Abstract
The induction of an immune response or tolerance is mediated by corresponding subsets of dendritic cells (DC). However, the property of tolerogenic DC is not clear. Recently, we have characterized a population of CD11c+ splenic DC derived from long-term mixed leucocyte culture (LT-MLC), which are able to proliferate upon stimulation and have a strong primary mixed leucocyte reaction (MLR)-stimulating activity in conventional MLR. In this study, we show that, in contrast to the irradiated ones, non-irradiated LT-MLC-derived DC induce polyclonal antigen-specific T-cell hyporesponsiveness when cocultured with allogeneic splenocytes for 3–11 days. The degree of the hyporesponsiveness increased with the length of coculture. Although these DC expressed major histocompatibility complex class II and B7 costimulatory molecules, which are down-regulated during coculture, they expressed very low or undetectable CD40 before and after coculture, respectively. The CD40-deficient DC spontaneously produce interleukin-10 (IL-10), but not IL-12. The skewed balance between IL-10 and IL-12 is associated with their capability to induce T-cell hyporesponsiveness, because a neutralizing antibody to IL-10, exogenous recombinant IL-12 or lipopolysaccharide (LPS) significantly blocked the hyporesponsiveness. Accordingly, infusion of a small number of non-irradiated LT-MLC-derived DC (5×105) significantly prolonged the survival of a vascularized heterotopic murine heart transplant, whereas irradiated DC accelerated graft rejection. These data suggest that CD40-deficient DC producing IL-10, but not IL-12 can induce T-cell hyporesponsiveness in vitro and in vivo.
INTRODUCTION
Professional antigen-presenting cells (APC), such as dendritic cells (DC), regulate immune responses by the induction of activated or tolerant T cells.1–4 Mature DC that express high levels of major histocompatibility complex (MHC) class II and costimulatory molecules, such as CD40, CD80 and CD86, provide two signals required for T-cell activation,5–7 and potently activate naive T cells.1,8 However, immature DC that express variable densities of MHC class II and costimulatory molecules may be immunogenic or tolerogenic, depending on the conditions in the context of immune responses.9
DC are highly heterogeneous in phenotype and function depending on the microenvironment in which they differentiate.4,10–13 For example, the cytokines granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) drive human monocytes to differentiate into intermediate (immature) DC, which may further differentiate into terminal (mature DC) in the presence of tumour necrosis factor-α (TNF-α),14,15 or into a subtype of DC, e.g. Langerhans’ DC in the presence of transforming growth factor-β (TGF-β),12 or into macrophages in the presence of M-CSF.16 In a transplantation model, infusion of B7-deficient donor-specific DC prolongs graft survival, whereas infusion of mature DC causes accelerated graft rejection.17 The DC regulated by IL-10 or prostaglandin (PG) E2 are capable of inducing T-cell hyporesponsiveness and T helper type 2 (Th2) T-cell differentiation, respectively.18–20 Thus, DC that differentiate in a particular cytokine milieu may determine the outcome of immune responses.17,21
T-cell anergy was initially described in vitro from the observation that cloned CD4+ T cells stimulated through their antigen receptor in the absence of APC-derived costimulatory signals lose the ability to produce IL-2 and proliferate upon antigen restimulation.22,23 However, it is difficult to explain the in vivo situations where costimulatory molecules on APC are accessible and T-cell anergy can also be induced.2,23,24 Although the phenotype and cytokine profiles produced by DC have been extensively studied in DC-producing systems,3,4,25 the identity of DC during tolerance induction is not clear. The current in vivo and in vitro tolerance induction models cannot be used to characterize DC phenotype and cytokine secretion profile during tolerance induction, because APC are either chemically fixed or irradiated in in vitro models,18,20 or difficult to isolate for characterization in in vivo models.9,24
To characterize the phenotype and cytokine profile of DC during tolerance induction, a novel in vitro system is required for study of unmodified DC, in which DC are not irradiated or chemically fixed, and may be influenced by responding T cells via feedback mechanisms. In this study, we developed a long-term culture system to induce anergic alloreactive T cells. Since classic cytokine-propagated bone-marrow DC are short-lived, we used long-lived CD11c+ DC generated in long-term mixed leucocyte culture (LT-MLC).25 They not only proliferate upon stimulation by apoptotic cells without the addition of exogenous cytokines but also exhibit potent activity to stimulate primary allogeneic T cells when they are irradiated in conventional mixed leucocyte reactions (MLR).25 These features of the LT-MLC-derived DC allow us to study the phenotype and cytokine profile of DC during interaction with T cells. Herein we report that, in contrast to the irradiated ones,25 non-irradiated LT-MLC-derived DC can induce T-cell hyporesponsiveness when they are cocultured long-term with allogeneic splenocytes without the need to add exogenous cytokines. The degree of the T-cell hyporesponsiveness correlates with the length of coculture, and the tolerogenicity of LT-MLC-derived DC is associated with a defect in CD40 expression and skewed production of IL-10 and IL-12 (IL-10high versus IL-12deficient). Consistently, infusion of live, but not irradiated, LT-MLC-derived DC into recipients can prevent acute allograft rejection in a murine heart transplantation model. These results suggest that the intrinsic properties of tolerogenic DC may be associated with a phenotype of CD40low/– and a polarized cytokine profile (IL-10high and IL-12deficient).
MATERIALS AND METHODS
Animals and medium
Eight- to 12-week-old male BALB/c (H-2d), C57BL/6 (H-2b) and C3H/HeN (H-2k) mice were purchased from Harlan Sprague Dawley Inc. (Indianapolis, IN) and housed in the Department of Animal Care, University of Western Ontario. Complete Dulbecco’s modified Eagle’s minimum essential medium (DMEM; D10F) or complete RPMI-1640 (R10F) (both from Gibco BRL, Gaithersburg, MD) medium were supplemented with 10% new-born calf serum (Gibco BRL), 2 mm glutamine, 50 μm 2-mercaptoethanol (2-ME), 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma, St Louis, MO).
Antibodies and cytokines
The following rat (except where indicated) monoclonal antibodies (mAb) against mouse antigens were generated from American Type Culture Collection (ATCC; Rockville, MD) cell lines, and purified from culture supernatant by protein-G affinity chromatography: hamster anti-mouse CD3 immunoglobulin G (IgG) CRL 1975 (145-2C11), anti-CD4 IgG2b TIB 207 (GK1.5), anti-CD8 IgG2b TIB 210 (2.43), hamster anti-mouse CD11c IgG HB224 (N418), hamster anti-mouse I-A/I-E antigens IgG HB225 (N22), anti-l-selectin IgG2a HB132 (MEL-14), anti-CD45RB IgG2a HB220 (MB23G2), anti-CD44 IgG1 TIB 242 (KM114), anti-IL-2R (CD25), IgM CRL 1878 (7D4), and anti-IgM IgG2b TIB 129 (331.12). The following rat anti-mouse mAb were purchased from PharMingen (San Diego, CA): fluorescein isothiocyanate (FITC)-conjugated anti-CD40, IgM (HM40-3), anti-B7-1 (CD80), IgG2a (GL1) and anti-B7-2 (CD86), IgG2b (1G10). Purified goat-neutralizing antibody to murine IL-10 (IgG), goat IgG and recombinant murine IL-2 and interferon-γ (IFN-γ) were purchased from R&D Systems (Minneapolis, MN). Recombinant murine IL-12 was obtained from Genetics Institute (Cambridge, MA). The recombinant cytokine preparations contained <0·1 ng/μg endotoxin. Lipopolysaccharide (LPS) was purchased from Sigma.
