Abstract
Eosinophils induce tissue injury by releasing granule-associated cytotoxic proteins, lipid mediators and superoxide anions in response to appropriate stimuli. Superoxide generation associated with respiratory burst is largely dependent on the assembly of the NADPH oxidase complex in the membrane, consisting of membrane-bound cytochrome b558 and translocated p47phox and p67phox. The activation of this complex is critically dependent on the translocation of GTP-bound Rac1, or its homologue Rac2, from the cytosol to the membrane in neutrophils. Rac expression has not yet been fully characterized in eosinophils. We proposed that eosinophils translate and express Rac2 and its GDP-dissociation inhibitor, RhoGDI. Furthermore, we hypothesized that Rac2 translocates along with p47phox and p67phox proteins from the cytosol to the plasma membrane during respiratory burst. By reverse transcription–polymerase chain reaction analysis and sequencing of the amplified product, guinea-pig eosinophils were found to express Rac2 mRNA, exhibiting 93% homology with the human Rac2 sequence. Rac1 mRNA was also detected in eosinophils but not its translated product. In contrast, Rac2 protein expression was detected using a specific antibody. In subcellular fractions, Rac2 was found to translocate, along with p47phox and p67phox, from cytosol to plasma membrane-associated fractions following phorbol myristate acetate stimulation, while RhoGDI remained within cytosolic fractions. These findings suggest that Rac2 is preferentially expressed and activated in eosinophils, and is likely to be a crucial regulator of the release of reactive oxygen species from these cells during inflammatory reactions.
INTRODUCTION
Eosinophils are major effector cells in atopic disorders and helminthic infections. They are recruited to sites of inflammation and activated in response to locally released chemotactic and immune stimuli. During activation, eosinophils discharge an array of cytotoxic substances, including lipid mediators, crystalloid granule proteins, and products of respiratory burst. Eosinophils are capable of elaborating relatively high concentrations of tissue-damaging superoxide and its toxic metabolites, collectively known as reactive oxygen species (ROS).1 The production of ROS is enhanced in eosinophils from atopic subjects2,3 and elevated products of respiratory burst have been detected in breath condensate samples from asthmatic individuals.4 It is likely that the generation of superoxide from eosinophils is an integral component in the pathophysiology of asthma, since their numbers increase in proportion to disease severity.5
A major component of superoxide generation in inflammatory cells is the membrane-associated NADPH oxidase complex.6,7 This oxidative enzyme complex is latent in resting cells, and is assembled within plasma and phagosomal membranes of neutrophils following phagocytosis of foreign bodies following cell stimulation.7 NADPH oxidase contains four proteins essential for its activation, as established in cell-free assays.1,8,9 They are cytochrome b558, p47phox, p67phox, and the GTPase Rac210. With the exception of cytochrome b558, these proteins are cytosolic in resting cells, with Rac2 bound to cytosolic Rho GDP-dissociation inhibitor (RhoGDI).10 Upon activation, the phox proteins bind to membrane-associated cytochrome b558, followed by Rac2 dissociation from RhoGDI and translocation to the complex to initiate superoxide generation.11,12 The association of Rac2 with NADPH oxidase is crucial for activation of the enzyme complex. Studies have shown that a dominant negative form of Rac2 inhibits superoxide production from NADPH oxidase.13
There is little evidence for Rac1 or Rac2 expression in eosinophils, although one report suggests that guinea-pig eosinophils express mainly Rac1, based on immunoblot analysis.14 Guinea-pig neutrophils and macrophages also express Rac1 as their dominant isoform.9,15 In contrast, human neutrophils express predominantly the Rac2 isoform which is required for NADPH oxidase activation.11,12,16
We proposed that guinea-pig eosinophils instead express Rac2, based on our preliminary findings obtained by reverse transcription–polymerase chain reaction (RT-PCR) and immunoblot analysis. We also hypothesized that Rac2 is activated and translocated to the plasma membrane during respiratory burst. To test these hypotheses, we prepared purified peritoneal guinea-pig eosinophils from suitably primed animals and stimulated them with phorbol myristate acetate (PMA), a potent inducer of superoxide generation in these cells.1,17 Rac1 and Rac2 mRNA expression was analysed by RT-PCR, and the PCR product was cloned and sequenced. The expression of Rac1, Rac2, RhoGDI, p47phox and p67phox was examined by Western blot analysis. Our results suggest that, in contrast to an earlier report,14 Rac2 protein is the dominant isoform expressed in eosinophils and that it is likely to be required for activation of NADPH oxidase.
MATERIALS AND METHODS
Materials
Catalase, ferricytochrome c (from horse heart), PMA, and superoxide dismutase (from bovine erythrocytes) (SOD), were acquired from Sigma (Oakville, Ont., Canada). Guanosine- 5′-O-(3-thiotriphosphate) (GTPγS) was obtained from Boehringer Mannheim, Laval, Québec, Canada. Rabbit polyclonal antiserum to recombinant human Rac2 was used as previously described.12 This antibody was highly specific for Rac2, as it failed to detect Rac1 in our preparations of guinea-pig macrophages (data not shown). Affinity-purified rabbit polyclonal antibodies to consensus peptide sequences from Rac1 and RhoGDI were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Monoclonal anti-Rac antibody was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). Rabbit polyclonal antisera raised against recombinant human p47phox and p67phox were kindly provided by Dr William Nauseef, University of Iowa, Iowa City, IA.
