Abstract
Differences in dermal mast cell prevalence for adult mice of different strains have been reported previously. In this study, the dermal mast cell prevalence for BALB/c and C57BL/6 mice at 6 weeks of age was similar but as BALB/c mice matured from 6 to 10 weeks of age, their dermal mast cell prevalence halved. In contrast, there was no significant difference in the dermal mast cell prevalence of 6‐ and 10‐week‐old C57BL/6 mice. These differences determined the degree of susceptibility of BALB/c and C57BL/6 mice of different ages to UVB (UV radiation of wavelength 280–320 nm)‐induced systemic immunosuppression. Expression of the receptor for stem cell factor, Kit protein, was examined on mast cells under conditions in which the dermal mast cell prevalence varied. A significant correlation was observed between Kit expression by mast cells from adult BALB/c, DBA/2 and C57BL/6 mice and dermal mast cell prevalence. In BALB/c mice, mast cell Kit expression decreased as the mice matured from 6 to 10 weeks of age and correlated with the reduction in dermal mast cell numbers. Kit levels on dermal mast cells from C57BL/6 mice were consistently higher than on mast cells from BALB/c mice although significant reductions in Kit were also measured with ageing from 6 to 10 weeks. We hypothesize that regardless of the extent of Kit expression, the dermal mast cell populations were maximally expanded in C57BL/6 mice. We suggest that BALB/c mice of 6 and 10 weeks of age are useful hosts in which to quantitatively evaluate mast cell involvement in a range of functional assays involving skin.
Introduction
Mast cells are derived from pluripotent haemopoietic cells in the bone marrow. Mast cell precursors in the blood are rare whilst mature mast cells are found in almost every vascularized tissue of the body. All histology text books describe the positioning of large numbers of mast cells in the dermis and subcutaneous fatty tissue of rodent and human skin. Mast cells can be purified by enzymatic digestion of both human and murine skin.1,2 However, difficulties arise during the isolation process with activation and degranulation of the cells. More commonly, experiments are performed with bone marrow‐derived mast cell precursors that are stimulated to develop into mast cells in the presence of interleukin (IL)‐3 and stem cell factor (SCF);3–5 further growth and differentiation signals have been provided by addition of factors including nerve growth factor,6 IL‐4,7 IL‐98 and IL‐10,7 to name but a few. It has been hypothesized that mast cell survival in tissues depends largely on the local production of SCF.9–11 The importance of SCF to mast cell survival has been highlighted in studies of mutant mice. Mice with mutations in the receptor for SCF have absent or low numbers of tissue mast cells, as do mice unable to produce SCF.12,13 Furthermore, the absence of mature mast cells in these mice can be reversed by reconstitution with mast cell precursors from non‐mutant mice or administration of SCF, respectively. In many patients with mastocytosis, the basis of their disease has been linked to a mutation in the c‐kit molecule.14
The purpose of skin mast cells is not fully known.15–17 First, they have been implicated in immunoglobulin E (IgE)‐dependent responses as characterizd by the wheal and flare of anaphylactic reactions. Second, mast cells can be degranulated by small cationic molecules in vitro, which suggests that they may have other roles in situ. There is strong evidence emerging that mast cells are important in innate immunity and this may be reflected in the type of molecules that cause their degranulation, e.g. complement molecules or bacterial products.15–17
In the mutant mice with defects in Kit or SCF, mast cell deficiencies develop during embryonic development.12,13 In this study, we observed age‐dependent changes in dermal mast cell numbers in BALB/c, but not in C57BL/6, mice. These changes occurred during the ageing of BALB/c mice from 6 to 10 weeks of age, i.e. mice near, or in, adulthood. This provides a unique window of time to study mast cell‐dependent responses in a quantitative manner. This opportunity was used to confirm the importance of dermal mast cells in determining the degree of susceptibility of mice to UVB (i.e. UV radiation of wavelength 280–320 nm)‐induced systemic immunosuppression. Finally, we examined Kit protein expression on dermal mast cells in mouse strains of varying age, and compared it with mast cell prevalence. The relationship between the two varied with the strain of the host.