Generation of DC from LT-MLC
DC were generated from unfractionated spleen cells in LT-MLC and were CD11c+ as described previously.25 Briefly, mice were killed in a CO2 chamber. Spleen cells (5×107–10×107) from C57BL/6 (H-2b) mice were cultured with irradiated (3000 rads) allogeneic spleen cells (5×107–10×107) from BALB/c (H-2d) in D10F in a 25-cm2 flask (Falcon, Cat. 3081, Becton Dickinson & Co. Lincoln Park, NJ). Adherent DC were observed after 2 weeks of culture, and they grew confluent with differential size of aggregates at 4–6 weeks. DC proliferated upon repetitive stimulation with 2×107–5×107 irradiated allogeneic splenocytes (every 7–10 days). Starting from 6–8 weeks, DC released from aggregates or dislodged by gentle pipetting were regularly harvested 4 days after stimulation by irradiated splenocytes and only CD11c-positive cells, as confirmed by fluorescence-activated cell sorter (FACS), were used for experiments. Please, note that CD11c is down-regulated upon coculture with non-irradiated allogeneic T cells as previous described25 (Fig. 6). The phenotype of these dendritic cells has been characterized in detail previously.25
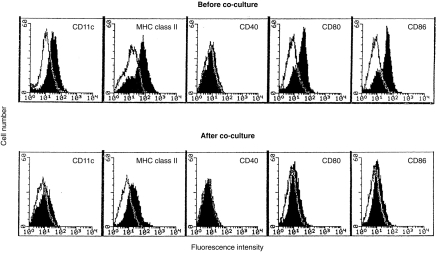
Figure 6.
Expression of CD40, CD80 and CD86 on LT-MLC-derived DC is down-regulated during coculture. DC harvested from the cocultures at day 11 were stained with mAbs to CD11c, I-A/I-E, CD40, CD80, or CD84, respectively, and then FITC conjugated secondary antibody as described in the Materials and Methods. The results were analysed by flow cytometry and are representative of three experiments (open histogram: control primary mAb).
Induction of T-cell unresponsiveness
To induce T-cell unresponsiveness, allogeneic spleen cells were cocultured with non-irradiated LT-MLC derived DC in D10F in a 25-cm2 flask for various times. DC (C57BL/6, H-2b) were harvested on day 4 after restimulation and were subcultured in 25-cm2 flasks (5×−10×105 cells/flask). Allogeneic spleen cells (BALB/c, H-2d) were added into the flasks (1×108 cells/flask) when the DC spread or grew more than 80–90% confluent. On day 3, 7, or 11 after coculture, spleen cells were harvested for phenotypic and functional analysis by flow cytometry and proliferation assay (see below), respectively, whereas most of the DC remained adherent on the surface of the flask. To remove contaminating DC, the harvested cells were incubated in R10F in a culture dish for 2 hr, or treated with mouse mAb to I-Ab/d plus low-toxic rabbit complement as described by the supplier (Cedarlane, Hornby, Ont.). The cell preparation consisted of CD4+ and CD8+ T (called DC-T) cells with DC being less than 2%. In the blocking experiments, goat anti-mouse IL-10 (20 μg/ml), recombinant murine IL-12 (1000 U/ml), IFN-γ (400 U/ml) or IL-2 (5 ng/ml) or LPS (1 μg/ml) were added into cocultures to block tolerance induction.
Preparation of Con A-T and MLR-T cells
Con A-activated T (Con A-T) cells were prepared and used as a control for DC-T cells that were obtained from coculture at day 7 or 11. In brief, spleen cells (5×106/ml) from BALB/c mice were cultured in D10F in the presence of Con A (5 μg/ml). After incubation for 4 days, 50% of the medium was replaced with fresh D10F. On day 6, blast cells were collected and washed to remove debris. The cells were re-cultured in 50% fresh D10F containing 5 μg/ml Con A. Con A-T were harvested at different incubation times matching those of DC-T cells and used as controls in proliferation assays. In addition, splenocytes (5×106 cells/flask) from BALB/c (H-2d) were cocultured for 3 days with equal numbers of irradiated splenocytes from C57BL/6 (H-2b). Viable cells (MLR-T cells) were isolated with lympholyte® gradient (Cedarlane, Hornby, Ont.), and used as controls for the 3-day DC-T cells.
T-cell proliferation and cytokine assays
DC-T, Con A-T, MLR-T, or splenic cells in various concentrations were stimulated with irradiated (3000 rads) allogeneic splenocytes (H-2b, 2×105 cells/well) in 200 μl R10F in round-bottomed 96-well plates (Falcon). In some experiments, exogenous murine IL-2 was added into cultures (5 ng/ml) to examine the reversibility of tolerized DC-T cells. All assays were performed in triplicates. The culture plates were incubated for 72–90 hr, and cell proliferation was measured by adding [3H]TdR (0·5 μCi/well) 16 hr before harvesting. IL-2 was measured by standard bioassay using the CTLL-2 cell line. IL-10 and IL-12 in coculture supernatants were analysed by enzyme-linked immunosorbent assay (ELISA)26 using PharMingen OptEIA™ sets for mouse IL-10 (Cat. 2657KK) and IL-12 (p40) (Cat. 2619KK).
Flow cytometry
Flow cytometric analysis of LT-MLC-derived DC and T cells was performed as described previously.25 Briefly, cells (2×105–5×105 for DC, 1×106 for T cells) in 100 μl of phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin (BSA) and 0·01% sodium azide were pretreated with the corresponding normal rat or mouse serum. The cells were stained with mAb to the corresponding surface antigen (2 μg/ml for purified IgG, 1:2 for culture supernatant) where indicated. Secondary antibody was FITC-conjugated IgG, either F(ab′)2 fragment of donkey anti-rat IgG (Jackson Immunoresearch Laboratories, Inc., West Grove, PA), goat anti-rat IgM (Sigma Chemical Co.) or F(ab′)2 fragment of goat anti-hamster IgG (PharMingen, San Diego, CA). All secondary reagents were diluted 1:300. Appropriate isotype-matched mAb were used as controls. Cells were incubated for 30 min at 4° for each step. After labelling, the cells were fixed in 0·5% paraformaldehyde in PBS. Flow cytometric analysis was performed on a FACScan® flow cytometer (Becton Dickinson, Mountain View, CA). Ten thousand events were acquired for each sample.
RNA extraction and reverse transcription–polymerase chain reaction (RT-PCR) analysis
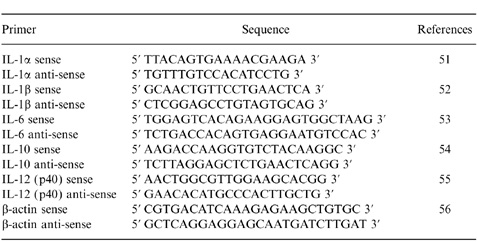
Total RNA was extracted from DC before and after coculture (on day 11) using Trizol (Gibco, BRL) according to the manufacturer’s instructions, quantified spectrophotometrically and stored at −70°. Sample or control RNA (1 μg) was reverse transcribed with SuperScript II reverse transcriptase (Gibco, BRL) at 42° for 50 min with 100 ng of random hexamer primer (Gibco, BRL) in a 20-μl reaction according to the manufacturer’s recommendations. After synthesis, the cDNA (0·8 μl) was subsequently amplified with oligonucleotides specific for cytokines tested (Table 1). The reaction cocktails contained 200 nm KCl of each oligonucleotide, 10 mm Tris–HCl, pH 8·3, 50 mm KCl, 0·001% gelatin, 50 mm MgCl2 and 0·125 units of Taq polymerase (Perkin-Elmer Cetus, Norwalk, CT) in a total volume of 20 μl. The reactions were cycled 35 times at 94° for 60 seconds, 57° for 60 seconds and 72° for 30 seconds in a DNA Thermal Cycler (Perkin-Elmer Cetus). Then 8 μl of the amplified samples were electrophoresed through a 1·5% agarose gel stained with ethidium bromide and subsequently scanned by Gel Doc 1000 (Bio-Rad).
