced strong antibody responses. Figure 1. shows examples of the predominant antibody pattern; a prominent reactivity to haptenized protein, but no or little reactivity to the non-modified protein.
Figure 1.
Antibody responses to modified MSA. C57BL/6J mice were immunized with indicated aldehyde-modified MSA. Serum IgG antibody titres to respective immunogen and native, non-modified albumin were measured by ELISA. But, butanal; ButRed, reduced butanal; MDA, malondialdehyde; Hept, heptadienal. Graphs show sera from individual mice, representative from two separate experiments. Values are means from duplicate wells, error bars are SEM.
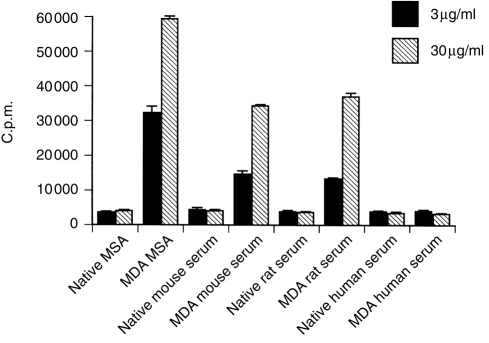
Since aldehyde conjugation of proteins may give rise to repeated epitopes through binding to several exposed lysine residues we tested whether T-cell-independent antibody responses occur by several criteria. All haptenizing antigens used induced low IgM titres but strong IgG1, IgG2a and IgG2b responses, suggesting a T-cell cytokine influence (data not shown). However, T-cell-independent antigens type 2 can induce both IgM and IgG in the absence of specific T-cell help.22 In the present study, the response to MDA-MSA at 7 days after primary immunization was very low (data not shown), while restimulation gave rise to high antibody titres (Fig. 1), indicative of a T-cell memory response. Finally, T-cell dependence was tested directly by immunization of athymic, nude mice lacking normal T cells. The mice used were back-crossed on a C57BL/6J background in order to allow comparison with C57BL/6J mice used in the experiments. No antibody responses were detected in nude mice, while normal C57BL/6J mice showed strong antibody titres (Fig. 2). In conclusion, the results clearly demonstrate the T-cell dependence of antibody responses to aldehyde-modified proteins.
Figure 2.
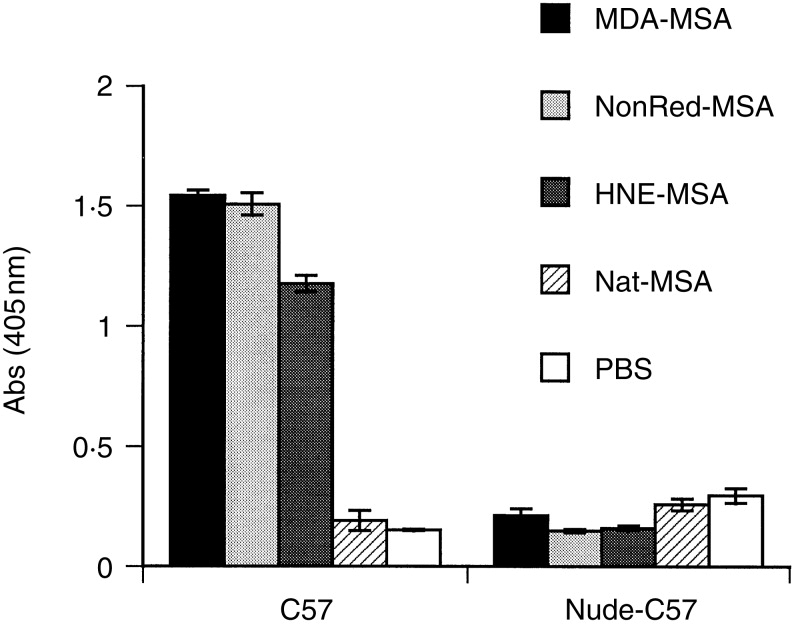
Antibody responses to modified MSA in normal C57BL/6J and T-cell-deficient nude mice. The mice were immunized with the indicated aldehyde-modified MSA, native MSA, or PBS. Serum was collected after the second booster immunization. IgG antibody titres to respective immunogen were measured by ELISA using serum diluted 1:500. MDA, malondialdehyde; NonRed, reduced nonanal; HNE, 4-hydroxynonenal; Nat, native MSA. Values are means from four animals in each group, error bars are SEM.