Materials and methods
Mice
Pathogen‐free, female BALB/c, DBA/2 and C57BL/6 mice were obtained from the Animal Resource Centre of the South Australian Department of Agriculture. All mice were not in hair growth, as determined by histology and increased skin‐fold thickness. Mice carrying the Wf mutation on both the C57BL/6 and DBA/2 background were obtained originally from the Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia.18 The homozygous mast cell‐depleted mice (Wf), the heterozygous mice (Wf/+) and the wild‐type mice (+/+), were the progeny of the mating of (C57BL/6Wf × DBA/2Wf) F1 hybrids, and the Wf/Wf, Wf/+ and +/+ mice were identified by coat colour. All experiments were performed according to the ethical guidelines of the National Health and Medical Research Council of Australia.
Immunohistochemical staining of mast cells
As previously described,19 samples of dorsal skin (two to four per mouse) were rolled and fixed in 10% buffered formalin. Prior to paraffin embedding, a vertical orientation of the roll was ensured. Sections (4 μm) were cut and stained immunohistochemically with either a rabbit polyclonal antihistamine antibody (Chemicon, Temecula, CA) or a rabbit polyclonal anti‐Kit antibody (0·2 μg/ml; M‐14, sc1494; Santa Cruz Biotechnology, Santa Cruz, CA). After incubation with a biotin‐conjugated sheep anti‐rabbit immunoglobulin (Silenus, Hawthorn, Australia) and peroxidase‐conjugated streptavidin (Dako, Carpinteria, CA), the reaction product was developed with the substrate 3,3′‐diaminobenzidine tetrahydrochloride (Sigma Chemical Co., St Louis, MO). Sections were counterstained with Lillie Mayer’s haematoxylin (diluted 1 : 1). Control sections were incubated with dilute rabbit serum (1 : 400) or no primary antibody.
Image analysis
As previously described,19 a video image analysis system (Video Pro 32; Leading Edge P/l, Adelaide, South Australia) was used to quantify histamine‐staining cells in 15 consecutive fields in each roll of skin. The epidermis was orientated horizontally and, using a ×10 objective and a ×2·5 camera eyepiece, fields covering the epidermis through to the subcutaneous fatty tissue (SCFT) were selected. Mast cells and area of dermis and SCFT per fixed field of 800‐μm width were measured (but expressed per mm); the coefficient of variation for measurement of mean dermal area for 15 fields was < 14%. Dermal mast cell prevalence was determined by dividing the mast cell numbers per mm horizontal field length by the dermal area for that field.
For quantification of Kit expression on mast cells, the integrated optical density (IOD, representing the total amount of Kit staining for a given mast cell) was measured, as well as the area for the stained cell. Two rolls of skin were examined from each mouse. For each roll, 50 dermal mast cells were captured systematically in consecutive fields using a ×40 objective and a ×5 camera eyepiece. For measurement of the image background in all measurements of optical density, readings were standardized using a bright field image of the background illumination. This allowed correction of non‐uniform illumination over a field of view, which would otherwise affect the discrimination of features by their grey value. In all the image analysis work, the identity of the sections for examination was coded, except for the positive controls that were sections from skin rolls prepared from two C57BL/6 mice.
UV irradiation
The UV source was a bank of FS40 sunlamps (Westinghouse Corp., Pittsburgh, PA) emitting a broad band of UV light, 250–360 nm, with 65% of the output in the UVB range (280–320 nm). A polyvinyl chloride plastic film (0·23 mm) was used to screen out wavelengths < 290 nm. The dose rate was monitored using a UVX radiometer with a UVX‐31 sensor (Ultraviolet Products Inc., San Gabriel, CA). For irradiation of the mice, a uniform dorsal area (8 cm2) was clean‐shaven, the ears protected with black adhesive insulation tape and the mice housed in individual compartments of perspex cages. The sunlamps were held 20 cm above the cages. The irradiation of the lamps, measured at the base of the perspex cages, was 7·2 W/m2.