Table 1.
Cytokine primer sequences
 |
Administration of DC and heterotopic vascularized heart transplantation
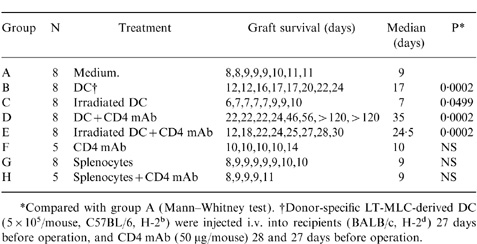
To evaluate the in vivo function of LT-MLC-derived DC, a vascularized murine heart transplantation model was employed.27 Graft rejection was defined as lack of grafted heart beating on palpation. Donor-specific DC (C57BL/6, H-2b) were harvested from LT-MLC after 6–8 weeks, washed twice, and re-suspended (2·5×106 cells/ml) in DMEM medium. In some cases, DC were irradiated (5000 rads). The DC (5×105 cells in 200 μl medium/mouse) were injected into recipients (BALB/c mice, H-2d) intravenously (i.v.) 27 days before operation. Equal numbers of donor-specific splenocytes were used as controls. In some cases, the recipients received i.v. mAb to CD4 (GK 1.5, 50 μg/mouse) at 28 and 27 days before operation (Table 2), as described previously.28 This protocol was chosen based on previous reports on donor-specific blood cell transfusions that induce tolerance.28
Table 2.
LT-MLC-derived DC prolonged allogeneic cardiac graft survival
 |
RESULTS
Splenic T (DC-T) cells exhibit a tolerogenic phenotype in long-term coculture with LT-MLC-derived DC
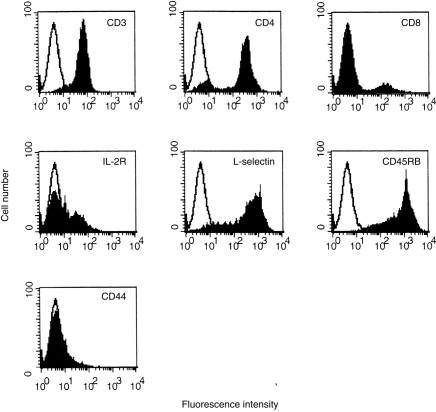
We have shown that LT-MLC-derived DC proliferate upon stimulation with irradiated allogeneic splenocytes and irradiated DC have potent MLR-stimulating activity.25 In this study, we examined the effect of non-irradiated LT-MLC-derived DC on activation and differentiation of alloreactive T cells. Allogeneic spleen cells were cocultured with non-irradiated LT-MLC-derived DC and harvested on days 3, 7 and 11, respectively. Flow cytometric analysis showed that, on day 3 of coculture, most of the splenic cells were CD3 positive (about 75–85%), and the rest were B220 or IgM positive (15–25%). Both CD4+ (45%) and CD8+ (24%) cells were recovered (data not shown). The cell composition was similar to that from time-matched conventional MLR (data not shown). On day 7 of culture, only T cells were recovered, as indicated by close to 100% of cells being CD3- positive. CD4+ T cells were about 80–90% and CD8+ cells were 10–20%. These cells were MEL-14high/moderate, CD45RBhigh/moderate, IL-2Rlow and CD44low (Fig. 1). The phenotype of IL-2Rlow is similar to that observed on tolerant T cells.20 Interestingly, the ratio and phenotypes of CD4+/CD8+ T cells remained stable after day 11 of coculture (data not shown), when the number of T cells recovered was about 5–10% of the added spleen cells. These T cells were not clonally restricted or oligoclonal, because polyclonal T-cell receptors were detected by RT-PCR with a distinct pattern from that of concanavalin A (Con A)-T cells (data not shown). The DC were required for the survival of alloreactive T cells in the coculture, since purified DC-T cells died within 1 week in the absence of DC, but survived more than 4 weeks without significant changes in cell numbers if DC were added back before they died (data not shown). These results suggest that LT-MLC-derived DC were required for the survival of DC-T cells in vitro, and the latter might be hyporesponsive upon antigen restimulation.
Figure 1.
Flow cytometric analysis of splenic T cells cocultured with LT-MLC-derived DC. Splenocytes (H-2d, BALB/c) were cocultured with LT-MLC-derived DC (H-2b, C57BL/6) for 7 days, harvested, and stained with mAb to CD3, CD4, CD8, IL-2R (CD25), CD62L (l-selectin), CD45RB, or CD44 as described in the Materials and Methods. The results are representative of three experiments (open histogram: control primary mAb). Similar results were observed after 11 days of coculture (not shown).
The degree of T-cell hyporesponsiveness induced by LT-MLC-derived DC correlates with the length of coculture
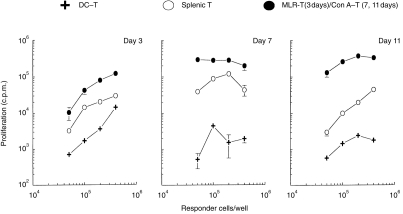
To confirm that DC-T cells were hyporesponsive upon antigen stimulation, we examined their proliferative response to alloantigens. DC-T cells (H-2d) were isolated from cocultures at day 3, 7, or 11 of coculture, and were re-stimulated with irradiated allogeneic splenocytes (H-2b). As shown in Fig. 2 (day 3), DC-T cells (H-2d) harvested on day 3 after coculture were hyporesponsive to specific alloantigens (H-2b), compared to T cells isolated from time-matched MLR-T cells. The proliferative response was also lower than that observed in primary MLR. More profound hyporesponsiveness of DC-T was observed on days 7 and 11 of coculture (Fig. 2). Thus, the degree of hyporesponsiveness induced by LT-MLC-derived DC correlated with the length of coculture, suggesting that the hyporesponsive T cells were preactivated. It was difficult to obtain sufficient time-matched MLR-T cells (at days 7 and 11) without addition of exogenous IL-2. To avoid potentially confounding effects of IL-2, we used time-matched Con A-T cells, which mainly consisted of CD4+ T cells (data not shown), as a control to exclude the possibility that the hyporesponsiveness of DC-T cells was caused by a non-specific effect of long-term culture. As shown in Fig. 2 (days 7 and 11), time-matched Con A-T cells had a strong proliferative response to allostimulation.
Figure 2.
Kinetics of the induction of T-cell hyporesponsiveness by LT-MLC-derived DC. DC-T cells (BALB/c, H-2d) isolated from the cocultures on days 3, 7 and 11 were restimulated with irradiated (3000 rads) allogeneic splenocytes (C57BL/6, H-2b) (2×105 stimulators/well) in 200 μl R10F in round-bottomed 96-well plates for 72–90 hr. Timing-matched activated T cells [MLR-T (day 3) and Con A-T (day 7 and 11)] and splenocytes were used as activated and naive T-cell controls, respectively. Proliferative response was measured by addition of [3H]TdR (0·5 μCi/well) 16–18 hr before harvesting. The results are expressed as mean c.p.m.±SD of triplicates from three to six experiments with similar results.
Next, the proliferative response of DC-T cells to third-party alloantigens was examined. As shown in Fig. 3, DC-T cells did not significantly proliferate upon stimulation by third-party alloantigens (H-2k). Their proliferative response to third-party antigens (H-2k) was even lower than to specific alloantigens (H-2b).
Figure 3.
