Abstract
Clinical signs of experimental autoimmune encephalomyelitis (EAE) are associated with the selective recruitment of CD4+ memory (CD45RBlow CD44high) T cells into the central nervous system (CNS). However, we have found that many of these recently recruited memory cells are CD44low, suggesting that the CD44 antigen may be involved in, and transiently lost during, the extravasation process. Indeed, administration of a CD44‐specific antibody (IM7.8.1) induced leucocyte CD44 shedding and both prevented the development and ameliorated the severity of established EAE by inhibiting mononuclear cell infiltration into the CNS. Trafficking of cells into lymph nodes, however, a property mainly of naïve cells, was essentially unaffected. In contrast, treatment with antibody to very late activation antigen‐4 (VLA‐4) prevented homing to both the CNS and to lymph nodes. This study contests previous reports that dismissed a role for CD44 in inflammation of the CNS and, coupled with observations in murine dermatitis and arthritis, suggests that CD44 is involved in the homing of primed lymphocytes to sites of inflammation. CD44 should therefore be considered a target for immunotherapy of T‐cell‐mediated inflammatory diseases, such as multiple sclerosis.
Introduction
Chronic relapsing experimental allergic encephalomyelitis (CREAE) is a T‐cell‐mediated autoimmune disease and shares many features with multiple sclerosis (MS), which is the major inflammatory, demyelinating disease of the central nervous system (CNS). CREAE is mediated by the action of CD4+ T cells, which are selectively recruited or retained within the CNS during disease.1,2 Histochemical staining of sections from lesion tissue suggested that these CD4+ cells are memory cells.2 However, as the memory phenotype in mice is defined by the relative expression of a number of cell‐surface antigens, flow cytometric analysis is required for accurate quantification. Naïve T cells (CD45RA+ CD45RO– in humans and CD45RBhigh CD44low in mice) circulate through lymphoid tissues. Extravasation from the blood through the high endothelial venules of lymphoid tissues is known to involve l‐selection (CD62L) as well as β1 and β2 integrins.3,4 Following activation, antigen‐primed cells switch to a CD45RA– CD45RO+ memory phenotype in humans, and to a CD45RBlow CD44high phenotype in mice, down‐regulate l‐selectin and up‐regulate other adhesion molecules, including lymphocyte function‐associated antigen‐1 (LFA‐1; CD11a) and very late activation antigen‐4 (VLA‐4; CD49d), and enter non‐lymphoid tissues.4,5 The development of EAE and MS is associated with the up‐regulation of vascular adhesion molecules, including intracellular adhesion molecule‐1 (ICAM‐1), vascular cell adhesion molecule‐1 (VCAM‐1), fibronectin and mucosal addressin cell adhesion molecule (MAdCAM)‐1, on CNS endothelia, which may facilitate the extravasation of leucocytes.6–8 Modelling studies in vitro clearly indicate a role for the receptor–ligand pairs LFA‐1–ICAM‐1 and VLA‐4–VCAM‐1 in lymphocyte adhesion to, and migration through, CNS endothelium.9–11 These systems, however, do not account for all lymphocyte adhesion,9,10 indicating the involvement of other receptor–ligand interactions.
The CD44 molecule may also be involved in controlling lymphocyte entry into the CNS. CD44 is strongly expressed on antigen‐activated T cells4,12 and on T cells with a transendothelial migratory capacity.13 CD44 was originally identified as a lymphocyte homing receptor, as a CD44‐specific monoclonal antibody (mAb) was able to inhibit, in vitro, the binding of human cell lines to addressins on high endothelial venules from lymphoid tissue and inflamed synovium.14,15 However when a CD44‐specific mAb IM7.8.1 (IM7) was administered to mice, it inhibited lymphocyte infiltration into a cutaneous hypersensitivity site16 and arthritic joints,17 whilst normal leucocyte migration into lymphoid tissue was unaffected. This suggests that CD44 plays a selective role in controlling lymphocyte trafficking to sites of inflammation.
Previous studies involving in vitro adhesion of leucocytes onto inflamed CNS vessels and mAb inhibition of adoptive EAE have, however, failed to support a role of CD44 in CNS inflammation.11,18 In such instances, interaction of VLA‐4 with VCAM‐1 has been shown to be critical in controlling lymphocyte entry into the CNS during EAE in rats, mice and guinea‐pigs.11,18,19 However, preliminary data led us to believe that CD44 expression was modulated on lymphocytes during entry into the CNS, suggesting that CD44 may yet be involved in the migration process. To address this question, the ability of the CD44‐specific mAb, IM7, to inhibit CREAE was examined.
Materials and methods
Animals
Male BALB/c (H‐2d, Thy1.2) mice were purchased from Bantin & Kingman (Hull, UK). Biozzi ABH (H‐2dq1, H‐2Ag7 Thy1.1) mice were from stock bred at the Royal College of Surgeons and the Institute of Ophthalmology. Water and the rat mouse‐1(expanded) (RM‐1(E)) diet were given to the mice ad libitum. For the generation of ABH.BALB/c.Thy1b mice (ABH Thy1.2), (ABH × BALB/c)F1 mice were backcrossed with ABH mice for 11 generations. At each generation, animals expressing Thy1.2 on Thy1+ dendritic epidermal cells were selected, following staining of 2 mm punch biopsy‐derived ear epidermal skin sheets with mAb specific for Thy1.2 (HO13.4; American Type Culture Collection [ATCC], Rockville, MD), as described previously.20 Animals were brother : sister mated and offspring were selected that were homozygous for the Thy 1b allele by the detection of Thy1.2 protein in skin sheet preparations. This was then confirmed by analysis of genomic DNA from tail skin, using the D9Nds2 (Thy1) microsatellite.21 Congenic ABH Thy1.2 mice were then inbred.