Aldehydes generated during lipid peroxidation induce MHC-restricted and protein sequence-dependent T-cell responses
A major antibody response in human atherosclerosis is directed to haptens in the form of aldehyde adducts on apolipoprotein B-10018,27–29 and it has been assumed that the T-cell response recognizes similar epitopes. This notion is supported by the demonstration that T-cell clones from human atherosclerotic plaques recognize oxidized LDL,16 but the T-cell epitope has not been characterized at a molecular level. The analysis of epitopes to oxidized LDL is hampered by the complexity of this large antigen, containing many different aldehyde adducts.1 To define T-cell receptor recognition of aldehyde adducts we therefore established T-cell hybridomas from mice immunized with modified MSA. Several MDA-MSA-responsive and HNE-MSA-responsive cell lines were identified. Figure 3 shows a strong, dose-dependent response to the modified antigen in MDA-specific (Fig. 3a) and HNE-specific (Fig. 3b) hybridomas.
Figure 3.
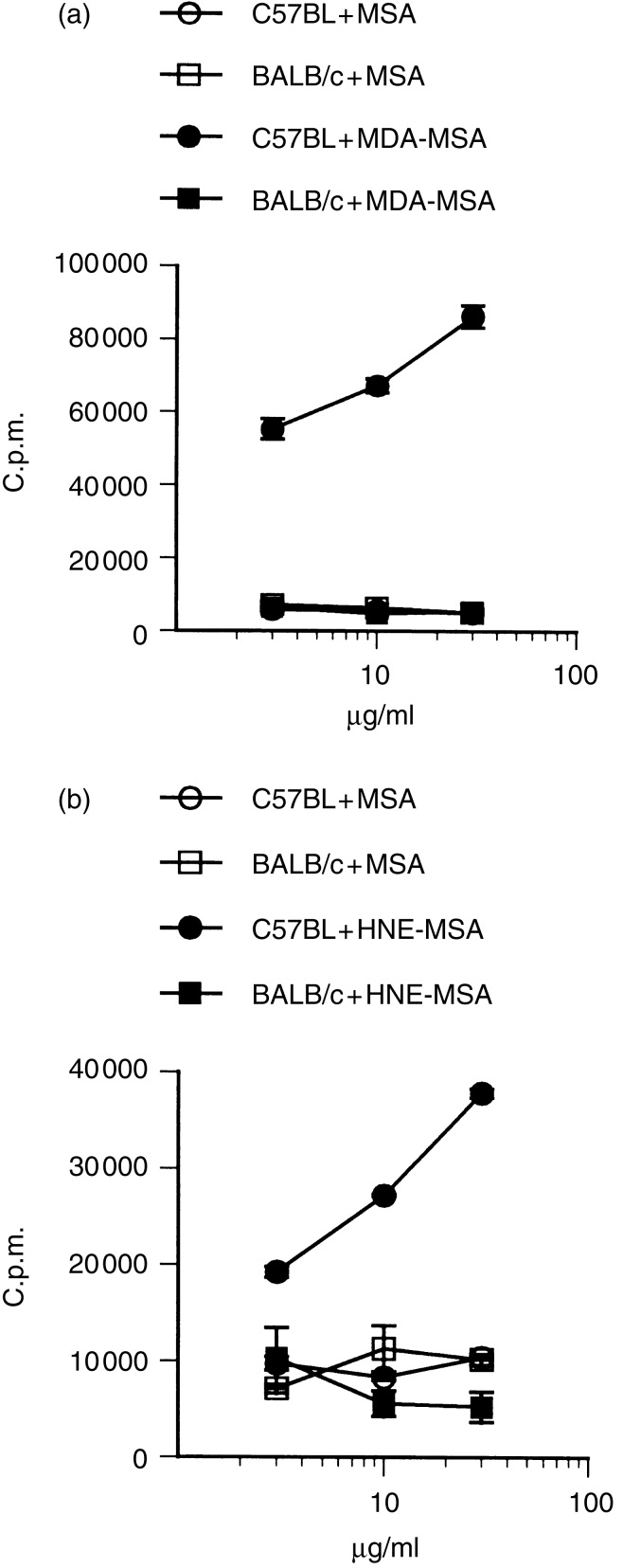
MHC-restricted antigen responses of aldehyde-specific T-cell hybridomas. T-cell hybridomas were established from mice immunized with MDA-MSA (a) or HNE-MSA (b). Antigen-specific responses were measured by 24-hr culture of hybridoma cells in the presence of autologous spleen cells or allogeneic BALB/c spleen cells and antigen as indicated. The supernatants were collected and their IL-2 content was assayed as radioactive thymidine incorporation of the IL-2-dependent cell line CTLL-2. Experiments were performed in triplicate, error bars are SEM.