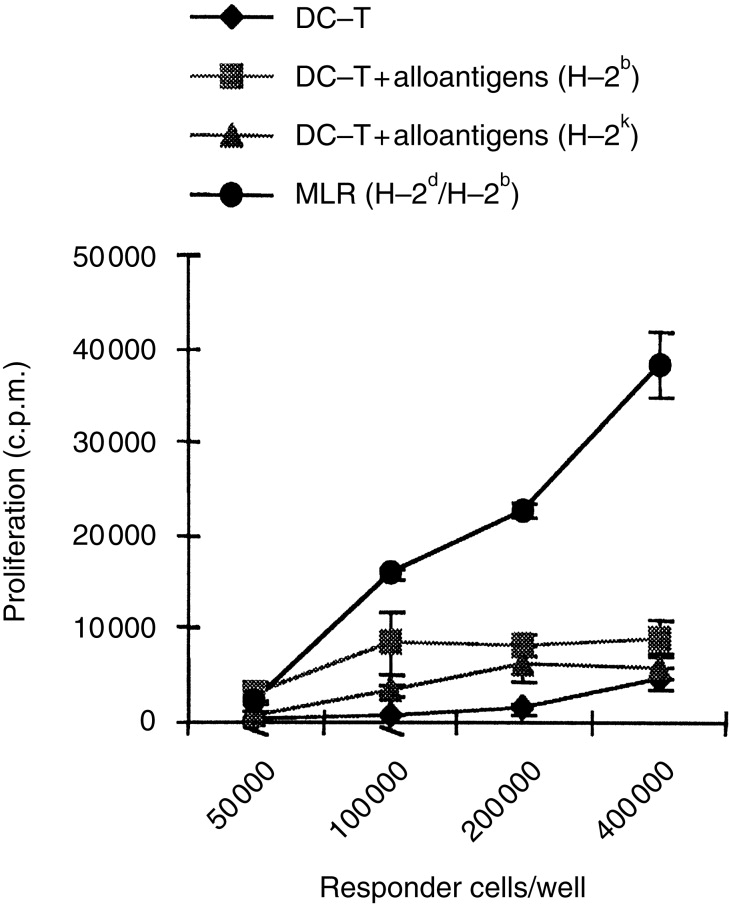
DC-T cells are unresponsiveness to third-party alloantigens. DC-T cells (H-2d, BALB/c) were harvested on day 11 after coculture with LT-MLC-derived DC and were restimulated with or without irradiated (3000 rads) antigen-specific allogeneic splenocytes (H-2b, C57BL/6) or non-antigen-specific allogeneic splenocytes (H-2k, C3H/NeN) (2×105 stimulators/well). Primary MLR (H-2d against H-2b) were used as control for normal baseline allogeneic response. The cells were cultured in 200 μl R10F in round-bottomed 96-well plates for 72–90 hr in 5% CO2 at 37°. The proliferative responses were measured by addition of [3H]TdR (0·5 μCi/well) 16–18 hr before harvesting. The results are expressed as mean c.p.m.±SD of triplicates from three experiments with similar results.
Exogenous IL-2 restores responsiveness of anergic DC-T cells
One mechanism of T-cell unresponsiveness is T-cell anergy. It is well known that anergic T cells fail to produce IL-2, which can be reversed by the addition of IL-2.22,23,29 To further confirm that the DC-T cells are anergic, we examined whether DC-T cells could produce IL-2 or whether exogenous IL-2 could reverse their hyporesponsiveness. DC-T cells harvested on day 11 were restimulated with specific alloantigens for 72 hr and the culture supernatants were examined for IL-2. Figure 4(a) shows that DC-T cells did not produce IL-2 upon re-stimulation with specific alloantigens. In contrast, naive splenic T cells produced high levels of IL-2 in response to allostimulation. Like classic anergic T cells,22,29 the hyporesponsiveness of DC-T cells was reversed when exogenous IL-2 was added to the culture in the absence or presence of irradiated allogeneic splenocytes (Fig. 4b). Taken together, these results confirm that non-irradiated LT-MLC-derived DC are able to induce T-cell anergy.
Figure 4.
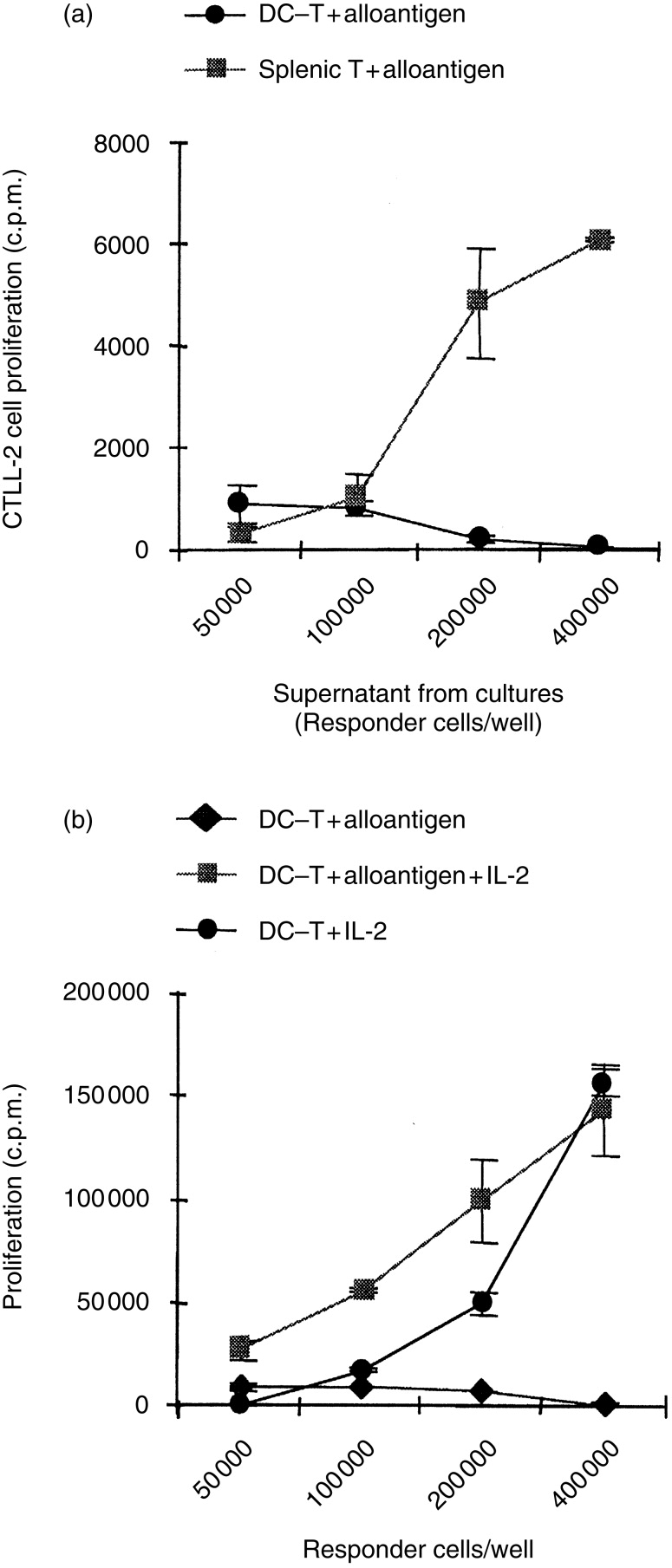
(a) DC-T cells are defective in IL-2 production. DC-T cells (H-2d, BALB/c) were isolated on day 11 after coculture with LT-MLC-derived DC, and restimulated with irradiated antigen-specific allogeneic splenocytes as described in Figure 2. Splenocytes were used as control. Fifty-microlitre culture supernatants were collected at 72 hr and tested for IL-2 production using bioassay. CTLL-2 cells, which were maintained in R10F supplemented with recombinant murine IL-2 were harvested, washed and resuspended in R10F (1×105/ml). The cell suspension (50 μl) was plated into round-bottomed 96-well plates (5000 cells/well) and 50 μl culture supernatants tested were added into each well. The cells were incubated overnight at 37° and 5% CO2 and CTLL-2 cell proliferative responses were measured by adding [3H]TdR (0·5 μCi/well) 6–8 hr before harvesting. The results are expressed as mean c.p.m.±SD of triplicates from at least three experiments. (b) Exogenous IL-2 can reverse hyporesponsiveness of DC-T cells. DC-T cells (H-2d, BALB/c) were isolated on day 11 after coculture with LT-MLC-derived DC and restimulated with or without irradiated antigen-specific allogeneic splenocytes (H-2b, C57BL/6, 2×105/well) in the presence or absence of recombinant murine IL-2 (5 ng/ml) in 200 μl R10F in round-bottomed 96-well plates. The proliferative responses were measured at 72–90 hr when cells were harvested. [3H]TdR (0·5 μCi/well) were added 16–18 hr before harvesting. The results are expressed as mean c.p.m.±SD of triplicates from five experiments.