Induction of CREAE
Mice were injected subcutaneously (s.c.) with 1 mg of ABH mouse spinal cord homogenate (SCH) emulsified in Freund’s complete adjuvant on days 0 and 7, as described previously.1,2 Disease was scored as follows: 0, normal; 1, limp tail; 2, impaired righting reflex; 3, partial paralysis; and 4, complete paralysis. Disease of a milder severity than the indicated grade was scored 0·5 lower, as described previously.1,2
Analysis of lymphocyte cell‐surface phenotypes in CREAE mice
Single‐cell suspensions were prepared from inguinal lymph nodes and peripheral blood.2 Mononuclear cells infiltrating the spinal cord were purified using density gradient centrifugation, as described previously.2 Briefly, spinal cords from perfused CREAE animals22 were homogenized and loaded into a discontinuous density gradient containing 100%, 80%, 40% and 30% Percoll (Amersham Pharmacia, Biotech Ltd, Little Chalfont, Bucks, UK). Following centrifugation at 400 g for 10 min at room temperature, leucocytes were removed from the 80% : 40% Percoll interface.2 In separate experiments, following the development of paralysis during acute‐phase CREAE, ABH mice were injected intravenously (i.v.) with 2 × 107 pooled auricular, cervical, axillary and inguinal lymph node cells from ABH Thy1.2 mice. Animals were perfused either 2 or 18 hr later, and single‐cell suspensions were prepared from the inguinal lymph nodes and spinal cord.2,22 Cells were stained by single, direct or indirect, double or triple direct immunofluorescence flow cytometry. Cells were incubated for 40 min at 4° with mAbs specific for l‐selectin (CD62L; clone MEL‐14; ATCC), VLA‐4 (α4 integrin/CD49d; clone R1/2; ATCC), CD44s (clone IM7.8.1; ATCC) or CD45RB (clone 16a), and detected with fluorescein isothiocyanate (FITC)‐conjugated rabbit anti‐rat immunoglobulin (Serotec Ltd, Kidlington, Oxford, UK). Rat mAb, conjugated directly to either FITC or phycoerythrin (PE), specific for mouse CD44 (IM7; BD Pharmingen, San Diego, CA), CD45RB (16a; Pharmingen), CD45RC (clone DN1.7; Pharmingen), mouse/human CD45RO (clone B011; Accurate Chemical & Scientific Corporation, Westbury, NY), Thy1.2 (CD90, clone H013.4; Pharmingen) and Tricolor‐conjugated CD4‐specific mAb (clone YTS 191; Caltag Laboratories, Burlingame, CA) were also used. These were diluted in 5% normal mouse serum in phosphate‐buffered saline (PBS). Following washing, the cells were either maintained on ice or fixed in 1% formaldehyde in PBS and analysed by flow cytometry.
In vivo mAb treatment
Animals were injected daily, intraperitoneally (i.p.), with 100 µg of the purified mouse CD44‐specific mAb, IM723 (rat immunoglobulin [Ig]G2b; ATCC), dissolved in 100 µl of PBS, either from day 12 postinjection (p.i.) or at the onset of clinical signs when animals exhibited a limp tail on days 15–16 p.i. As controls, mice were treated with vehicle or were injected with a rat IgG2b mAb specific for mouse CD8 (clone YTS169.4), which has previously been shown to have no effect on the clinical course of CREAE.1 A group of animals were killed on day 18 p.i. when peripheral blood, spleens and inguinal lymph nodes were removed for histological analysis and the assessment of CD44 antigen shedding. Brains and spinal cords were removed, fixed in 10% formal saline and processed for routine histology. Longitudinal sections were cut and stained with haematoxylin and eosin.
Analysis of CD44 shedding
Lymph nodes and spleens were removed from animals treated in vivo with either IM7 or rat immunoglobulin (Serotec) and stained with PE‐conjugated CD44‐specific mAb (clone KM201; Serotec). This mAb recognizes an epitope in the N‐terminal hyaluronic acid‐binding region of CD44, distinct from that recognized by IM7.17 In addition, cells were double‐ or triple labelled, as described above, with mAbs reactive with mouse B cells (clone OX‐6; Serotec) and CD4+ T cells, which were gated to detect naive (CD45RBhigh) and memory (CD45RBlow) T cells. In addition, cells were stained with a mAb specific for CD11a (M17/4; Pharmingen) or rabbit polyclonal Ab specific for either mouse/human α4 (CD49d) or β1 (CD29) integrins (Chemicon International Ltd, Harrow, UK), detected by FITC‐conjugated mAb specific for rabbit immunoglobulin (Dako Ltd, High Wycombe, Bucks, UK).
Culture of lymph node cells with IM7 in vitro and transfer to CREAE mice
RPMI‐1640 medium (Gibco, Paisley, Strathclyde, UK) supplemented with l‐glutamine, 1% sodium pyruvate, 50 µg/ml gentamicin (Sigma, St Louis, MO) and 10% heat‐inactivated fetal calf serum (FCS; Sigma) was used to culture lymph node cells. Thy1.2 congenic lymph node cells (8 × 106/well) were cultured for 4 hr, at 37° in 5% CO2, in six‐well tissue clusters (Corning Costar Corp., Cambridge, MA), precoated overnight with 200 µg/well IM7 or rat immunoglobulin (Serotec) in sodium carbonate buffer, pH 9·6. An aliquot of cells from the mAb treatments was assayed for CD44, LFA‐1 and VLA‐4 expression by flow cytometry. The cells were then washed and 1 × 107 cells injected i.v. into mice with paralytic EAE. Animals were perfused 6 hr following transfer and the number of CD4+ and Thy1.2+ cells in inguinal lymph nodes and spinal cords were assessed by flow cytometry. In a separate experiment, cells were incubated with saturating levels of VLA‐4‐specific mAb (PS/2; ATCC) prior to injection. Animals were perfused 18 hr later and the percentage number of infiltrating Thy1.2+ cells was determined.