Assay of contact hypersensitivity
For assay of systemic contact hypersensitivity (CHS) responses, 5 days after UVB irradiation, mice (five per group) were sensitized on the shaved ventral skin with 100 μl of freshly prepared 5% (w/v) 2,4,6‐trinitrochlorobenzene (TNCB; Tokyo Kasei Kogyo. Ltd, Tokyo, Japan) in acetone. Five days later, and after coding the identities of the mouse groups, a CHS response was elicited by applying 10 μl of freshly prepared 1% TNCB in acetone to each of the ventral and dorsal surfaces of both ears. Twenty‐four hours after challenge, the ear thickness was measured using a micrometer (Mitutoyo Corp., Tokyo, Japan) and the extent of ear swelling for each mouse was calculated by subtracting the ear thickness measurement obtained before challenge. From this value was subtracted the mean swelling measured in mice that were challenged, but not sensitized, with TNCB (≈ 0·03 mm).
Statistical analysis
A multiple comparison procedure using a one‐way analysis of variance and Fisher’s least significant difference test was used to determine the statistical significance (P < 0·05) of differences between groups of mice of different age or strain.
Results
Age‐related changes in dermal mast cell prevalence
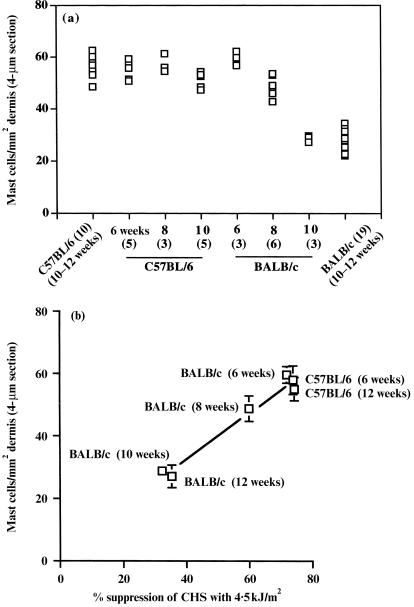
We have previously reported that dermal mast cell prevalence varies in the skin of adult C57BL/6, DBA/2 and BALB/c mice with mean prevalences of 59·9, 44·8 and 27·0 mast cells per mm2 dermis, respectively.19 More importantly, these differences were important in determining the susceptibility of the different mouse strains to a mast cell‐dependent response, i.e. susceptibility to UVB‐induced systemic immunosuppression.19 However, BALB/c mice do not always have a low dermal mast cell prevalence as shown in (Fig. 1a), where significantly increased levels were detected in 6‐week‐old mice. With ageing from 6 to 10 weeks, the dermal mast cell prevalence decreased significantly (P < 0·05 among 6‐, 8‐ and 10‐week‐old mice). In contrast, the level of mast cells remained high in C57BL/6 mice (Fig. 1a). These changes in dermal mast cell prevalence in BALB/c mice are reflected in an altered susceptibility with ageing to UVB‐induced systemic immunosuppression (Fig. 1b). There was a significant difference (P < 0·05) in the percentage suppression by 4·5 kJ/m2 UVB among BALB/c mice of 6, 8 and 10 weeks of age.
Figure 1.
(a) Dermal mast cell prevalence in C57BL/6 and BALB/c mice of different ages. The dermal mast cell prevalence was evaluated in mice of 6, 8 and 10 weeks of age and compared with the prevalence published for a sample of 10 C57BL/6 and 19 BALB/c mice of 10–12 weeks of age.19 The number of mice studied is shown in parenthesis. (b) Relationship between dermal mast cell prevalence in C57BL/6 and BALB/c mice of different ages and their susceptibility to UV radiation of wavelength 280–320 nm (UVB; 4·5 kJ/m2) for systemic suppression of chronic hypersensitivity (CHS) responses. C57BL/6 mice of 6 and 12 weeks of age and BALB/c mice of 6, 8, 10 and 12 weeks of age (five mice per group) were irradiated with UVB (4·5 kJ/m2) and the percentage suppression of a CHS response measured (mean value shown). The mean mast cell number per mm2 dermal area is as shown in (a). The mean ± SD value (if sufficiently large) for each group of mice is shown.