Preparation of eosinophils
Male Dunkin Hartley guinea-pigs (≥400 g) were primed for peritoneal eosinophil production by intraperitoneal (i.p.) injection of 1 ml sterile horse serum (Gibco/BRL Life Technologies, Grand Island, NY) three times per week, with a minimum of 8 weeks of injections carried out before collection of cells by peritoneal lavage. Eosinophils were collected and purified by centrifugation down a discontinuous Percoll gradient, as previously described.18 Typical eosinophil yields were ≈2·5×107 per animal (≥95% purity) as determined using Kimura’s stain.19
RT-PCR
Total RNA was extracted from batches of eosinophils reaching ≥97% purity by following the manufacturer’s instructions for the QIAGEN QIAShredder and RNeasy MiniKit (QIAGEN Inc, Mississauga, Ont., Canada) using 2×106 cells per extraction (producing ≈1 μg total RNA). The RT-PCR reaction was carried out using Moloney murine leukaemia virus (MMLV) reverse transcriptase (Gibco/BRL) and Taq polymerase (Gibco/BRL). The positive control for the reaction was β-actin as described previously.20 Primer sequences used for detecting Rac1/2 mRNA were as follows: degenerate Rac1/2 primers [product size=406 base pairs (bp)]: forward 2–24 bp 5′-TGCAGGCCATCAAGTGTGTGGTG-3′; reverse 385–407 bp 5′-GGAGCCAGCTTCTTCTCCTTCAG-3′; and Rac2-specific primers (product size=577 bp): forward 3–25 bp 5′-GCAGGCCATCAAGTGTGTGGTGG-3′; reverse 556–578 bp 5′-TAGAGGAGGCTGCAGGCGCGCTT-3′.
Both the reverse transcription and amplification were carried out in a PTC 100 Programmable Thermal Controller (M-J Research, Inc, Watertown, MA). After denaturing at 94° for 1 min, the reaction was conducted for 30 cycles at 94° for 45 seconds, 57° for 1 min (Rac1/2 primers) or 56° for 1 min (Rac2-specific primers), and 72° for 30 seconds before a final elongation step at 72° for 7 min. Products were visualized by electrophoresis on a 1·2% agarose gel and staining of gels in ethidium bromide.
Cloning and sequencing of cDNA bands
The amplified product was subcloned into the pCR™2.1 plasmid vector using the T/A cloning kit (Invitrogen Corporation, Carlsbad, CA). Plasmid DNA was isolated with the Wizard Plus Mini-prep kit (Promega Corporation, Madison, WI). Double-stranded DNA sequencing was performed using M13 and Rac2-specific forward and reverse primers. Sequencing was conducted using an ABI 373A automated sequencer (Applied Biosystems, Foster City, CA) by a dideoxy-chain-termination method.
Measurement of superoxide release from eosinophils
Superoxide generation in intact cells was measured at 25° by spectrophotometric assay of ferricytochrome c reduction in 1·0-ml microcuvettes containing 2×106 cells and 50 μm cytochrome c in phosphate-buffered saline (PBS), pH 7·4 supplemented with 1·2 mm MgCl2, 5 mm KCl, 0·5 mm CaCl2, 5 mm glucose, and 0·1% bovine serum albumin (BSA) (PBS+). The mixture was blanked at 550 nm in a Beckman DU 640 spectrophotometer (Beckman Instruments Inc, Mississauga, Ont., Canada) before adding 500 ng/ml PMA and recording the time-dependent release of superoxide from eosinophils. Superoxide dismutase-inhibitable absorbance was calculated using ε=2·11×104 m−1 cm−1 for reduced cytochrome c.
Subcellular fractionation
Purified peritoneal eosinophils (≥5×107 cells) were washed at ambient temperature then prewarmed to 37° in 5 ml PBS+ with 250 U/ml catalase and 50 U/ml SOD for 5 min. At the start of the reaction, PMA (final concentration 500 ng/ml) was added and the incubation continued at 37°. The reaction was terminated after 8 min by the addition of 10 ml ice-cold PBS+ and the cells were washed in ice-cold HEPES-buffered sucrose (0·25 m sucrose, 10 mm HEPES, pH 7·4, and 1 mm EGTA). Resting or stimulated cells (50×106) were subjected to homogenization by 10–20 passages through a 12-μm clearance in a ball-bearing homogenizer (EMBL, Heidelberg, Germany), with resulting organelles separated by a linear Nycodenz density gradient as previously described.18 Normally, 16 fractions, each of 0·8 ml, were collected and stored at −80° until used for each preparation.
Cell-free superoxide release
Superoxide release in Nycodenz gradient fractions prepared from PMA-stimulated eosinophils was measured at 25° using a modification of a procedure described for neutrophil fractions.12,21 Reaction mixtures were prepared by combining the cell-free assay buffer (10 mm Na2HPO4, 10 mm NaH2PO4, 130 mm NaCl, 2 mm EGTA, 2 mm MgCl2, and 2·7 mm KCl, pH 7·2) with 10 μm flavin adenine dinucleotide, 50 μm cytochrome c, 2 mm sodium azide, 200 μm NADPH, 10 μm GTPγS, 100 μm sodium dodecyl sulphate, 20 μl membrane fraction and 20 μl cytosol in a 1·0-ml cuvette. The resulting absorbance change was recorded against time at 550 nm. In separate reactions, 8 μg/ml SOD was added to determine the baseline absorbance change in fractions containing maximal superoxide-generating capacity.