Statistical analysis
Statistical differences between CREAE groups were assessed using the two‐sample test of Wilcoxon. Differences between groups for trafficking experiments were analysed using the Student’s t‐test, incorporating the F‐test to determine equality of variances.
Results
Selective homing of memory T cells in the CNS during CREAE
In lymph nodes and peripheral blood, the majority of CD4+ T cells expressed a naïve CD44low CD45RBhigh phenotype (Table 1). In contrast, the majority of the cells isolated from the CNS during acute‐phase CREAE expressed a memory CD44high CD45RBlow phenotype (Table 1). This phenotype was maintained during both the acute‐phase paralysis (grade 4) on days 17–19 (p.i.), of SCH in Freund’s adjuvant, and the postacute phase (grade 2) on days 19–21 p.i. (Table 1). Consistent with the selective loss of l‐selectin by memory T cells,4,5 the majority of CD4+ T cells extracted from the CNS were l‐selectin negative (4·8 ± 1·9% MEL‐14+ compared with 82·0 ± 5·0% in lymph nodes and 46·0 ± 13·0% in peripheral blood; n = five to eight mice), but surprisingly the expression of CD49d/VLA‐4 was low (19·5 ± 7·7% of CD4+ CD49dhigh T cells) on CNS‐extracted CD4+ cells compared with that in the periphery (65·9 ± 3·9% in lymph node and 63·5 ± 11·9% in peripheral blood n = five to eight mice).24 In an attempt to further confirm the distinct migration pathways of naïve and memory T cells in CREAE, ABH mice with paralytic CREAE were injected with lymph node cells, with no selected specificity for myelin antigens, derived from ABH Thy1.2 congenic mice, which contained 85–90% CD45RBhigh CD4+ T cells. Consistent with the resident lymph node population (Table 1), the majority of the cells homing into the lymph node were also of the CD44low CD45RBhigh naïve phenotype (Table 2). In contrast, the majority of cells homing into the CNS were CD45RBlow T cells (Table 2), which again was reflective of those already resident within the CNS (Table 1). Although this may represent a phenotype generated within the CNS, these 102–103 CNS‐trafficking cells probably represent the selective migration of the minor CD45RBlow population within the injected lymph node cells. Interestingly, in contrast to the CD44high phenotype of memory cells established within the CNS (Table 1), the newly extravasated memory cells were mainly CD44low (Table 2). The high expression of CD44 on memory T cells and its potential modulation during entry into the CNS suggested that CD44 may be involved in the migration of antigen‐primed T cells into the CNS. To explore this possibility, the CD44‐specific IM7 mAb was administered to mice to examine its effect on the development of CREAE.
Table 1.
Selective recruitment of CD4+ memory/primed T cells to the central nervous system (CNS) during experimental allergic encephalomyelitis (EAE)
| Antigen expression by CD4+ T lymphocytes (%) | |||
|---|---|---|---|
| Disease phase | Tissue examined | CD44high | CD45RBhigh |
| Acute EAE | Lymph | 20·1 ± 8·7 | 77·0 ± 12·5 |
| Postacute | node | 7·1 ± 3·1 | 68·9 ± 11·1 |
| Acute EAE | Peripheral | 8·1 ± 8·0 | 78·0 ± 11·6 |
| Postacute | blood | 7·6 ± 4·6 | 91·6 ± 4·9 |
| Normal | 13·3 ± 13·5 | 65·9 ± 17·8 | |
| Acute EAE | Spinal | 87·2 ± 12·4 | 14·2 ± 16·6 |
| Postacute | cord | 66·1 ± 10·1 | 6·9 ± 1·6 |
Table 2.
Comparison of the trafficking profiles of CD4+ T cells in the central nervous system (CNS) and lymph nodes during experimental allergic encephalomyelitis (EAE)
| Antigen expression by CD4+ T lymphocytes (%) | |||
|---|---|---|---|
| Tissue examined | Time (hr) | CD44high | CD45RBhigh |
| Lymph node | 2 | 22·4 ± 17·6 | 73·1 ± 10·6 |
| 18 | 10·2 ± 8·4 | 89·8 ± 11·9 | |
| Spinal cord | 2 | 42·6 ± 2·5 | 27·9 ± 17·4 |
| 18 | 25·1 ± 12·9 | 21·9 ± 11·5 | |
Following the development of paralysis during acute‐phase chronic‐relapsing experimental allergic encephalomyelitis (CREAE), Biozzi AB/H (Thy1.1+) mice were injected intravenously (i.v.) with 2 × 10
lymph node cells expressing Thy
1.2 antigen. Animals were perfused either 2 or 18 hr later and single‐cell suspensions were prepared from the inguinal lymph nodes and spinal cords. The levels of CD44 and CD45RB antigen expressed by transferred Thy1.2+, CD4+ T cells was assessed using flow cytometry. The results represent the mean ± SD percentage antigen expression by Thy1.2+, CD4+ cells isolated from a minimum of five individual samples.
Anti‐CD44 IM7 mAb induces the loss of CD44 antigen from lymphocytes
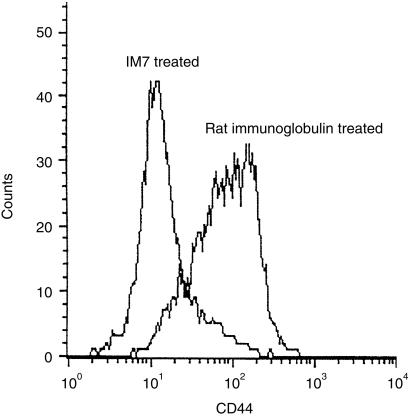
Direct analysis of CD44 expression with the anti‐CD44 mAb, KM201, showed that lymph node cells from IM7‐treated CREAE mice had markedly reduced levels compared with lymph node cells from control Ab‐treated mice (Fig. 1). A similar down‐regulation of CD44 expression was demonstrated on splenocytes, as described previously.17 H‐2A+ B cells and CD4+ T cells, both CD45RB memory and naïve phenotypes, were shown to lose expression of CD44 following IM7 treatment in vivo, whereas levels of LFA‐1 (CD11a), VLA‐4 (CD49d), β1 (CD29) and β2 (CD18) integrins, H‐2A, ICAM‐1 (CD54), CD3, CD4, CD8, CD45RB and CD25 were unaffected, as described previously.17,25 The ratios of granulocytes, lymphocytes and monocytes were essentially the same in all animals, indicating that the decrease in CD44 expression was not the result of a selective depletion of CD44+ cells.