LT-MLC-derived DC produce IL-10, but not IL-12
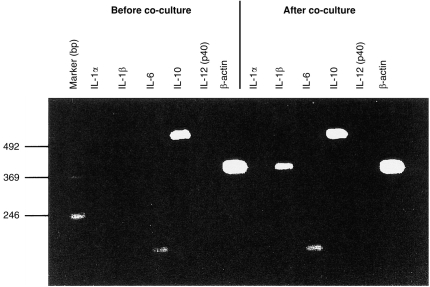
While the irradiated LT-MLC-derived DC are stimulatory in the conventional MLR,25 the same non-irradiated type of DC were tolerizing (Fig. 2 and 4), suggesting that these DC may secrete the tolerogenic cytokine IL-10 during tolerance induction. Using RT-PCR, we compared the cytokine production profile of LT-MLC-derived DC before and after coculture with allogeneic spleen cells. As shown in Fig. 5, IL-10 and IL-6 mRNA were constitutively expressed in DC before coculture, whereas mRNAs for IL-1α, IL-1β and IL-12 (p40) were not detected. IL-10 mRNA exhibited a higher level than IL-6 mRNA. Interestingly, these cytokine profiles remained stable after coculture with allogeneic spleen cells except for IL-1β mRNA, which was up-regulated. The up-regulation of IL-1β mRNA suggested that the DC had been activated during coculture.30,31 Consistent with the RT-PCR results, IL-10 protein (91·3±3·98 pg/ml, n=5), but not IL-12, was detected by enzyme-linked immunosorbent assay (ELISA) in the supernatants from day 11 of coculture. The results suggest that the polarized production of IL-10 and IL-12 by LT-MLC-derived DC contributes to the induction of T-cell hyporesponsiveness.
Figure 5.
LT-MLC-derived DC express high levels of IL-10 mRNA, but not IL-12 mRNA upon activation. LT-MLC-derived DC were harvested before and after coculture (on day 11) with allogeneic splenocytes, and mRNAs of cytokines IL-1α, IL-1β, Il-6, IL-10 and IL-12 (p40) were examined using RT-PCR. The results are representative experiment of two experiments.
LT-MLC-derived DC are defective in CD40 expression after activation
To address why LT-MLC-derived DC are unable to produce IL-12, we examined CD40 expression on DC before and after coculture with T cells, since CD40-mediated signalling not only stimulates DC to produce IL-12,32 but also up-regulates expression of B7 molecules.33 Before coculture with allogeneic splenocytes, the DC expressed CD11c, MHC class II and costimulatory molecules CD80 and CD86 (Fig. 6). This phenotype is consistent with that of stimulatory DC in conventional MLR.25 However, they expressed a very low level of CD40. On day 11 of coculture, all surface molecules tested above were down-regulated on the DC, especially for CD40, which was undetectable (Fig. 6). These results suggest that the inability of LT-MLC-derived DC to produce IL-12 during coculture may be correlated with a deficient IL-12-inducing signal provided by CD40 costimulatory molecules.34,35
Exogenous IL-12 and LPS bypass the T-cell hyporesponsiveness induced by LT-MLC-derived DC
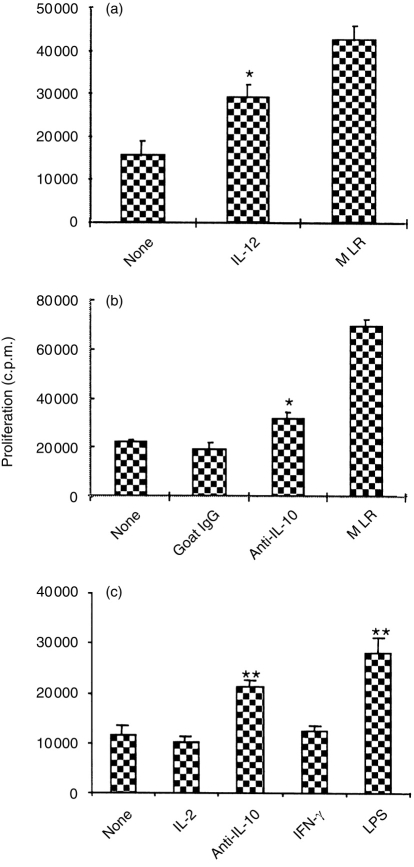
To further confirm that the skewed production of IL-10 and IL-12 by the CD40-deficient DC was correlated with induction of T-cell hyporesponsiveness, exogenous IL-12, anti-IL-10 antibody and LPS were used to correct the imbalance leading to T-cell hyporesponsiveness. Allogeneic splenocytes (H-2d) were cocultured with LT-MLC-derived DC (H-2b) for 3 days in the presence of the indicated reagents. Then, DC-T cells were isolated and re-stimulated with irradiated antigen-specific allogeneic splenocytes (H-2b). As shown in Fig. 7(a), in the presence of exogenous IL-12 the proliferative response of DC-T cells to alloantigens significantly increased >90% compared to that of DC-T cells from the cocultures in the absence of exogenous IL-12. This suggests that IL-12 is required for maintaining the responsiveness of primarily activated T cells. Since LT-MLC-derived DC constitutively secrete IL-10, a neutralizing antibody to IL-10 was used to block the induction of T-cell hyporesponsiveness. As expected, anti-IL-10 antibody significantly blocked T-cell hyporesponsiveness, compared to controls (Fig. 7b,c). The slight blockade by anti-IL-10 in some experiments was not surprising because non-irradiated DC may continuously produce IL-10 to antagonize the antibody. Since LPS can up-regulate CD40 expression on DC and stimulate them to produce IL-12,36,37 we examined whether LPS could block the induction of T-cell hyporesponsiveness. Adding LPS into the cocultures strikingly promotes proliferative responses of DC-T cells upon alloantigen re-stimulation. However, IL-2 and IFN-γ had no effects in blocking tolerance induction (Fig. 7c). In accordance, the DC isolated from LPS-stimulated cocultures expressed higher levels of CD40 and IL-12 (data not shown). Taken together, these results suggest that the balance between IL-10 and IL-12 in the context of an immune response may determine the responsiveness of DC-T cells.
Figure 7.