To determine whether recognition of aldehyde-modified proteins occurs by classical presentation of MHC-presented peptides, MHC restriction and protein sequence dependence of the T-cell hybridomas was tested. Figure 3 shows that MDA-specific and HNE-specific T-cell hybridomas, derived from C57BL/6J (I-Ab) mice, failed to respond to antigen in the presence of BALB/c (I-Ad and I-Ed) spleen cells, indicating MHC-restriction.
Protein sequence dependence was tested by addition of modified serum albumin from species with varying sequence homology to mouse albumin. The responses were compared with both the commercial MSA-preparation and fresh mouse serum. Hybridomas reacted to modified rat serum, but not with modified human (Fig. 4) or bovine serum (not shown). This indicates sequence-dependent cross-reactivity with protein from the two closely related rodents. The conclusion is supported by sequence alignment of the known 438-amino acid sequence of mouse albumin with albumin from the other species. This demonstrated 45 amino acid mismatches with rat albumin while human and bovine albumin displayed 120 and 124 mismatches, respectively.
Figure 4.
Protein sequence dependence of T-cell antigen responses. T-cell hybridomas were established and assayed as in Fig. 3. Sensitivity to protein sequence was tested for the MDA-MSA-specific hybridoma M20 by incubation with native or MDA-modified whole serum from different species. Experiments were performed in triplicate, error bars are SEM.
Immunization with aldehyde-modified homologous protein breaks B-cell self tolerance
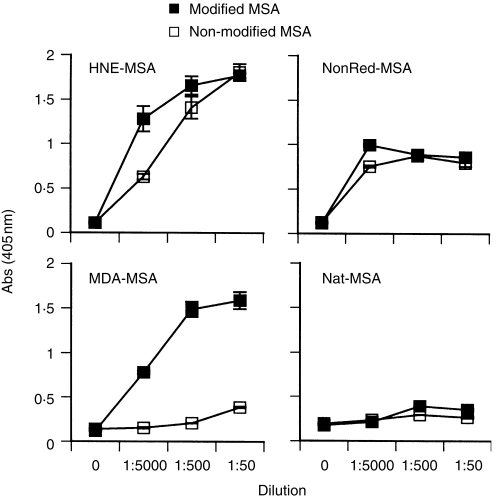
The mouse is expected to be tolerant to self albumin. In agreement with this, immunization with non-modified MSA induced no detectable antibody response (Fig. 5). Most haptenized MSA preparations, such as MDA-MSA, induced antibody responses only against the relevant hapten (Fig. 5). In contrast, HNE-MSA and reduced nonanal-MSA induced a response also to non-modified MSA (Fig. 5). The titre of this response was high, approaching the titres to the haptenized antigens and was reproducible in repeated experiments. The effect was not due to the adjuvant used, since PBS-immunized mice showed no response (data not shown). Neither was it caused by contaminating non-murine antigen since no response was seen after immunization with non-modified MSA (Fig. 5).
Figure 5.
Antibody responses to modified and native MSA. Mice were immunized with the indicated antigens. Serum was collected after the second booster immunization and the IgG antibody titres to respective immunogen and native MSA were measured by ELISA. MDA, malondialdehyde; NonRed, reduced nonanal; HNE, 4-hydroxynonenal; Nat, native MSA. In the last panel, reactivity to MDA-MSA is shown as an irrelevant antigen. Values are means of duplicate wells, error bars are SEM.