Marker enzyme assays
Activities of alkaline phosphodiesterase (APDE, plasma membrane marker), eosinophil peroxidase (crystalloid granule marker), and β-hexosaminidase (marker for secretory/small granules and lysosomes) were determined in supernatants and pellets using modifications of microtitre plate assays previously described.18 Cytosolic activity was determined using a modification of a microtitre plate end-point assay for lactate dehydrogenase (LDH).22
Western blot analysis
Samples of cell homogenates and fractions were subjected to electrophoretic separation on acrylamide gels and transferred to Immobilon polyvinyl difluoride membrane blots as previously described.18 Immediately after transfer, blots were blocked for 1 hr in 5% goat serum, 5% milk in Tris-buffered saline (TBS), pH 7·6 containing 0·2% Tween-20 (TBS-Tween). Primary rabbit polyclonal antibodies to Rac1 (1:500, 2 hr), Rac2 (1:500, 2 hr), RhoGDI (1:500, 1 hr), p47phox (1:1000, 1 hr), and p67phox (1:1000, 2 hr) were incubated at room temperature before washing in TBS-Tween. Secondary antibody, 1:5000 donkey anti-rabbit immunoglobulin G (IgG) antibody conjugated to horseradish peroxidase (Amersham Canada Ltd, Oakville, Ont., Canada), was added to detect primary antibody labelling. Chemiluminescence was developed by addition of SuperSignal substrate solution (Pierce, Rockford, IL) and blots were exposed to film for between 1 and 60 min (Hyperfilm ECL, Amersham). The resulting bands were scanned and quantified in a gel scanner (ImageMaster DTS, Pharmacia, Uppsala, Sweden).
Confocal laser scanning microscopy
Cytospins of eosinophils (100 μl of 0·5×106 cells/ml in RPMI supplemented with 20% fetal calf serum) were made by spinning slides in a Cytospin 2 (Shandon Ltd, Astmoor, Runcorn, UK) centrifuge (70 g for 2 min) followed by fixing in 2% paraformaldehyde in PBS for 6 min, as optimized in our previous report.23 Immunoreactivity to Rac2 was detected using 20 μg/ml rhodamine-conjugated goat anti-rabbit antibody (Molecular Probes, Eugene, OR). Slides were examined using a ×100 objective under a Leica confocal laser scanning microscope (CLSM; Heidelberg, Germany), and images were collected and processed as described previously.23,24
RESULTS
RT-PCR, cloning and sequencing of the PCR product
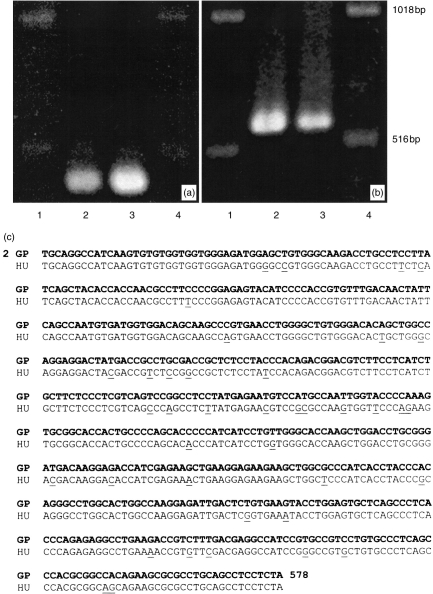
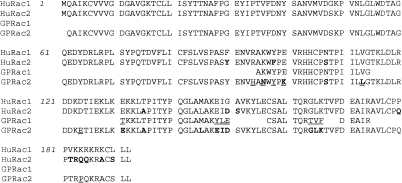
Guinea-pig eosinophils were used in this study since they are more abundant than human eosinophils and are more consistently reproducible in experimental analysis. Samples of cDNA prepared from purified guinea-pig eosinophils (≤97% purity) were amplified by PCR using one set of degenerate Rac primers and another set of Rac2-specific primers based on human Rac sequences. As shown in Fig. 1(a), the degenerate primer pair amplified a product (406 bp) from total eosinophil RNA. Cloning and sequencing of this product generated a partial sequence which was highly homologous to human Rac1 (97%; GenBank access. no. M29870.1; data not shown). Using Rac2-specific primers, the PCR reaction generated a product of size 577 bp (Fig. 1b). After cloning, sequencing of the 577 bp product from two different clones (Genbank access. no. AF085341) revealed 93% identity with human Rac2 mRNA (Genbank access. no. NM_002872.1; Fig. 1c). The differences between the two species appeared to be randomly distributed throughout the sequences. No more than 97% homology between cDNA sequences purified from two different clones was achieved. Interestingly, the homology of guinea-pig Rac2 mRNA sequence declined to 88% when compared with murine Rac2 mRNA (GenBank access. no. X53247). At the level of the predicted amino acid sequence, the homology between guinea-pig and human Rac2 rose to 96% (Fig. 2), with six significant changes at positions 94, 96, 98, 100, 103 and 184.
Figure 1.
Detection by RT-PCR analysis of Rac mRNA expression in guinea-pig eosinophils. (a) PCR products (406 bp) obtained using degenerate primers for Rac mRNA, and (b) Rac2-specific primers (577 bp). In both cases, lane 1, DNA ladder; lane 2, guinea-pig eosinophils; lane 3, human neutrophils; and lane 4, DNA ladder. In lanes 2 and 3, total RNA samples from 2×106 cells were purified, reverse transcribed, and subjected to 30 cycles of amplification. (c) Rac2 PCR-amplified fragment sequence from guinea-pig eosinophils. Comparison of PCR product (577 bp) from guinea-pig eosinophils (GP, bold letters) corresponding to nucleotides 2–578 revealed 93% homology to human Rac2 (HU, Genbank access code no. NM_002872.1). A single underline corresponds to differences between the sequences. Sequence analysis was carried out using the BLAST sequencing facility available from the National Institutes of Health, Bethesda, MD, USA.