Exogenous IL-12, neutralizing antibody to IL-10, or LPS blocks DC-induced T-cell hyporesponsiveness. Splenocytes (H-2d, BALB/c) were cocultured with DC in D10F in 25 cm2 flasks in the presence or absence (controls) of recombinant murine IL-12 (1000 U/ml) (a), neutralizing goat antibody to IL-10 (20 μg/ml) (b and c), IL-2 (5 ng/ml), IFN-γ (400 U/ml) or LPS (1 μg/ml) (c) for 3 days. Goat IgG (20 μg/ml) was used as a control for anti-IL-10 antibody (b). Then, DC-T cells were isolated and restimulated (4×105 cells/well) with irradiated (3000 rads) antigen-specific allogeneic splenocytes (2×105 cells/well) (H-2b, C57BL/6) in 200 μl R10F in flat-bottomed 96-well plates. Secondary MLR was used as positive controls. Proliferative responses were determined at 72–90 hr by adding [3H]TdR (0·5 μCi/well) 16–18 hr before harvesting. The results are expressed as mean c.p.m.±SD of a representative from three experiments. *P≤0·05; **P<0·01, compared to controls (analysis of variance).
An infusion of LT-MLC-derived DC prolongs the survival of cardiac allografts
To confirm the in vitro finding that LT-MLC-derived DC can induce T-cell hyporesponsiveness, we investigated whether DC could induce T-cell hyporesponsiveness in vivo in a murine heterotopic vascularized cardiac transplantation model.28 Donor-specific DC (5×105) (C57BL/6, H-2b) were injected i.v. alone into recipients (BALB/c, H-2d) 27 days before operation, or in combination with two doses of CD4 mAb (GK1.5, 50 μg/mouse) given i.v. at 27 and 28 days before operation. As shown in Table 2, pretreatment with donor-specific DC significantly prolonged cardiac allografts [median survival time (MST): 17 days versus control 9 days]. A synergistic effect was observed when DC treatment was combined with CD4 mAb, and 25% of the grafts survived more than 100 days (MST=35 days). The CD4 mAb treatment alone did not prolong the survival of cardiac grafts (MST=10 days), compared with untreated animals (MST=9 days). The increased survival of the cardiac grafts was actively mediated by live DC, because treatment with irradiated DC did not prolong the graft survival, in fact, allograft rejection was slightly, but significantly, accelerated (MST=7 versus 9 days, P=0·05). Interestingly, treatment with irradiated DC under the coverage of CD4 mAb significantly prolonged graft survival (MST=24·5 days), suggesting that antigenic signals provided by irradiated DC could be modulated by immunosuppressants such as CD4 mAb. The prolonged graft survival was specifically mediated by DC, since infusion of donor-specific spleen cells alone or in combination with CD4 mAb had no effect on graft survival (MST=9 days, respectively). In addition, mice that accepted heart grafts for >100 days were grafted with donor-specific (C57BL/6, H-2b) and third-party (C3H/HeN, H-2k) skin grafts simultaneously. The donor-specific skin grafts survived significantly longer than third-party grafts (8 versus 28 days). These results are consistent with the in vitro observation that irradiated LT-MLC-derived DC are immunogenic, whereas the non-irradiated are tolerogenic.
DISCUSSION
We have reported previously that LT-MLC-derived CD11c+ DC can proliferate upon antigen stimulation and survive in vitro long-term.25 In this study, we further investigate the effect of DC proliferation on T-cell responses and characterize the phenotype and cytokine production profiles of DC during interaction with T cells. There are three major findings in this study. First, DC proliferation in the context of immune responses are required for the induction of T-cell tolerance, since irradiated LT-MLC-derived DC are immunogenic, while non-irradiated LT-MLC derived DC are tolerogenic, as observed in vitro (ref. 25 and Fig. 2) and in vivo (Table 2). Secondly, tolerogenic DC express MHC class II and B7 stimulatory molecules, but not CD40. This phenotype may allow tolerogenic DC to prime naive T cells, a process required for tolerance induction,23 but not to produce IL-12, a critical costimulator for T-cell activation.30 Thirdly, tolerogenic DC constitutively express high levels of IL-10 and have a skewed balance between IL-10 and IL-12 production. Interestingly, this polarized cytokine profile does not change with DC activation (Fig. 5). This is a demonstration that endogenous cytokines play important roles in the regulation of DC function.
Our DC-mediated T-cell tolerance induction system is different from that previously reported by Lu et al.38 They used GM-CSF-propagated, short-lived bone-marrow-derived DC. These DC seem to be defective in expression of MHC class II and B7-2, but not B7-1 costimulatory molecules and are not stimulatory in conventional MLR.38 In contrast, long-lived LT-MLC-derived DC express sufficient levels of MHC class II and B7 costimulatory molecules and are potentially stimulatory in MLR (ref. 25 and Fig. 6). However, these DC have the ability to induce T-cell hyporesponsiveness in vitro and in vivo (Fig. 2 and 4, and Table 2). This seems to contradict the previous finding that these DC are potent stimulators when they were irradiated in the conventional MLR.25 However, the coculture condition used in this study is different from that in the conventional MLR. In the conventional MLR, LT-MLC-derived DC were irradiated and did not proliferate upon antigen stimulation.25 They might continue to mature to the terminal stage upon interaction with T cells3,4,12,34 and display stimulatory activity.25,32,34 However, non-irradiated DC can proliferate upon interaction with T cells25 and may not differentiate further into terminal mature DC. This hypothesis is supported by the findings that CD11c expression on the DC was down-regulated after coculture and that these DC are less mature compared to those before coculture as indicated by the down-regulation of MHC class II and B7 molecules (Fig. 6). The level of CD11c expression is correlated with the degree of DC maturation, since cytokines TNF-α and IL-4, and LPS, which can drive DC maturation,15,37 can up-regulate CD11c expression on LT-MLC-derived DC (our unpublished observations). Secondly, the degree of T-cell hyporesponsiveness induced by LT-MLC-derived DC correlates with the length of coculture (Fig. 2), suggesting that the non-irradiated DC were initially stimulatory. The prolonged coculture not only satisfied the need for T-cell priming or activation in the early stage, which is thought to be necessary for the induction of T-cell tolerance,39–41 but also provided long-lived immature DC that may continuously produce cytokines such as IL-10 to support a tolerogenic environment at the later stage of immune responses. Consistent with in vitro observations, infusion of donor-specific irradiated LT-MLC-derived DC significantly accelerated heart graft rejection. In contrast, infusion of non-irradiated LT-MLC-derived DC significantly prolonged graft survival (Table 2). Thus, non-irradiated DC are substantially different from irradiated DC in the regulation of immune responses in vitro and in vivo. It is likely that under physiological conditions, stimulatory DC may differentiate into tolerizing DC, as long as they do not differentiate into the terminal mature stage.3,6,10,14
Why can LT-MLC-derived DC be switched from stimulatory to tolerizing DC? Analysis of cytokine profiles of the DC before and after coculture revealed that these DC constitutively express IL-10 and IL-6, but not IL-12. Interestingly, this cytokine profile did not change even after DC activation, as indicated by up-regulation of IL-1β mRNA (Fig. 5), an activation marker for DC and macrophages.30,31 Although it has been shown that IL-10 is a critical factor for the induction of T-cell tolerance,18,20 it is not clear whether tolerogenic DC can produce IL-10 in the context of an immune response. In this study we clearly show that LT-MLC-derived DC spontaneously produce IL-10 throughout the tolerance induction. Autocrine IL-10 may act on DC themselves, and thus inhibit IL-1235,42 and B7 molecule expression.43,44 IL-12 is an important cytokine for T-cell activation and differentiation.30 The inability of LT-MLC-derived DC to produce IL-12 leads to an IL-10-dominated environment in the context of an immune response and favours the induction of T-cell hyporesponsiveness. This polarized cytokine production profile (IL-10high, IL-12deficient) determines the tolerogenicity of DC. This conclusion is further supported by the fact that addition of exogenous IL-12 or anti-IL-10 antibody significantly inhibited T-cell hyporesponsiveness induced by LT-MLC-derived DC (Fig. 7).