Figure 1.
Anti‐CD44 monoclonal antibody (mAb) IM7 induces the loss of CD44 from lymphocytes in vivo. Animals were injected intraperitoneally (i.p.), daily for 5 days, with 100 µg of either IM7 mAb or rat immunoglobulin. Inguinal lymph nodes were removed 12 hr after the last injection and the level of CD44 on CD4+ T cells was analysed by direct immunofluorescence using CD44‐specific KM201 mAb. The y‐axis shows cell number and the x‐axis is mean fluorescence intensity. Background staining gave an intensity of less than 10 fluorescence units.
Anti‐CD44 IM7 mAb prevents and ameliorates CREAE
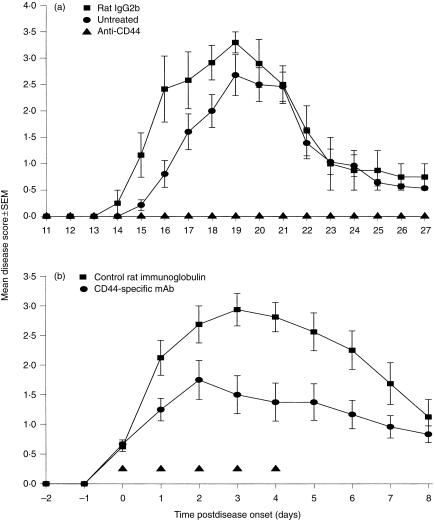
Mice treated daily from day 12 p.i., shortly before the onset of disease, with 100 µg of control rat IgG2b mAb (n = 8) and untreated animals (n = 14) all developed severe clinical disease (mean score of 3·1 ± 0·4, day of onset 15·7 ± 1·3; and mean clinical score 3·0 ± 0·6, day of onset 16·5 ± 1·3; respectively) between days 14 and 20 p.i. (Fig. 2a). In contrast, treatment with anti‐CD44‐specific IM7 mAb (n = 8) completely prevented (P < 0·002) the development of disease (Fig. 2a). Following cessation of treatment on day 21 p.i., although two animals developed disease on day 28 p.i., even by day 40 p.i. only three of eight animals had exhibited CREAE. In contrast, all 22 control animals had developed a paralytic relapse episode (between days 33 and 40 p.i.). IM7 was also shown to have a strong therapeutic effect when administered to mice (five injections of 100 µg) after the onset of clinical EAE. In all 12 mice injected, IM7 was shown to markedly reduce (P < 0·05) the mean daily disease score compared with control rat immunoglobulin‐treated mice (Fig. 2b).
Figure 2.
Anti‐CD44 monoclonal antibody (mAb) IM7 inhibits the development and ameliorates the severity of established clinical disease. (a) Mice were injected with mouse spinal cord homogenate (SCH) in Freund’s adjuvant on days 0 and 7 and then injected intraperitoneally (i.p.), daily from day 12 postinjection (p.i.), with 0·1 ml of phosphate‐buffered saline (PBS) (untreated) (•), isotype rat immunoglobulin G2b (IgG2b) (YTS 169) control (▪) or CD44‐specific mAb IM7 (▴). (b) Animals were injected with SCH in Freund’s adjuvant on days 0 and 7. Following the onset of clinical disease on days 13–15 p.i., chronic‐relapsing experimental allergic encephalomyelitis (CREAE) mice were treated (▴) with five daily i.p. injections of 100 µg of anti‐CD44 mAb IM7 (•) or rat immunoglobulin control (▪). For both (a) and (b), the results represent the mean ± SEM clinical score of animals within each group following the start of mAb treatment.
CD44 is involved in leucocyte extravasation into the CNS
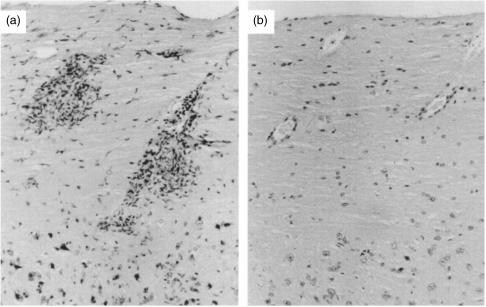
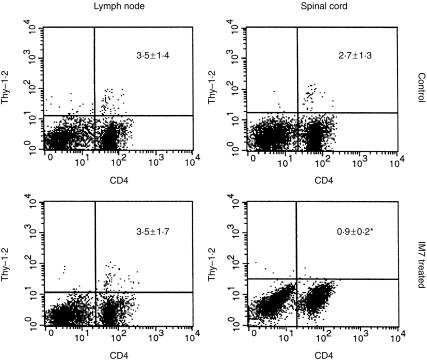
As the development of CREAE is associated with the influx of mononuclear cells into the CNS and resolution of acute‐phase clinical EAE is associated with the rapid resolution of histological lesions2, further mice were treated with mAb IM7 in order to assess the histological effect of CD44‐specific mAb treatment. Animals were examined on day 18 p.i. when the acute‐phase paralysis seen in controls (all eight affected) was just starting to resolve. Anti‐CD44 mAb IM7 treatment was again effective, with four of seven completely protected from clinical disease and three of seven animals showing only mild disease (mean score of 1·4 ± 0·3). In comparison, control mice showed substantial mononuclear cell infiltration along the entire length of the spinal cord, in the CNS white matter (Fig. 3a), whereas IM7‐treated animals without clinical disease were essentially free of mononuclear CNS infiltration (Fig. 3b). The IM7‐treated mice with mild disease (three of 15 mice in total) had only very small numbers of mononuclear cells within the CNS (results not shown). Thus, CD44 appeared to prevent the influx of mononuclear cells into the CNS of mice. This was definitively shown in short‐term in vivo trafficking studies (Fig. 4). When ABH Thy1.2 lymph node cells were cultured on immobilized IM7 in vitro, CD44 expression was reduced compared with rat IgG‐treated cells, whilst levels of CD11a and CD49d were unaffected (results not shown).17,25 When 1 × 107 lymph node cells were injected into mice, the IM7‐treated cells had a significantly reduced capacity to migrate into the CNS compared with control cells (2·7% ± 1·3% compared with 0·9% ± 0·2%; P < 0·005), whereas migration to lymph nodes was unaffected (Fig. 4). The results suggest an important role for CD44 in primed memory lymphocyte migration into inflammatory sites in the CNS. A VLA‐4‐specific mAb (PS/2), however, unlike IM7, markedly inhibited trafficking of cells both into the CNS (0·3% ± 0·1%) and into lymph node tissue (0·1% ± 0·1%).