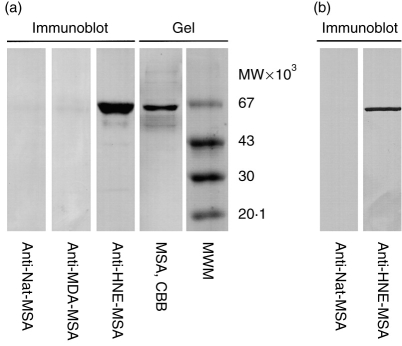
The target of the autoantibodies was further characterized by SDS–PAGE Western blot. The autoreactive sera reacted with the MSA preparation used for immunization (Fig. 6a). as well as to IgG-depleted albumin freshly isolated from mouse serum (Fig. 6b). HNE-MSA-immune serum, but not MSA-immune serum, showed strong reactivity to the albumin band. This demonstates that autoantibodies were generated against the self protein. Any autoantibodies recognizing circulating albumin would be expected to be blocked by binding to the large excess of antigen in serum. Accordingly, anti-HNE-MSA serum did not react with freshly isolated IgG-depleted mouse serum by ELISA. The reactivity to non-modified commercial MSA by ELISA and denatured mouse albumin by Western blot probably reflects recognition of epitopes not exposed in the freshly isolated albumin. However, the same MSA in the absence of modified epitopes, failed to induce autoantibody formation, clearly demonstrating the requirement of covalent modification for autoantibody formation.
Figure 6.
Western blot showing mouse serum reactivity to mouse albumin (a) Non-modified MSA (Sigma); (b) fresh IgG-depleted mouse albumin. Albumin was subjected to SDS–PAGE and electroblotted to nitrocellulose membranes. The membrane strips were incubated with indicated serum from immunized mice diluted 1:50, and detected with alkaline phosphatase-labelled secondary anti-IgG antibody. CBB, Coomassie brilliant blue staining; MWM, molecular weight markers.
DISCUSSION
Atherosclerosis, in man as well as in animal models, is associated with an antibody response to modified LDL. Hydroxynonenal- and malondialdehyde-modified lysine residues have been shown to be two major epitopes11–13 in this response. These highly reactive aldehydes are generated during lipid peroxidation and bind primarily to lysine residues in adjacent proteins.9,12,20 Also T-cell responses to modified LDL have been detected16 and it has been suggested that T cells also may recognize modified epitopes. This notion is supported by recent studies defining T-cell recognition of other haptens at a molecular level.30,31
The aim of the present study was to investigate molecular interactions and effects of defined aldehyde modifications on T- and B-cell immune responses. Mice were immunized with MSA modified with a series of aldehydes, known to be generated during copper-induced oxidation of LDL.1 Several features of the resulting antibody responses, e.g. isotype switch, enhanced secondary response, as well as the inability of T-cell-deficient mice to mount an antibody response, firmly establish the T-cell dependence of autoantibody responses to peroxidation-derived epitopes. This conclusion is supported by the successful establishment of T-cell hybridomas reactive to modified proteins. Antigen assays revealed a strong, dose-dependent, MHC-restricted and protein sequence-dependent response to the relevant modified antigen, but not to native protein. These data represent the first direct demonstration of T-cell recognition of naturally occurring aldehyde modifications. This may result from either direct recognition of modified hapten epitopes31 or recognition of native epitopes which are presented de novo as a consequence of altered processing of modified proteins.23,32
For most aldehydes tested, the antibody response was directed only to the modified murine serum albumin used for immunization. However, some aldehyde modifications resulted in strong antibody reactivity also to the non-modified protein. This indicates that the normal tolerance to the homologous protein is broken at the B-cell level. B-cell tolerance to self proteins is maintained through several mechanisms, the first occurring in the bone marrow where immature B cells principally reactive to membrane-bound self antigens are deleted.33 Self-reactive B cells escaping this selection are kept anergic by the requirement of cognate interaction with T cells.33 Specific T-cell help to self-reactive B cells is normally lacking due to clonal deletion during thymic maturation.34 In agreement with this, introduction of a single non-self T-cell epitope in a self protein may lead to break of B-cell tolerance and autoantibody formation.35 By similar mechanisms, immunization with heterologous protein may induce autoantibodies reacting with the self protein.36 In the present study, the absence of an autoantibody response after immunization with native MSA can be explained by the absence of T cells reactive to native MSA. Our demonstration of a T-cell response to modified antigens provides a mechanism for antigen-specific help to B cells reactive to hapten-MSA as well as to B cells recognizing non-modified MSA. Both types of B cells may effectively take up and present modified, or cryptic native, epitopes to T cells which in turn provide cytokine help for B-cell differentiation. In addition to providing T-cell epitopes, aldehyde modifications might also induce conformational changes in vivo, exposing hidden B-cell epitopes on self antigens and thus directing autoantibody responses to the inflamed tissue.