Figure 2.
Comparison of the predicted amino acid sequence for guinea-pig Rac2 with those of a partial sequence for guinea-pig Rac1 (as shown in Pick et al.35) and human Rac1 and Rac2. Underlined regions show differences in sequences between guinea-pig and human proteins, while those shown in bold (in the Rac2 sequences) indicate differences between the two isoforms. The predicted guinea-pig Rac2 sequence differs by a single amino acid from the human Rac2 sequence in the C-terminus (position 184).
Superoxide generation
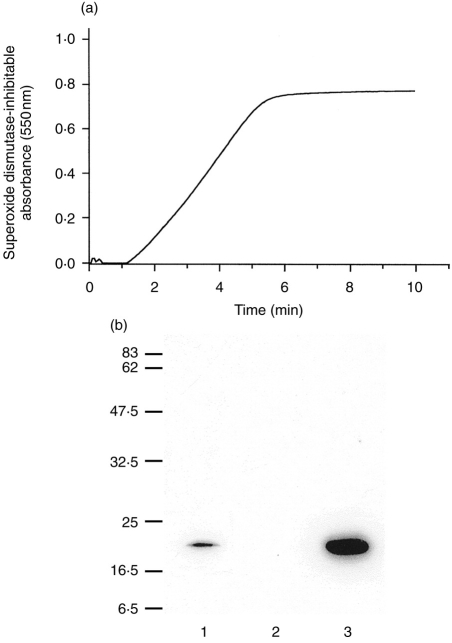
Purified eosinophils were resuspended in PBS+ containing 50 μm cytochrome c and their superoxide generation in response to 500 ng/ml PMA was continuously measured at 550 nm. Previous experiments were performed to determine that the rate of response to this dose of PMA was supramaximal (data not shown). The SOD-inhibitable cytochrome creduction measured in response to PMA in eosinophils reached a plateau after 6–7 min of stimulation (Fig. 3a), producing 4·7 nmol superoxide ions/min/106 cells at the peak rate of absorbance change between 3 and 4 min of incubation in this representative plot. The plateau of cytochrome c reduction was due to the consumption of cytochrome c in the assay rather than the interruption of superoxide production. This was concluded since reducing the levels of cytochrome c in the assay caused a decrease in the amplitude of response without a concurrent change in the rate of reduction (data not shown). This response was typical of eight measurements taken from three different batches of eosinophils.
Figure 3.
(a) Continuous kinetic recording of a PMA-induced respiratory burst response in guinea-pig eosinophils. Purified eosinophils (2×106) were treated with 500 ng/ml PMA and the absorbance change was monitored by superoxide dismutase-inhibitable reduction of cytochrome c (deducted from the trace shown here). This trace is representative of eight separate measurements. (b) Western blot analysis of Rac2 immunoreactivity in eosinophils in comparison with human PMNs. Lane 1, postnuclear supernatant from homogenized guinea-pig eosinophils (12 μg); lane 2, whole NIH 3T3 cells (10 μg); and lane 3, cytosol from human polymorphonuclear cells (10 μg). Positions of molecular weight markers are shown at left.
Expression of Rac2 protein in eosinophils
Eosinophils were found to express an immunoreactive protein which migrated at the same relative molecular weight as that of Rac2 protein detected in PMN cytosol using a rabbit polyclonal antiserum (Fig. 3b). NIH 3T3 cells were negative for Rac2 immunoreactivity. We also used a polyclonal antibody to a polypeptide homologous to the C-terminus of human Rac2 (#sc-96 from Santa Cruz Biotechnology, Inc.) to confirm these findings. However, we were unable to obtain an immunoreactive band at the predicted molecular weight for Rac2 in the positive control (recombinant Rac2 protein) using this antibody preparation (data not shown).
Using two commercially supplied antibodies to Rac1 (#sc-95 and #sc-217 from Santa Cruz Biotechnology, Inc) we were unable to detect Rac1 protein expression by Western blot analysis in guinea-pig eosinophils (data not shown) although the positive control (human recombinant Rac1) was detected. A monoclonal antibody from Upstate Biotechnology demonstrated cross-reactivity to both Rac1 and Rac2, and was unable to differentiate between these in recombinant samples. Guinea-pig macrophages, which express predominantly the Rac1 isoform, were positive for Rac1 using the monoclonal antibody while showing no immunoreactivity with the same Rac2-specific antiserum used above (data not shown).