Figure 3.
Anti‐CD44 monoclonal antibody (mAb) IM7 inhibits lymphocyte entry into the central nervous system (CNS). Following the development of chronic‐relapsing experimental allergic encephalomyelitis (CREAE) 13–15 days after the injection of spinal cord homogenate (SCH) in Freund’s complete adjuvant, mice were given five daily injections of phosphate‐buffered saline (PBS) (a) or 100 µg of anti‐CD44 mAb IM7 (b). On day 18 postinjection (p.i.), spinal cords were removed, and sections were made and stained with haematoxylin and eosin. (a) A section from a control mouse with EAE containing large numbers of infiltrating cells. (b) A section from a corresponding area from a clinically well IM7‐treated mouse. Note the lack of infiltrating mononuclear cells.
Figure 4.
In vitro shedding of CD44 prevents T‐cell migration into inflammatory central nervous system (CNS) lesions. Following the development of paralysis during acute‐phase chronic‐relapsing experimental allergic encephalomyelitis (CREAE), Biozzi AB/H (Thy1.1+) mice were injected intravenously (i.v.) with 1 × 107 lymph node cells expressing Thy1.2 antigen, which had been treated with immobilized rat immunoglobulin or CD44‐specific IM7 monoclonal antibody (mAb) for 4 hr at 37°. Animals were perfused 6 hr later and single‐cell suspensions were prepared from the inguinal lymph nodes and spinal cord from each individual animal. Cells were gated to detect lymphocytes, and a dot‐plot was created showing double immunofluorescence flow cytometry of transferred Thy1.2+ T cells (fluorescence intensity on y‐axis) and the CD4+ T‐cell population (fluorescence intensity on x‐axis).
Discussion
The therapeutic effect of anti‐CD44 treatment in CNS autoimmune disease, shown here, and murine dermatitis16 and arthritis,17 indicates a common principle of cell interactions that govern migration during inflammatory processes. This contrasts with two previous studies that have failed to demonstrate a role for CD44 in the trafficking of lymphocytes to the CNS during EAE, which was thought to be a function mainly of VLA‐4–VCAM‐1 interactions.11,18 However, preliminary studies examining the levels of CD44 on lymphocytes within the CNS led us to believe that CD44 may yet be involved in lymphocyte entry to the CNS.
Herein, it was shown that CD4+ lymphocytes, within lymph nodes of CREAE and normal mice, were mainly of the naïve phenotype whereas those within the inflamed CNS tissue of mice with established CREAE were predominantly memory cells, as found previously in studies of both CNS24,26,27 and non‐CNS inflammatory diseases.4,28 The majority of these cells are not activated, as assessed by interleukin (IL)‐2 receptor (CD25) expression,2 suggesting that antigen‐primed/memory cells, irrespective of their specificity, are selectively recruited to such inflammatory sites. Although encephalitogenic cells and other recruited primed T cells established within the CNS are CD44high,24,26 it was shown herein that the newly recruited memory CD45RBlow T cells were, like naïve cells, predominantly CD44low, suggesting that during entry of primed T cells into the CNS, CD44 expression is transiently lost. In support of this we have shown that activated T cells down‐regulate their CD44 expression following migration across rat brain endothelial cells in vitro (P. Adamson, F. R. Brennan, D. Baker et al., unpublished). Likewise, a similar cleavage of CD44 and, notably, l‐selectin on activated granulocytes has also been observed during diapedesis through endothelium.29–31l‐selectin is involved during the rolling and migration of naïve T cells through lymphoid endothelium.4,32,33 Following T‐cell activation, l‐selectin is down‐modulated, whereas CD44 is up‐regulated and redistributed to adhesion points.34 The up‐regulation of CD44 then mediates ligand (hylaronate) binding.17,33 Recently, CD44 has been shown to mediate rolling of antigen‐activated T cells on endothelium,33 and hence it is attractive to speculate that CD44 may assume some or all of the function of l‐selectin on memory cells after it is lost following transformation from the naïve to the memory phenotype. Thus, lymphocytes exhibit high expression of CD44 for optimizing lymphocyte attachment17,33 and/or migration across normal or inflamed CNS endothelium, and these CD44 molecules may be cleaved following coupling with their ligand. Following retention of these cells in the CNS tissue or exiting from the lymph node into the periphery, CD44 re‐expression takes place. In agreement with this, soluble CD44 can be detected in the sera of normal mice and humans35,36 and the concentration of soluble CD44 is increased in the blood during inflammatory disease in humans and mice.17,35,36 Thus, this cleavage of surface adhesion molecules may represent a mechanism controlling adhesion/de‐adhesion processes during extravasation of leucocytes to both normal and inflamed endothelium.37
Lymphocytes (including memory T cells) from in vivo IM7‐treated CREAE mice, displayed reduced CD44 expression compared with control mAb‐treated lymphocytes, whereas the expression of other lymphocyte adhesion molecules, such as LFA‐1 and VLA‐4, thought to be critical for CNS migration, was unaffected. This treatment prevented the development of clinical signs, even following disease onset, and inhibited the accumulation of inflammatory cells in the CNS. That CD44 inhibited migration across CNS endothelia was confirmed when it was shown that CD4+ lymphocytes treated with immobilized IM7 in vitro lost CD44 expression17 and were unable to traffic to the CNS when transferred to CREAE mice. Importantly, lymphocyte trafficking to lymph nodes was essentially unaffected by IM7 treatment, arguing against a role for CD44 in normal lymphocyte homing,16,17 at least with respect to naïve T cells. This contrasts with the soluble VLA‐4‐specific (PS/2) mAb, which inhibits extravasation of transferred T cells into both the CNS and lymph node tissues. The CD44 loss is mediated by proteases and is a transient event,38 as re‐expression can be achieved on lymphocytes within 24 hr both in vitro and in vivo.17,38 This suggests that repeated administration of this mAb may be required for an optimal therapeutic effect, and is supported by the observation that a single i.p. injection of 500 µg of IM7 on day 12 p.i. failed to inhibit the development of EAE (all six affected; mean group score 2·8 ± 0·6; day of onset 19·5 ± 1·5) compared with saline (0·5 ml)‐injected animals (11 of 14 affected; mean group score 2·3 ± 0·4; day of onset 17·4 ± 1·4).