Our results with naturally occurring peroxidation-derived aldehydes add biological relevance to previous studies showing autoantibody formation after immunization with chemically modified self proteins,24,37 and have several important implications. In addition to atherosclerosis, lipid peroxidation has been demonstrated in many different pathophysiological situations, including infections,4,38 and a number of inflammatory conditions.2,3,6,7,39–45
The generated aldehydes have numerous effects on cells and tissues such as cytotoxicity and genotoxicity.46 The present study provides further evidence that lipid peroxidation products can induce T-cell responses16 and adds the potential of certain aldehydes to induce autoimmunity by breaking B-cell tolerance to non-modified proteins. Our proposed mechanism may contribute to the occurrence of transient autoantibody responses which are commonly observed in infections and potentially result in overt autoimmune disease. Recent studies have also demonstrated increased lipid peroxidation in a number of autoimmune conditions, including multiple sclerosis,3,40 rheumatoid arthritis,2,42 diabetes,5,43,44 and systemic lupus erythematosus.45 Lipid peroxidation may be an early phenomenon in these diseases, or may be induced secondary to inflammation. In either case, peroxidation-induced T-cell responses to modified self proteins, as well as ensuing generation of antibodies to native proteins, may play a role during disease development.
Acknowledgments
We thank Ingrid Törnberg for excellent technical assistance. This work was supported by grants from the Swedish Medical Research Council (project no. 6816), the Heart and Lung foundation, King Gustaf V 80th Anniversary Fund, the Johnsson foundation, the Nanna Svartz foundation, the Swedish Society of Medicine, the Å ke Wiberg foundation, the Magnus Bergvall foundation, the Lars Hierta foundation and the Karolinska Institute funds.
Abbreviations
- HNE
4-hydroxynonenal
- LDL
low-density lipoprotein
- MDA
malondialdehyde
- MSA
mouse serum albumin
References
- 1.Esterbauer H, Ramos P. Chemistry and pathophysiology of oxidation of LDL. Rev Physiol Biochem Pharmacol. 1996;127:31. doi: 10.1007/BFb0048264. [DOI] [PubMed] [Google Scholar]
- 2.Selley ML, Bourne DJ, Bartlett MR, et al. Occurrence of (E)-4-hydroxy-2-nonenal in plasma and synovial fluid of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1992;51:481. doi: 10.1136/ard.51.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naidoo R, Knapp ML. Studies of lipid peroxidation products in cerebrospinal fluid and serum in multiple sclerosis and other conditions. Clin Chem. 1992;38:2449. [PubMed] [Google Scholar]
- 4.Goode HF, Cowley HC, Walker BE, Howdle PD, Webster NR. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit Care Med. 1995;23:646. doi: 10.1097/00003246-199504000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Griesmacher A, Kindhauser M, Andert SE, et al. Enhanced serum levels of thiobarbituric-acid-reactive substances in diabetes mellitus. Am J Med. 1995;98:469. doi: 10.1016/s0002-9343(99)80347-7. [DOI] [PubMed] [Google Scholar]
- 6.Paradis V, Kollinger M, Fabre M, Holstege A, Poynard T, Bedossa P. In situ detection of lipid peroxidation by-products in chronic liver diseases. Hepatology. 1997;26:135. doi: 10.1053/jhep.1997.v26.pm0009214462. [DOI] [PubMed] [Google Scholar]
- 7.Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci USA. 1996;93:2696. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strohmaier H, Hinghofer SH, Schaur RJ. Detection of 4-hydroxynonenal (HNE) as a physiological component in human plasma. J Lipid Med Cell. 1995;11:51. doi: 10.1016/0929-7855(94)00027-a. [DOI] [PubMed] [Google Scholar]