Subcellular fractionation and Western blot analysis of NADPH oxidase components
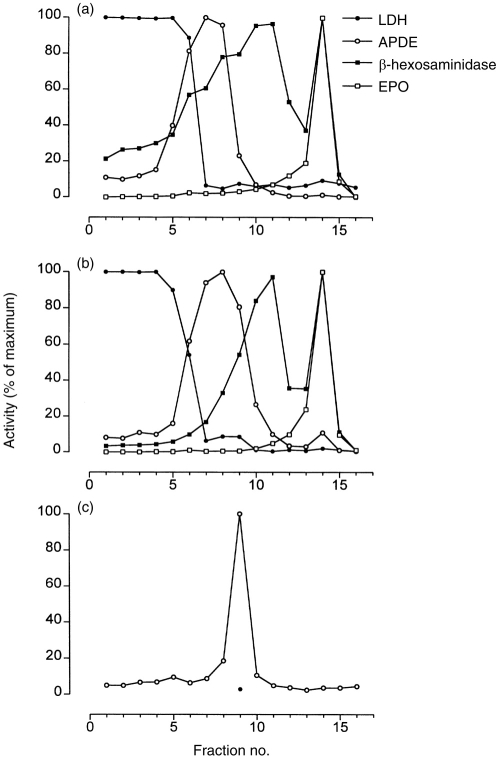
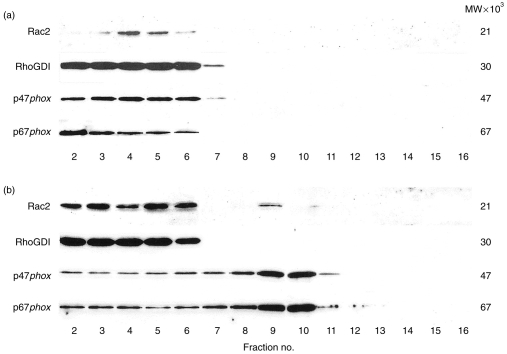
Eosinophils were subfractionated and the resulting fractions were analyzed for marker enzyme activities (Fig. 4a). The cytosolic fractions, detected by LDH activity, occurred above the gradient, while plasma membrane-associated fractions (APDE activity) were found in low-density regions of the Nycodenz gradient. Secretory granules, using an assay for eosinophil peroxidase, were found to sediment at higher densities as determined by the refractive index of each fraction (data not shown).18
Figure 4.
Subcellular distribution of intracellular compartments in density gradients prepared from eosinophils. Guinea-pig eosinophils (5×107), control unstimulated (a) or PMA-stimulated (500 ng/ml) for 8 min at 37° (b), were fractionated and eosinophil peroxidase (crystalloid granules), β-hexosaminidase (secretory/small granules and lysosomes), APDE (plasma membrane) and LDH activities were measured to determine positions of intracellular compartments in the gradient. (c) SOD-inhibitable superoxide-generating activity in fractions from PMA-stimulated cells. In (c), all fractions were measured in the absence of SOD (○) while fraction eight was additionally incubated in the presence of SOD (•). The specific activity of the peak superoxide-generating fraction was 890·3 nmol superoxide/min/mg of membrane protein. Activities from marker enzyme assays are depicted as percentages of maximal activity for the respective assay in fractions from the gradient. Fraction 1 contains 0% Nycodenz while fraction 16 contains 45% Nycodenz. These results are representative of three separate experiments.
In unstimulated eosinophils, immunoreactive bands corresponding to Rac2, RhoGDI, p47phox and p67phox proteins were found to co-localize with cytosolic fractions as determined by peak LDH activity (Fig. 4a and Fig. 5a).
Figure 5.
Western blot analysis of fractions from guinea-pig eosinophils. A total of 5×107 purified eosinophils were subfractionated before stimulation (a) and after stimulation for 8 min with PMA (b). Fractions were subjected to Western blot analysis and antibodies to Rac2, RhoGDI, p47phox and p67phox were employed as described in the Materials and Methods to determine the subcellular localization of NADPH oxidase components in unstimulated and activated cells. These results are representative of three separate experiments.
To assess the intracellular localization of NADPH oxidase components in eosinophils during respiratory burst, cells were stimulated with 500 ng/ml PMA for 8 min and the reaction was terminated for subcellular fractionation. The peak activities of marker enzyme activities did not shift significantly following 8 min stimulation with 500 ng/ml PMA (Fig. 4b), with the exception of β-hexosaminidase which was reduced in the peak area after stimulation.
Fractions co-localizing with the heavy plasma membrane region of the gradient were found to exhibit cell-free superoxide generation which was inhibitable by SOD (8 μg/ml) (Fig. 4c).
Immunoreactivities to Rac2, p47phox and p67phox proteins translocated from the cytosol to the same plasma membrane-associated fraction exhibiting peak superoxide-generating activity in PMA-stimulated eosinophils (Fig. 5b). In this representative sample, about 7% of the total cellular Rac2 was found to translocate to this fraction, while RhoGDI immunoreactivity remained associated with the cytosolic fractions. Approximately 18% of the total cellular content of each p47phox and p67phox protein also translocated to this superoxide-generating fraction. The intensity of Rac2 immunoreactivity appeared to be elevated in the cytosolic fractions of PMA-stimulated eosinophils over that of unstimulated cells (Fig. 5a). These observations were reproducibly obtained from three separate subcellular fractionations of PMA-stimulated guinea-pig eosinophils.
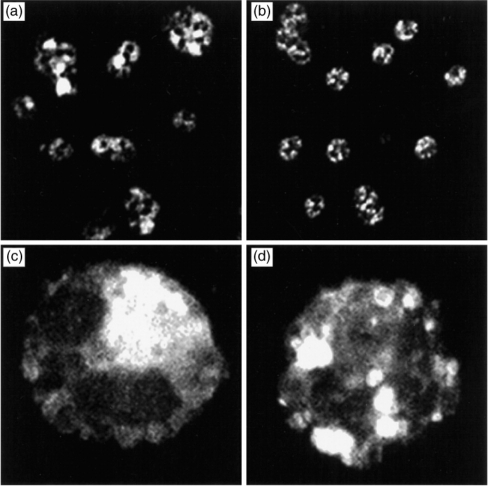
Confocal laser scanning microscopy of Rac2 immunofluorescence
Confocal images of Rac2 immunofluorescence were obtained from resting and PMA-stimulated cells (Fig. 6). In resting eosinophils, Rac2 was distributed in the cytosolic compartment of the cell (Fig. 6a,c). Following 8 min of stimulation by 500 ng/ml PMA, the pattern of Rac2 immunoreactivity was strikingly redistributed from the cytosol to ‘pocket-like’ formations throughout the cell (Fig. 6b,d). These findings confirm those of Western blot analysis and suggest that translocation of Rac2 had occurred during respiratory burst.