The clinical efficacy of anti‐CD44 immunotherapy in this EAE model contrasts with previous studies.18,39 Using the same anti‐CD44 mAb IM7, Baron et al. were unable to inhibit adoptive transfer of EAE to H‐2u mice using myelin‐specific T‐cell clones and concluded that VLA‐4, but not CD44, was critical for lymphocyte entry into the CNS.18 However, lymphocytes in that study were treated with mAb in vitro prior to transfer. Even if the CD44 was shed in vivo, it would have been re‐expressed prior to the development of clinical disease, which occurred at least 5–7 days post‐transfer,18 and may account for the lack of efficacy. However, a recent study40 has confirmed our results, demonstrating that anti‐CD44 antibodies delivered in vivo can prevent EAE induced by myelin‐specific T‐cell clones, although treatment of established disease was not examined, or the ability of the antibody to induce CD44 shedding. Cross‐linking of CD44, achieved by IM7 immobilization, triggers CD44 shedding and these IM7‐treated, CD44– human lymphocytes have a reduced capacity to bind to non‐CNS endothelial cells in vitro, compared with CD44‐bearing cells.38 Furthermore, cleavage of CD44 with bromelain reduced lymphocyte binding to human umbilical vein endothelial cells.41 The role of CD44 in lymphocyte:CNS endothelial cell adhesion and transmigration is currently under investigation. As IM7 inhibited lymphocyte migration into the CNS, even in the presence of the other adhesion molecules known to be involved in binding to and/or migration across endothelium, such as LFA‐1 and VLA‐4,9,10,42 there is a possibility that rather than disrupting CD44 interactions with its ligands on endothelial cells (hyaluronic acid, fibronectin, osteopontin),17,35,43 IM7 may be modulating other adhesion pathways, such as those mediated by integrins. Indeed, molecular cross‐talk between CD44 and LFA‐1 has been shown to be involved in cell–cell adhesion44,45 and in homotypic aggregation.46
The ability of mAb IM7 to prevent leucocyte migration during inflammatory diseases of the CNS (described herein), skin16 and joints,17,47 suggested to us that CD44 is involved in the non‐organ‐specific homing of antigen‐primed lymphocytes to sites of inflammation. However, in a recent study we have shown that IM7 fails to prevent the induction of experimental autoimmune thyroiditis48 and in fact exacerbates the disease. The contrasting effects of IM7 in different models of autoimmune disease may reflect different mechanisms of disease induction and indicate that a greater understanding of the function of CD44, the dynamics of its expression and the specific effects of individual anti‐CD44 antibodies are needed before CD44‐based antibody therapy can enter the clinic.
Acknowledgments
The authors would like to thank Professor J. L. Turk, Professor T. T. Glant and Dr K. Mikecz for their assistance. This work was supported financially by the Multiple Sclerosis Society of Great Britain and Northern Ireland and from the Mary E. McKinnel Trust.