- 9.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 10.Uchida K, Stadtman ER. Covalent attachment of 4-hydroxynonenal to glyceraldehyde-3-phosphate dehydrogenase. A possible involvement of intra- and intermolecular cross-linking reaction. J Biol Chem. 1993;268:6388. [PubMed] [Google Scholar]
- 11.Rosenfeld ME, Palinski W, Ylä-Herttuala S, Butler S, Witztum JL. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arterioscler Thromb. 1990;10:336. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- 12.Ylä-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14:32. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 13.Palinski W, Ord VA, Plump AS, Breslow JL, Steinberg D, Witztum JL. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994;14:605. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 14.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 15.Zhou X, Stemme S, Hansson GK. Evidence for a local immune response in atherosclerosis: CD4+ T cells infiltrate lesions of apolipoprotein-E-deficient mice. Am J Pathol. 1996;149:359. [PMC free article] [PubMed] [Google Scholar]
- 16.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized LDL. Proc Natl Acad Sci USA. 1995;92:3893. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansson GK. Atherosclerosis: cell biology and lipoproteins. Curr Opin Lipidol. 1998;9:73. doi: 10.1097/00041433-199802000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Palinski W, Rosenfeld ME, Ylä-Herttuala S, et al. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci USA. 1989;86:1372. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammer A, Kager G, Dohr G, Rabl H, Ghassempur I, Jürgens G. Generation, characterization, and histochemical application of monoclonal antibodies selectively recognizing oxidatively modified apoB-containing serum lipoproteins. Arterioscler Thromb Vasc Biol. 1995;15:704. doi: 10.1161/01.atv.15.5.704. [DOI] [PubMed] [Google Scholar]
- 20.Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou X, Paulsson G, Stemme S, Hansson GK. Hypercholesterolemia is associated with a T Helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J Clin Invest. 1998;101:1. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mond JJ, Vos Q, Lees A, Snapper CM. T cell independent antigens. Curr Opin Immunol. 1995;7:349. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 23.Abraham R, Chodhury A, Basu SK, Bal V, Rath S. Disruption of T cell tolerance by directing a self antigen to macrophage-specific scavenger receptors. J Immunol. 1997;158:4029. [PubMed] [Google Scholar]
- 24.Weigle WO. The induction of autoimmunity in rabbits following injection of heterologous or altered homologous thyroglobulin. J Exp Med. 1965;121:289. doi: 10.1084/jem.121.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fields R. The measurement of amino groups in proteins and peptides. Biochem J. 1971;124:581. doi: 10.1042/bj1240581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981;153:1198. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ylä-Herttuala S, Palinski W, Rosenfeld ME, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salonen JT, Ylä-Herttuala S, Yamamoto R, et al. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. The Lancet. 1992;339:883. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 29.Holvoet P, Perez G, Zhao Z, Brouwers E, Bernar H, Collen D. Malondialdehyde-modified low density lipoproteins in patients with atherosclerotic disease. J Clin Invest. 1995;95:2611. doi: 10.1172/JCI117963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nalefski EA, Rao A. Nature of the ligand recognized by a hapten- and carrier-specific, MHC-restricted T cell receptor. J Immunol. 1993;150:3806. [PubMed] [Google Scholar]