Figure 6.
Confocal laser scanning microscopy of cytospins prepared from guinea-pig eosinophils. (a) and (c) Rac2 immunoreactivity in resting eosinophils using rhodamine-conjugated goat anti-rabbit antibody. (b) and (d) Eosinophils were stimulated for 8 min with 500 ng/ml PMA and tested for Rac2 immunoreactivity. (a) and (b), low magnification (original magnification ×63) (c) and (d) high magnification (original magnification ×100).
DISCUSSION
Rac2, as well as its closely related homologue Rac1, is a Rho-related guanine nucleotide-binding protein which is essential for activation of the NADPH oxidase complex in whole cells25 and in cell-free reconstitution assays.8,9 In this study, we have demonstrated that guinea-pig eosinophils transcribe and translate mRNA encoding the GTP-binding protein Rac2. Interestingly, guinea-pig Rac2 mRNA showed closer homology with human rather than murine sequences for Rac2. Using degenerate Rac primers, we obtained a PCR product that exhibited 97% homology with the human Rac1 sequence, indicating that guinea-pig eosinophils also express Rac1 mRNA. However, eosinophils appear to express Rac2 instead of Rac1 protein, as determined by immunoblot analysis, in contrast to an earlier report demonstrating predominantly Rac1 (with some Rac2) expression in these cells using the same technique.14 Our study is strengthened by the inclusion of RT-PCR and confocal microscopy analyses to demonstrate the expression of Rac2 mRNA and protein. The preferential expression of Rac2 over Rac1 in eosinophils is similar to human neutrophils which mainly express Rac2 protein.11,12,16 Rac1 is the dominant isoform expressed in macrophages and neutrophils from guinea-pig, and has been shown to stimulate NADPH oxidase activity in these cells.9,15 These findings suggest that a differential pattern of expression of Rac1 and Rac2 isoforms occurs in a cell-specific manner within the guinea-pig.
In resting eosinophils, Rac2 is likely to be associated with RhoGDI in the cell cytosol, since their immunoreactivities co-eluted with cytosolic fractions in Western blot analysis, in agreement with observations in neutrophils.11,12 The putative function of RhoGDI is to prevent the dissociation of GDP from Rac2, and thus maintain the GTPase in an inactive form. Once cell activation occurs, Rac2 is dissociated from RhoGDI and translocates to the plasma membrane where it activates NADPH oxidase to form superoxide.11 In our findings, Rac2 was translocated from the cytosol to the heavy plasma membrane region of the gradient in PMA-stimulated eosinophils, suggesting that Rac2 associates with the membrane in these cells via its isoprenyl group attached to the C-terminus of the protein.26 This is supported by our finding that superoxide-generating capacity resides within the same membrane fraction containing Rac2 immunoreactivity. In addition, the phagocytic oxidases p47phox and p67phox translocated to the same plasma membrane-associated fractions following PMA stimulation. It is important to emphasize that these fractions produced from subcellular fractionation are contaminated with other cell organelles, including small vesicles, Golgi apparatus and endosomal structures.18 However, an earlier report has shown that most of the superoxide-generating capacity of the eosinophil resides in the plasma membrane.1 Images obtained from confocal microscopy suggest that translocation of Rac2 has occurred within stimulated cells to highly focused structures which may be associated with the plasma membrane.
The increased intensity of Rac2 immunoreactivity observed in the cytosolic fractions of PMA-stimulated eosinophils over that of unstimulated eosinophils was observed reproducibly in three separate subcellular fractionations as well as in immunofluorescence images. Thus, it is unlikely that intensification of Rac2 immunoreactivity may be due to an artefact. Whether this may be caused by conformational changes in the structure of Rac2, such as modifications to its isoprenylation state, or an absolute increase in the level of Rac2 within the cell, remains unknown. Rac2 is isoprenylated at its C-terminus27 and the rabbit polyclonal antiserum used in this study is likely to be directed against this region of the protein, since it appears to distinguish between Rac1 and Rac2 (which are highly divergent in their C-termini).
The intracellular signalling pathways leading to Rac2 stimulation are currently unknown. PMA is thought to trigger protein kinase C-mediated phosphorylation of regulatory proteins upstream of Rac2, subsequently causing activation of a Rac2-specific guanine exchange factor (GEF). Potential candidates for GEFs acting on Rac proteins include Vav, Dbl, Tiam and Ost2,8–31 although it is not yet established whether any of these act on Rac2.