Glossary
Abbreviations
- CNS
central nervous system
- CREAE
chronic‐relapsing experimental allergic encephalomyelitis
References
- 1.O’Neill JK, Baker D, Davidson AN, et al. Control of immune‐mediated disease of the central nervous system with monoclonal (anti‐CD4) antibodies. J Neuroimmunol. 1993;45:1. doi: 10.1016/0165-5728(93)90157-t. [DOI] [PubMed] [Google Scholar]
- 2.Allen SJ, Baker D, O’Neill JK, Davison AN, Turk JL. Isolation and characterization of cells infiltrating the spinal cord during the course of chronic relapsing experimental allergic encephalomyelitis in the Biozzi AB/H mouse. Cell Immunol. 1993;146:335. doi: 10.1006/cimm.1993.1031. [DOI] [PubMed] [Google Scholar]
- 3.Gallatin WM, Weissman IL, Butcher EC. A cell‐surface molecule involved in organ‐specific homing of lymphocytes. Nature. 1983;304:30. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 4.Bradley LM, Watson SR. Lymphocyte migration into tissue: the paradigm derived from CD4 subsets. Curr Opin Immunol. 1996;8:312. doi: 10.1016/s0952-7915(96)80118-x. [DOI] [PubMed] [Google Scholar]
- 5.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 6.O’Neill JK, Butter C, Baker D, et al. Expression of vascular addressins and ICAM‐1 by endothelial cells in the spinal cord during chronic relapsing experimental allergic encephalomyelitis in the Biozzi AB/H mouse. Immunology. 1991;72:520. [PMC free article] [PubMed] [Google Scholar]
- 7.Barten DM, Ruddle NH. Vascular cell adhesion molecule‐1 modulation by tumor necrosis factor‐alpha in experimental allergic encephalomyelitis. J Neuroimmunol. 1994;51:123. doi: 10.1016/0165-5728(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 8.Sobel RA, Mitchell ME, Fronden G. Intracellular adhesion molecule‐1 (ICAM‐1) in cellular immune reactions in the central nervous system. Am J Pathol. 1990;136:1309. [PMC free article] [PubMed] [Google Scholar]
- 9.Male D, Rahman J, Pryce G, Tamatani T, Miyasaka M. Lymphocyte migration into the CNS modelled in vitro: roles for LFA‐1, ICAM‐1 and VLA‐4. Immunology. 1994;81:366. [PMC free article] [PubMed] [Google Scholar]
- 10.Greenwood J, Wang Y, Calder VL. Lymphocyte adhesion and transendothelial migration in the central nervous system: the role of LFA‐1, ICAM‐1, VLA‐4 and VCAM‐1. Immunology. 1994;86:408. [PMC free article] [PubMed] [Google Scholar]
- 11.Yednock TA, Cannon C, Fritz LC, Sanchez‐Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4β1 integrin. Nature. 1992;356:63. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 12.Budd RC, Cerottini J‐c, Horvath C, et al. Distinction of virgin and memory T lymphocytes. Stable acquisition of the pgp‐1 glycoprotein concomitant with antigen‐stimulation. J Immunol. 1987;138:3120. [PubMed] [Google Scholar]
- 13.Brezinschek RI, Lipsky PE, Galea P, Vita R, Oppenheimer‐Marks N. Phenotypic characterization of CD4+ T cells with a transendothelial migratory capacity. J Immunol. 1995;154:3062. [PubMed] [Google Scholar]
- 14.Jalkanen ST, Steere AC, Fox RI, Butcher EC. A distinct endothelial cell recognition system that controls lymphocyte traffic into inflamed synovium. Science. 1986;233:556. doi: 10.1126/science.3726548. [DOI] [PubMed] [Google Scholar]
- 15.Jalkanen S, Bargatze RF, De Los Toyos J, Butcher EC. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85 kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987;105:983. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camp RL, Scheynius A, Johansson C, Pure E. CD44 is required for optimal contact allergic responses but is not required for normal leukocyte extravasation. J Exp Med. 1993;178:497. doi: 10.1084/jem.178.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikecz K, Brennan FR, Kim JH, Glant TT. Anti‐CD44 treatment abrogates tissue oedema and leukocyte infiltration in murine arthritis. Nature Med. 1995;1:458. doi: 10.1038/nm0695-558. [DOI] [PubMed] [Google Scholar]
- 18.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA. Surface expression of α4 integrin by CD4 + T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kent SJ, Karlik SJ, Cannon C, et al. A monoclonal antibody to alpha 4 integrin suppresses and reverses active encephalomyelitis. J Neuroimmunol. 1995;58:1. doi: 10.1016/0165-5728(94)00165-k. [DOI] [PubMed] [Google Scholar]
- 20.Lui GY, Baker D, Fairchild S, et al. Complete characterization of the expressed immune response genes in Biozzi AB/H mice: Structural and functional identity between Biozzi AB/H and NOD A‐region molecules. Immunogenetics. 1993;37:296. doi: 10.1007/BF00187458. [DOI] [PubMed] [Google Scholar]
- 21.Baker D, Rosenwasser JK, O’Neill JK, Turk JL. Genetic analysis of experimental allergic encephalomyelitis in mice. J Immunol. 1995;155:4046. [PubMed] [Google Scholar]
- 22.Butter C, Baker D, O’Neill JK, Turk JL. Mononuclear cell trafficking and plasma protein extravasation into the CNS during chronic relapsing experimental allergic encephalomyelitis in Biozzi AB/H mice. J Neurol Sci. 1991;104:9. doi: 10.1016/0022-510x(91)90209-p. [DOI] [PubMed] [Google Scholar]
- 23.Trowbridge LS, Lesley J, Schilte K, Hyman R, Trotter J. Biochemical characterization and cellular distribution of a polymorphic, murine cell‐surface glycoprotein expressed on lymphoid cells. Immunogenetics. 1982;15:299. doi: 10.1007/BF00364338. [DOI] [PubMed] [Google Scholar]
- 24.Barten DM, Clark RB, Ruddle NH. Presence of T cells with activated and memory phenotypes in inflammatory spinal cord lesions. J Immunol. 1995;155:5409. [PubMed] [Google Scholar]
- 25.Mikecz K, Brennan FR, Yao J, Nuki G, Glant TT. Anti‐CD44 may have a therapeutic potential in rheumatoid arthritis. Trans Orthoped Res Soc. 1996;22:755. [Google Scholar]