- 31.Martin S, von Bonin A, Fessler C, Pflugfelder U, Weltzien HU. Structural complexity of antigenic determinants for class I MHC-restricted, hapten-specific T cells. Two qualitatively differing types of H-2Kb-restricted TNP epitopes. J Immunol. 1993;151:678. [PubMed] [Google Scholar]
- 32.Griem P, Panthel K, Kalbacher H, Gleichmann E. Alteration of a model antigen by Au (III) leads to T cell sensitization to cryptic peptides. Eur J Immunol. 1996;26:279. doi: 10.1002/eji.1830260202. [DOI] [PubMed] [Google Scholar]
- 33.Cornall RJ, Goodnow CC, Cyster JG. The regulation of self-reactive B cells. Curr Opin Immunol. 1995;7:804. doi: 10.1016/0952-7915(95)80052-2. [DOI] [PubMed] [Google Scholar]
- 34.Kruisbeek AM, Amsen D. Mechanisms underlying T-cell tolerance. Curr Opin Immunol. 1996;8:233. doi: 10.1016/s0952-7915(96)80062-8. [DOI] [PubMed] [Google Scholar]
- 35.Dalum I, Jensen MR, Hindersson P, Elsner HI, Mouritsen S. Breaking of B cell tolerance toward a highly conserved self protein. J Immunol. 1996;157:4796. [PubMed] [Google Scholar]
- 36.Young JL, Hooper DC. Characterization of autoreactive helper T cells in a murine model of autoimmune haemolytic disease. Immunology. 1993;80:13. [PMC free article] [PubMed] [Google Scholar]
- 37.Yamashita U, Takami T, Hamaoka T, Kitagawa M. The role of hapten-reactive T lymphocytes in the induction of autoimmunity in mice. II. Termination of self-tolerance to erythrocytes by immunization with hapten-isologous erythrocytes. Cell Immunol. 1976;25:32. doi: 10.1016/0008-8749(76)90094-0. [DOI] [PubMed] [Google Scholar]
- 38.Leib SL, Kim YS, Chow LL, Sheldon RA, Tauber MG. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J Clin Invest. 1996;98:2632. doi: 10.1172/JCI119084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demling R, Lalonde C, Ikegami K, Picard L, Nayak U. Alpha-tocopherol attenuates lung edema and lipid peroxidation caused by acute zymosan-induced peritonitis. Surgery. 1995;117:226. doi: 10.1016/s0039-6060(05)80090-x. [DOI] [PubMed] [Google Scholar]
- 40.Toshniwal PK, Zarling EJ. Evidence for increased lipid peroxidation in multiple sclerosis. Neurochem Res. 1992;17:205. doi: 10.1007/BF00966801. [DOI] [PubMed] [Google Scholar]
- 41.Savenkova ML, Mueller DM, Heinecke JW. Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein. J Biol Chem. 1994;269:20394. [PubMed] [Google Scholar]
- 42.Yahya MD, Pinnas JL, Meinke GC, Lung CC. Antibodies against malondialdehyde (MDA) in MRL/lpr/lpr mice: evidence for an autoimmune mechanism involving lipid peroxidation. J Autoimmun. 1996;9:3. doi: 10.1006/jaut.1996.0002. [DOI] [PubMed] [Google Scholar]
- 43.Suarez PW, Strynadka K, Rabinovitch A. Destruction of rat pancreatic islet beta-cells by cytokines involves the production of cytotoxic aldehydes. Endocrinology. 1996;137:5290. doi: 10.1210/endo.137.12.8940348. [DOI] [PubMed] [Google Scholar]
- 44.Traverso N, Menini S, Cosso L, et al. Immunological evidence for increased oxidative stress in diabetic rats. Diabetologia. 1998;41:265. doi: 10.1007/s001250050902. [DOI] [PubMed] [Google Scholar]
- 45.Grune T, Michel P, Sitte N, et al. Increased levels of 4-hydroxynonenal modified proteins in plasma of children with autoimmune diseases. Free Rad Biol Med. 1997;23:357. doi: 10.1016/s0891-5849(96)00586-2. [DOI] [PubMed] [Google Scholar]
- 46.Schaur RJ, Zollner H, Esterbauer H. Biological effects of aldehydes with particular attention to 4-hydroxynonenal and malonaldehyde. In: Vigo-Pelfrey C, editor. Membrane Lipid Oxidation. III. Boca Raton, FL: CRC Press; 1989. pp. 141–163. [Google Scholar]