In vivo studies have shown that Rac2 is critical for regulating the release of ROS from granulocytes. Using a bcr-null mutant mouse model, elevated Rac2 activity was detected in bcr−/− neutrophils, apparently due to loss of Rac2 inhibition.32 The bcr gene product is a physiological GTPase-activating protein (GAP) for Rac2.33 Elevated Rac2 activity in bcr- null mice led to a substantial increase in ROS production and widespread tissue damage in reaction to lipopolysaccharide.32
Superoxide generation in allergic inflammation, particularly asthma, is likely to be of strong physiological importance. Products of respiratory burst, such as H2O2 and thiobarbituric acid-reactive products, are elevated 26-fold in the bronchoalveolar lavage fluids from asthmatics over those of normal, healthy controls.4 In addition, peripheral blood eosinophils obtained from asthmatic subjects exhibit an enhanced ability to generate superoxide in childhood3 and nocturnal asthma.2 Hypodense eosinophils from patients with asthma have been demonstrated to generate significantly more superoxide in response to PMA than normodense eosinophils or neutrophils from the same subjects.34 Eosinophils may be a major source of ROS in asthma as their numbers broadly correlate with severity of disease in airway mucosa and peripheral blood.5
In summary, our findings demonstrate that eosinophils express the GTP-binding protein Rac2 along with RhoGDI and components of the NADPH oxidase complex. We also showed that Rac2 was translocated from the cytosol to plasma membrane-associated regions following stimulation by PMA. The results arising from these studies will contribute to our understanding of the mechanisms underlying eosinophil activation, and may ultimately provide new strategies aimed at preventing or modulating the release of toxic eosinophil-derived mediators in allergic inflammation and asthma.
Acknowledgments
The authors would like to thank Dr William Nauseef for his kind gift of rabbit polyclonal antisera to human recombinant p47phox and p67phox proteins and Dr Mark T. Quinn for his helpful advice and suggestions in the course of this study. In addition, we would like to extend our gratitude to Ms Stacey C. Hagen, University of Alberta, for her technical assistance with the project, and Dr Vera Chlumecky, Department of Cell Biology and Anatomy, University of Alberta, for her assistance in confocal laser scanning microscopy. This work was supported by the Alberta Lung Association, Canada; the Medical Research Council, Canada; Alberta Heritage Foundation for Medical Research, Canada; the Health Research Council of New Zealand; the National Child Health Research Foundation of New Zealand; the Asthma and Respiratory Foundation of New Zealand; the Wellington Medical Research Foundation; the Marjorie Barclay Trust, New Zealand; and NIH Grant number HL 48008. P.L. is a Parker B. Francis Fellow in Pulmonary Research; R.M. is an Alberta Heritage Senior Medical Scholar.
Abbreviations
- APDE
alkaline phosphodiesterase
- GTPγS
guanosine-5′-O-(3-thiotriphosphate)
- LDH
lactate dehydrogenase
- Rac2
ras-related C3 botulinum toxin substrate
- RhoGDI
Rho GDP-dissociation inhibitor
- ROS
reactive oxygen species
- SOD
superoxide dismutase
References
- 1.Bolscher BG, Koenderman L, Tool AT, Stokman PM, Roos D. NADPH: O2 oxidoreductase of human eosinophils in the cell-free system. FEBS Lett. 1990;268:269. doi: 10.1016/0014-5793(90)81025-j. [DOI] [PubMed] [Google Scholar]
- 2.Jarjour NN, Busse WW, Calhoun WJ. Enhanced production of oxygen radicals in nocturnal asthma. Am Rev Respir Dis. 1992;146:905. doi: 10.1164/ajrccm/146.4.905. [DOI] [PubMed] [Google Scholar]
- 3.Schauer U, Leinhaas C, Jager R, Rieger CH. Enhanced superoxide generation by eosinophils from asthmatic children. Int Arch Allergy Appl Immunol. 1991;96:317. doi: 10.1159/000235515. [DOI] [PubMed] [Google Scholar]
- 4.Antczak A, Nowak D, Shariati B, Krol M, Piasecka G, Kurmanowska Z. Increased hydrogen peroxide and thio-barbituric acid-reactive products in expired breath condensate of asthmatic patients. Eur Respir. 1997;10:1235. doi: 10.1183/09031936.97.10061235. [DOI] [PubMed] [Google Scholar]
- 5.Corrigan CJ, Kay AB. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992;13:501. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- 6.DeLeo FR, Quinn MT. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J Leukoc Biol. 1996;60:677. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- 7.Segal AW, Shatwell KP. The NADPH oxidase of phagocytic leukocytes. Ann N Y Acad Sci. 1997;832:215. doi: 10.1111/j.1749-6632.1997.tb46249.x. [DOI] [PubMed] [Google Scholar]
- 8.Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac2. Science. 1991;254:1512. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- 9.Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- 10.Bokoch GM, Knaus UG. The role of small GTP-binding proteins in leukocyte function. Curr Opin Immunol. 1994;6:98. doi: 10.1016/0952-7915(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 11.Abo A, Webb MR, Grogan A, Segal AW. Activation of NADPH oxidase involves the dissociation of p21rac from its inhibitory GDP/GTP exchange protein (rhoGDI) followed by its translocation to the plasma membrane. Biochem J. 1994;298:585. doi: 10.1042/bj2980585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinn MT, Evans T, Loetterle LR, Jesaitis AJ, Bokoch GM. Translocation of Rac correlates with NADPH oxidase activation. Evidence for equimolar translocation of oxidase components. J Biol Chem. 1993;268:20983. [PubMed] [Google Scholar]