- 26.Zeine R, Owens T. Direct demonstration of the infiltration of murine central nervous system by Pgp‐1/CD44high, CD45RBlow, CD4+ T cells that induce experimental allergic encephalomyelitis. J Neuroimmunol. 1992;40:57. doi: 10.1016/0165-5728(92)90213-5. [DOI] [PubMed] [Google Scholar]
- 27.Engelhardt B, Conley FK, Kilshaw PJ, Butcher EC. Lymphocytes infiltrating the CNS during inflammation display a distinctive phenotype and bind to VCAM‐1 but not MAdCAM‐1. Int Immunol. 1995;7:481. doi: 10.1093/intimm/7.3.481. [DOI] [PubMed] [Google Scholar]
- 28.Koch AE, Hronfeld‐Harrington LB, Szekanecz Z, et al. In situ expression of cytokines and cellular adhesion molecules in the skin of patients with systemic sclerosis. Their role in early and late disease. Pathobiology. 1993;61:239. doi: 10.1159/000163802. [DOI] [PubMed] [Google Scholar]
- 29.Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil mac‐1 and MEL‐14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 30.Kuijpers TW, Hakkert BC, Hart MH, Roos D. Neutrophil migration across monolayers of cytokine‐prestimulated endothelial cells: a role for platelet‐activating factor and IL‐8. J Cell Biol. 1992;117:565. doi: 10.1083/jcb.117.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bazil V, Strominger JL. Metalloproteases and serine protease are involved in cleavage of CD43, CD44 and CD16 from stimulated granulocytes. Induction of l‐selectin via CD16. J Immunol. 1994;152:1314. [PubMed] [Google Scholar]
- 32.Von‐Adrian VH, Hasslen SR, Nelson RD, Erlandsen SL, Butcher EC. A central role for microvillus receptor presentation in leukocyte adhesion under flow. Cell. 1995;82:989. doi: 10.1016/0092-8674(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 33.Degrendele HC, Picker LJ, Siegelman P. CD44 and its ligand hyaluronate mediate rolling under physiologic flow: a novel lymphocyte‐endothelial cell primary adhesion pathway. J Exp Med. 1996;183:1119. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.del‐Pozo MA, Sanchez‐Mateos P, Nieto M, Sanchez‐Madrid F. Chemokines regulate cellular polarization and adhesion receptor‐redistribution during lymphocyte interaction with endothelium and extracellular matrix: involvement of cAMP signalling pathways. J Cell Biol. 1995;131:495. doi: 10.1083/jcb.131.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haynes BF, Hale LP, Patton KL, Martin ME, McCallum RM. Measurement of an adhesion molecule as an indicator of inflammatory disease activity. Up‐regulation of the receptor for hyaluronate (CD44) in rheumatoid arthritis. Arthritis Rheum. 1991;34:1434. doi: 10.1002/art.1780341115. [DOI] [PubMed] [Google Scholar]
- 36.Katoh S, McCarthy JB, Kincade PW. Characterization of soluble CD44 in the circulation of mice. Levels are affected by immune activity and tumor growth. J Immunol. 1994;153:3440. [PubMed] [Google Scholar]
- 37.Bazil V. Physiological enzymatic cleavage of leukocyte membrane molecules. Immunol Today. 1995;16:135. doi: 10.1016/0167-5699(95)80130-8. [DOI] [PubMed] [Google Scholar]
- 38.Brennan FR, Mikecz K, Glant TT, et al. CD44 expression in rheumatoid arthritis and modulation by specific antibody: implications for lymphocyte adhesion to endothelial cells and synoviocytes in vitro. Scand J Immunol. 1997;45:213. doi: 10.1046/j.1365-3083.1997.d01-382.x. [DOI] [PubMed] [Google Scholar]
- 39.Laman JD, Maassen CB, Schellekens MM, et al. Therapy with antibodies to CD40L (CD154) and CD44‐variant isoforms reduces experimental autoimmune encephalomyelitis induced by a proteolipid protein peptide. Mult Scler. 1998;4:147. doi: 10.1177/135245859800400312. [DOI] [PubMed] [Google Scholar]
- 40.Brocke S, Piercy C, Steinman L, Weissman IL, Veromaa T. Antibodies to CD44 and integrin alpha4, but not l‐selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc Natl Acad Sci USA. 1999;96:6896. doi: 10.1073/pnas.96.12.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munzig E, Eckert K, Harrach T, Graf H, Maurer HR. Bromelain protease F9 reduces the CD44 mediated adhesion of human peripheral blood lymphocytes to human umbilical vein endothelial cells. FEBS Lett. 1994;351:215. doi: 10.1016/0014-5793(94)00860-4. [DOI] [PubMed] [Google Scholar]
- 42.Oppenheimer‐Marks N, Davis LS, Tompkins Bogue D, Ramberg J, Lipsky PE. Differential utilization of ICAM‐1 and VCAM‐1 during adhesion and transendothelial migration of human T lymphocytes. J Immunol. 1991;147:2913. [PubMed] [Google Scholar]
- 43.Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor–ligand interaction between CD44 and osteopontin (Eta‐1) Science. 1996;271:509. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 44.Bruyzeel I, Koopman G, van der Raaij LM. CD44 antibody stimulates adhesion of peripheral blood T cells to keratinocytes through the leukocyte function‐associated antigen‐1/intracellular adhesion molecule‐1 pathway. J Invest Dermatol. 1993;100:424. doi: 10.1111/1523-1747.ep12472106. [DOI] [PubMed] [Google Scholar]
- 45.Fujii K, Tanaka Y, Hubscher S, Saito K, Ota T, Eto S. Cross‐linking of CD44 on rheumatoid synovial cells up‐regulates VCAM‐1. J Immunol. 1999;162:2391. [PubMed] [Google Scholar]
- 46.Koopman G, van Kooyk Y, de Graaff M, Meyer CJ, Figdor CG, Pals ST. Triggering of the CD44 antigen on T lymphocytes promotes T cell adhesion through the LFA‐1 pathway. J Immunol. 1990;145:3589. [PubMed] [Google Scholar]
- 47.Mikecz K, Dennis K, Shi M, Kim JH. Modulation of hyaluronan receptor (CD44) function in vivo in a murine model of rheumatoid arthritis. Arthritis Rheum. 1999;42:659. doi: 10.1002/1529-0131(199904)42:4<659::AID-ANR8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 48.Parish NM, Brennan FR, Cooke A. Anti‐CD44 treatment does not prevent the extravasation of autopathogenic T cells to the thyroid in experimental autoimmune thyroiditis. Immunology. 1999;97:533. doi: 10.1046/j.1365-2567.1999.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]