CD40 is another important costimulatory molecule in the regulation of DC maturation.7,8,32 Ligation of CD40 not only triggers secretion of IL-1232,34 but also up-regulates expression of B733 and other costimulatory molecules.45 During an immune response, CD40+ DC may engage with activated CD40L+ T cells to amplify their costimulatory activity by triggering IL-12 production. Since CD40 signalling may antagonize IL-10 signalling, we predicted that tolerogenic DC may be defective in CD40 signalling. In confirmation of that hypothesis, LT-MLC-derived DC expressed a very low level of CD40 before coculture, which was further down-regulated during coculture. This may explain why IL-12 was still absent even when the DC were activated. Thus, up-regulation of CD40 may block tolerance induction. As expected, addition of LPS to the coculture significantly blocked tolerance induction (Fig. 7), because LPS is able to promote CD40 expression36 and IL-12 production37 by LT-MLC-derived DC (data not shown). It is not clear why CD40 expression is not up-regulated in the coculture when the DC were activated (Fig. 6). Further investigation of this issue is ongoing in our laboratory.
Notably, addition of neutralizing anti-IL-10 antibody into the cocultures sometimes slightly reverses T-cell hyporesponsiveness (Compare Fig. 7b and c). This, however, is not surprising. In cocultures, the DC continuously produce IL-10 to antagonize the antibody and it is difficult to completely block endogenous IL-10. In addition, the antibody itself does not have the ability to correct defects in CD40 expression and IL-12 production by tolerogenic DC, even though IL-10 is completely blocked by the antibody. In contrast, in the IL-10-treated culture system, completely blocking exogenous IL-10 may prevent DC differentiation into tolerogenic ones.18 Thus, it is possible that anti-IL-10 antibody treatment does not reverse T-cell hyporesponsiveness in our system as much as in the IL-10-treated system.18
To our surprise, DC-T cells showed unresponsiveness to third-party alloantigens (Fig. 3). However, this is consistent with the in vivo observation by Fu et al. that infusion of cytokine-induced bone-marrow-derived immature DC significantly prolonged the survival of third-party grafts in a murine heart transplantation model.17 There are several potential explanations for why this may be the case. First, antigen-specific DC-T cells are anergic, and could inhibit other populations that may respond to third-party alloantigens.46,47 In agreement with this hypothesis, we found that anergic DC-T cells potently suppressed primary MLR by 90%. This suppression appears to be mediated by soluble factor(s), which were produced by DC-T cells rather than DC (our unpublished observations). Alternatively, DC-T cell populations may contain predominantly antigen-specific alloreactive T cells with few third-party-specific T cells that may have died in culture.48
Consistent with in vitro results, LT-MLC-derived DC induce significant hyporesponsiveness in vivo in a vascularized heart transplantation model. Mice pretreated with an infusion of donor-specific LT-MLC-derived DC 1 month before transplantation had a significantly prolonged graft survival. This was an active process because treatment with irradiated DC at 27 days before operation (Table 2) or non-irradiated DC at the operation day did not prolong graft survival (data not shown). The tolerogenic effect of DC was enhanced under the coverage of a mAb that partially depleted CD4 cells: approximately 25% of these recipients survived >120 days. Donor-specific skin grafts survived significantly longer than third-party skin grafts (8 days versus 28 days). This effect was actively mediated by DC rather than by CD4 mAb, as treatment with CD4 mAb alone or in combination with splenocytes had no effect on graft survival (Table 2). In addition, the effect of LT-MLC-derived DC in prolonging graft survival cannot be attributed to a blood transfusion effect, since the infusion of splenocytes alone did not have such an effect. Further studies are underway to see if prolonged, specific unresponsiveness can be achieved by additional manipulations of the protocol, as peripheral tolerance can be modulated at multiple levels depending on environmental stimuli.49,50
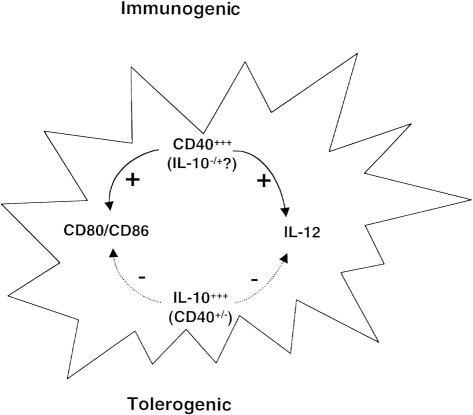
In summary, we have characterized the properties of tolerogenic DC. The ability of tolerogenic DC to proliferate upon interaction with T cells may be required for maintaining their tolerogenicity, otherwise they may differentiate into immunogenic DC as in the case of the irradiated DC (ref. 25 and Table 2). Under physiological conditions, stimulatory DC may differentiate into tolerogenic DC, depending on the cytokine milieu of the immune response. The balance of signals between IL-10 and CD40 or IL-12 (Fig. 8) may determine the outcome of an immune response.
Figure 8.
Mechanisms of dendritic cell differentiation into tolerogenic or immunogenic APC. CD40 and IL-10 may play critical roles in determining whether DC are tolerogenic or immunogenic. CD80/CD86 and IL-12 are regulated by signals from CD40 or IL-10. The products of infectious agents such as LPS may modulate CD40 expression.
Acknowledgments
This work was partially supported by the Medical Research Council of Canada Grant MT-11457, the Kidney Foundation of Canada and the London Health Sciences Center. Dr David Grant is an Ontario Ministry of Health Career Scientist. The authors are grateful to Dr S. Wolf at the Genetics Institute, who provided us with reagents for IL-12, Dr A. Abbas at Harvard University, who critically reviewed the manuscript, and Dr A. M. Jevnikar at the University of Western Ontario, who provided cytokine primers. We also acknowledge the expert advice of Dr R. Mukherjee.