- 13.Xu X, Barry DC, Settleman J, Schwartz MA, Bokoch GM. Differing structural requirements for GTPase-activating protein responsiveness and NADPH oxidase activation by Rac. J Biol Chem. 1994;269:23569. [PubMed] [Google Scholar]
- 14.Someya A, Nishijima K, Nunoi H, Irie S, Nagaoka I. Study on the superoxide-producing enzyme of eosinophils and neutrophils – comparison of the NADPH oxidase components. Arch Biochem Biophys. 1997;345:207. doi: 10.1006/abbi.1997.0252. [DOI] [PubMed] [Google Scholar]
- 15.Bromberg Y, Shani E, Joseph G, Gorzalczany Y, Sperling O, Pick E. The GDP-bound form of the small G protein Rac1 p21 is a potent activator of the superoxide-forming NADPH oxidase of macrophages. J Biol Chem. 1994;269:7055. [PubMed] [Google Scholar]
- 16.Heyworth PG, Bohl BP, Bokoch GM, Curnutte JT. Rac translocates independently of the neutrophil NADPH oxidase components p47phox and p67phox. Evidence for its interaction with flavocytochrome b558. J Biol Chem. 1994;269:30749. [PubMed] [Google Scholar]
- 17.Petreccia DC, Nauseef WM, Clark RA. Respiratory burst of normal human eosinophils. J Leukoc Biol. 1987;41:283. doi: 10.1002/jlb.41.4.283. [DOI] [PubMed] [Google Scholar]
- 18.Lacy P, Thompson N, Tian M, et al. A survey of GTP-binding proteins and other potential key regulators of exocytotic secretion in eosinophils. J Cell Sci. 1995;108:3547. doi: 10.1242/jcs.108.11.3547. [DOI] [PubMed] [Google Scholar]
- 19.Kimura I, Moritani Y, Tanizaki Y. Basophils in bronchial asthma with reference to reagin-type allergy. Clin Allergy. 1973;3:195. doi: 10.1111/j.1365-2222.1973.tb01321.x. [DOI] [PubMed] [Google Scholar]
- 20.Gilchrist M, MacDonald AJ, Neverova I, Ritchie B, Befus AD. Optimization of the isolation and effective use of mRNA from rat mast cells. J Immunol Methods. 1997;201:207. doi: 10.1016/s0022-1759(96)00230-x. [DOI] [PubMed] [Google Scholar]
- 21.Bromberg Y, Pick E. Activation of NADPH-dependent superoxide production in a cell-free system by sodium dodecyl sulfate. J Biol Chem. 1985;260:13539. [PubMed] [Google Scholar]
- 22.Bach MK, Brashler JR, Petzold EN, Sanders ME. Superoxide production by human eosinophils can be inhibited in an agonist-selective manner. Agents Actions. 1992;35:1. doi: 10.1007/BF01990944. [DOI] [PubMed] [Google Scholar]
- 23.Mahmudi-Azer S, Lacy P, Bablitz B, Moqbel R. Inhibition of nonspecific binding of fluorescent-labelled antibodies to human eosinophils. J Immunol Methods. 1998;217:113. doi: 10.1016/s0022-1759(98)00105-7. [DOI] [PubMed] [Google Scholar]
- 24.Lacy P, Levi-Schaffer F, Mahmudi-Azer S, et al. Intracellular localization of interleukin-6 in eosinophils from atopic asthmatics and effects of interferon γ. Blood. 1998;91:2508. [PubMed] [Google Scholar]
- 25.Dorseuil O, Vazquez A, Lang P, Bertoglio J, Gacon G, Leca G. Inhibition of superoxide production in B lymphocytes by rac antisense oligonucleotides. J Biol Chem. 1992;267:20540. [PubMed] [Google Scholar]
- 26.Kreck ML, Freeman JL, Abo A, Lambeth JD. Membrane association of Rac is required for high activity of the respiratory burst oxidase. Biochemistry. 1996;35:15683. doi: 10.1021/bi962064l. [DOI] [PubMed] [Google Scholar]
- 27.Kinsella BT, Erdman RA, Maltese WA. Carboxyl-terminal isoprenylation of ras-related GTP-binding proteins encoded by rac1, rac2, and ralA. J Biol Chem. 1991;266:9786. [PubMed] [Google Scholar]
- 28.Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 29.Han J, Luby-Phelps K, Das B, et al. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 30.Ridley AJ. Signal transduction through the GTP-binding proteins Rac and Rho. J Cell Sci. 1994;18(Suppl):127. doi: 10.1242/jcs.1994.supplement_18.19. [DOI] [PubMed] [Google Scholar]
- 31.MacAra IG, Lounsbury KM, Richards SA, McKiernan C, Bar-Sagi D. The Ras superfamily of GTPases. FASEB J. 1996;10:625. doi: 10.1096/fasebj.10.5.8621061. [DOI] [PubMed] [Google Scholar]
- 32.Voncken JW, Van Schaick H, Kaartinen V, et al. Increased neutrophil respiratory burst in bcr-null mutants. Cell. 1995;80:719. doi: 10.1016/0092-8674(95)90350-x. [DOI] [PubMed] [Google Scholar]
- 33.Diekmann D, Brill S, Garrett MD, et al. Bcr encodes a GTPase-activating protein for p21rac. Nature. 1991;351:400. doi: 10.1038/351400a0. [DOI] [PubMed] [Google Scholar]
- 34.Sedgwick JB, Geiger KM, Busse WW. Superoxide generation by hypodense eosinophils from patients with asthma. Am Rev Respir Dis. 1990;142:120. doi: 10.1164/ajrccm/142.1.120. [DOI] [PubMed] [Google Scholar]
- 35.Pick E, Gorzalczany Y, Engel S. Role of the rac1 p21-GDP-dissociation inhibitor for rho heterodimer in the activation of the superoxide-forming NADPH oxidase of macrophages. Eur J Biochem. 1993;217:441. doi: 10.1111/j.1432-1033.1993.tb18264.x. [DOI] [PubMed] [Google Scholar]