Abbreviations
- APC
antigen-presenting cells
- DC
dendritic cells
- LT-MLC
long-term mixed leucocyte culture
- MLR
mixed leucocyte reaction
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Parijs LV, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Lanzavecchia A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J Exp Med. 1999;189:611. doi: 10.1084/jem.189.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rissoan M-C, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:183. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 5.Linsley PS. Distinct roles for CD28 and cytotoxic T lymphocyte-associated molecule-4 receptors during T cell activation. J Exp Med. 1995;182:289. doi: 10.1084/jem.182.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rescigno M, Winzler C, Delia D, Mutini C, Lutz M, Ricciardi CP. Dendritic cell maturation is required for initiation of the immune response. J Leukoc Biol. 1997;61:415. [PubMed] [Google Scholar]
- 7.Grewal IS, Flavell RA. The role of CD40 ligand in co-stimulation and T-cell activation. Immunol Rev. 1996;153:85. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 8.Mackey MF, Gunn JR, Maliszewski C, Kikutani H, Noelle RJ, Barth RJ. Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J Immunol. 1998;161:2094. [PubMed] [Google Scholar]
- 9.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157:1406. [PubMed] [Google Scholar]
- 10.Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998;160:4587. [PubMed] [Google Scholar]
- 11.Lu L, Woo J, Rao AS, et al. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen. J Exp Med. 1994;179:1823. doi: 10.1084/jem.179.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geissmann F, Prost C, Monnet J-P, Dy M, Brousse N, Hermine O. Transforming growth factor-β1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans’ cells. J Exp Med. 1998;187:2417. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winzler C, Rovere P, Rescigno M, et al. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185:317. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hausser G, Ludewig B, Gelderblom HR, Tsunetsugu-Yokota Y, Akagawa KS, Meyerhans A. Monocyte-derived dendritic cells represent a transient stage of differentiation in the myeloid lineage. Immunobiology. 1997;197:534. doi: 10.1016/S0171-2985(97)80085-X. [DOI] [PubMed] [Google Scholar]
- 15.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akagawa KS, Takasuka N, Nozaki Y, et al. Generation of CD1+RelB+ dendritic cells and tartrate-resistant acid phosphatase-positive osteoclast-like multinucleated giant cells from human monocytes. Blood. 1996;88:4029. [PubMed] [Google Scholar]
- 17.Fu F, Li Y, Qian S, et al. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86−) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62:659. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772. [PubMed] [Google Scholar]
- 19.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type-2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28. [PubMed] [Google Scholar]
- 20.Groux HM, de Bigler VJ, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonysamy MA, Steptoe RJ, Khanna A, Rudert WA, Subbotin VM, Thomson AW. Flt-3 ligand increases microchimerism but can prevent the therapeutic effect of donor bone marrow in transiently immunosuppressed cardiac allograft recipients. J Immunol. 1998;160:4106. [PubMed] [Google Scholar]
- 22.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? J Exp Med. 1996;184:1. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malvey E-N, Telander DG, Vanasek TL, Mueller DL. The role of clonal anergy in the avoidance of autoimmunity: inactivation of autocrine growth without loss of effector function. Immunol Rev. 1998;165:301. doi: 10.1111/j.1600-065x.1998.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 24.Perez VL, Parijs LV, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 25.Gao JX, Madrenas J, Zeng W, Zhong R, Grant D. Generation of dendritic cell-like antigen-presenting cells in long-term mixed leukocyte culture: phenotypic and functional studies. Immunology. 1997;91:135. doi: 10.1046/j.1365-2567.1997.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cameron MJ, Arreaza GA, Zucker P, et al. IL-4 prevents insulitis and insulin-dependent diabetes mellitus in nonobese diabetic mice by potentiation of regulatory T helper-2 cell function. J Immunol. 1998;159:4686. [PubMed] [Google Scholar]
- 27.Zhang Z, Zhu L, Quan D, et al. Pattern of liver, kidney, heart, and intestine allograft rejection in different mouse strain combinations. Transplantation. 1996;62:1267. doi: 10.1097/00007890-199611150-00016. [DOI] [PubMed] [Google Scholar]
- 28.Bushell A, Pearson TC, Morris PJ, Wood KJ. Donor-recipient microchimerism is not required for tolerance induction following recipient pretreatment with donor-specific transfusion and anti-CD4 antibody. Evidence of a clear role for short-term antigen persistence. Transplantation. 1995;59:1367. doi: 10.1097/00007890-199505270-00001. [DOI] [PubMed] [Google Scholar]
- 29.Mueller DL, Jenkins MK. Molecular mechanisms underlying functional T-cell unresponsiveness. Curr Opin Immunol. 1995;7:375. doi: 10.1016/0952-7915(95)80113-8. [DOI] [PubMed] [Google Scholar]
- 30.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 31.Rutherford MS, Witsell A, Schook LB. Mechanisms generating functionally heterogeneous macrophages: chaos revisited. J Leukoc Biol. 1993;53:602. doi: 10.1002/jlb.53.5.602. [DOI] [PubMed] [Google Scholar]
- 32.Cella M, Scheidegger D, Palmer LK, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Wilson JM. CD40 ligand-dependent T cell activation: requirement of B7–CD28 signaling through CD40. Science. 1996;273:1862. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 34.Mackey MF, Barth RJ, Noelle RJ. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J Leukoc Biol. 1998;63:418. doi: 10.1002/jlb.63.4.418. [DOI] [PubMed] [Google Scholar]
- 35.Kock F, Stanzl U, Jennewein P, et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and donwregulation by IL-4 and IL-10. J Exp Med. 1999;184:741. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suri RM, Austyn JM. Bacterial lipopolysaccharide contamination of commercial collagen preparations may mediate dendritic cell maturation in culture. J Immunol Methods. 1998;214:149. doi: 10.1016/s0022-1759(98)00048-9. [DOI] [PubMed] [Google Scholar]
- 37.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of co-stimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919. [PubMed] [Google Scholar]
- 38.Lu L, McCaslin D, Starzl TE, Thomsone AW. Bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7dim, B7− induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation. 1995;60:1539. doi: 10.1097/00007890-199560120-00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkins MK. The role of cell division in the induction of clonal anergy. Immunol Today. 1992;13:69. doi: 10.1016/0167-5699(92)90137-V. [DOI] [PubMed] [Google Scholar]
- 40.Vidard L, Colarusso LJ, Benacerraf B. Specific T-cell tolerance may be preceded by a primary response. Proc Natl Acad Sci USA. 1994;91:5627. doi: 10.1073/pnas.91.12.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Risdon G, Daddy J, Horie M, Broxmeyer HE. Alloantigen priming induces a state of unresponsiveness in human umbilical cord blood T cells. Proc Natl Acad Sci USA. 1995;92:2413. doi: 10.1073/pnas.92.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Human dendritic cell responses to lipopolysaccharide and CD40 ligation are differentially regulated by interleukin-10. Eur J Immunol. 1997;27:1848. doi: 10.1002/eji.1830270805. [DOI] [PubMed] [Google Scholar]
- 43.Willems FA, Marchant JP, Delville C, et al. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur J Immunol. 1994;24:1007. doi: 10.1002/eji.1830240435. [DOI] [PubMed] [Google Scholar]
- 44.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224. [PubMed] [Google Scholar]
- 45.Lee HH, Dempsey PW, Parks TP, Zhu X, Baltimore D, Cheng G. Specificities of CD40 signaling: involvement of TRAF2 in CD40-induced NF-kappaB activation and intercellular adhesion molecule-1 up-regulation. Proc Natl Acad Sci USA. 1999;96:1421. doi: 10.1073/pnas.96.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Groux H, O’Garra A, Bigler M, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 47.Lombardi G, Sidhu S, Batchelor R, Lechler R. Anergic T cells as suppressor cells in vitro. Science. 1994;264:1587. doi: 10.1126/science.8202711. [DOI] [PubMed] [Google Scholar]
- 48.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 49.Arnold B, Schonrich G, Hammerling GJ. Multiple levels of peripheral tolerance. Immunol Today. 1993;14:12. doi: 10.1016/0167-5699(93)90317-E. [DOI] [PubMed] [Google Scholar]
- 50.Taams LS, van Eden W, Wauben MHM. Dose-dependent induction of distinct anergic phenotypes: multiple levels of T cell anergy. J Immunol. 1999;162:1974. [PubMed] [Google Scholar]
- 51.Lomedico PT, Gubler U, Hellmann CP, et al. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. Nature. 1984;312:458. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- 52.Gray PW, Glaister D, Chen E, Goeddel DV, Pennica D. Two interleukin 1 genes in the mouse: cloning and expression of the cDNA for murine interleukin 1 beta. J Immunol. 1986;137:3644. [PubMed] [Google Scholar]
- 53.Chiu CP, Moulds C, Coffman RL, Rennick D, Lee F. Multiple biological activities are expressed by a mouse interleukin 6 cDNA clone isolated from bone marrow stromal cells. Proc Natl Acad Sci USA. 1988;85:7099. doi: 10.1073/pnas.85.19.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mosman TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI [published erratum appears in Science 1990 October 26, 250 (4980): 494] Science. 1990;248:1230. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- 55.Wolf SF, Temple PA, Kobayashi M, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074. [PubMed] [Google Scholar]
- 56.Alonso S, Minty A, Bourlet Y, Buckingham M. Comparison of three actin-coding sequences in the mouse, evolutionary relationships between the actin genes of warm-blooded vertebrate. J Mol Evol. 1986;23:11. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]