Kit expression on dermal mast cells of adult mice of different strains
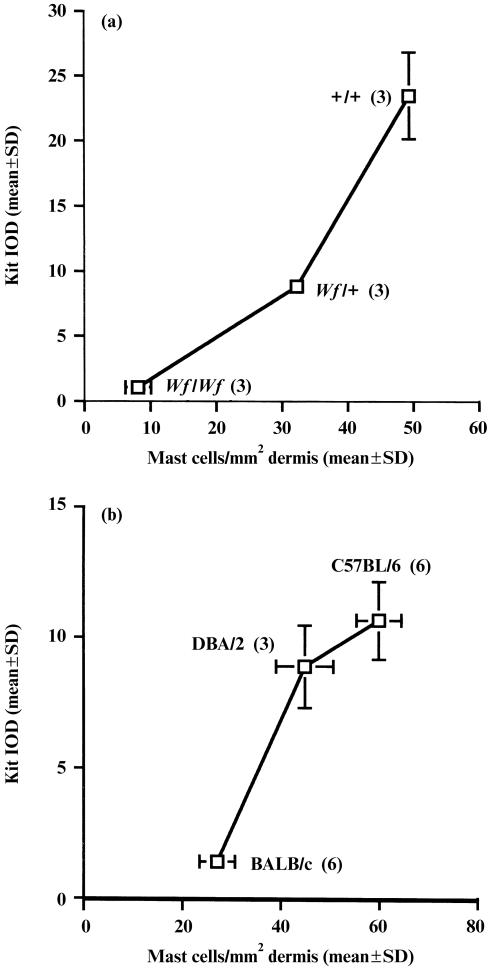
Levels of Kit, the receptor for SCF, were examined on dermal mast cells from different mouse strains. First, Kit expression was examined on residual mast cells in the dermis of mast cell‐depleted Wf/Wf mice. These mutant mice have an abnormality at the W locus, which thus encodes low or absent levels of Kit protein. We have previously reported that Wf/Wf mice have ≈ 10% of the dermal mast cell complement detected in wild‐type mice.19 On examination of Kit expression in the residual mast cells, very low levels were measured whilst heterozygous Wf/+ mice expressed levels of Kit that were intermediate between Wf/Wf and wild‐type +/+ mice (Fig. 2a). On examination of mature C57BL/6, DBA/2 and BALB/c mice, a positive correlation was detected between the mean Kit expression on dermal mast cells and mast cell prevalence, with a significant difference determined between adult C57BL/6 and BALB/c mice (P < 0·05; Fig. 2b).
Figure 2.
(a) Relationship between mast cell expression of Kit protein and dermal mast cell prevalence in Wf/Wf, Wf/+ and +/+ mice. Dorsal skin from three mice in each group was stained immunohistochemically for Kit and histamine. For each mouse, the mean integrated optical density (IOD) for Kit expression on 100 mast cells was recorded. In a similar manner, for each mouse the mean mast cell number per mm2 dermal area was measured from 30 to 60 fields. (b) Relationship between mast cell expression of Kit protein and dermal mast cell prevalence in C57BL/6, DBA/2 and BALB/c mice of 10–12 weeks of age. The number of mice studied is shown in brackets. The mean ± SD (if sufficiently large) for all measurements is shown.
Kit expression on dermal mast cells from mice of different ages
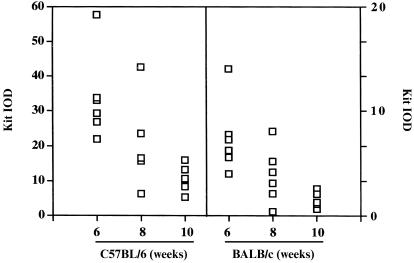
Next, mast cells from C57BL/6 and BALB/c mice of 6, 8 and 10 weeks of age were examined for Kit expression. In both strains, Kit expression was significantly higher on cells from 6‐week‐old than from 10‐week‐old mice (P < 0·05; Fig. 3). Although dermal mast cell numbers were very similar on 6‐week old mice from both strains, Kit expression was significantly higher on mast cells from C57BL/6 mice. Thus, on C57BL/6 mice the correlation between Kit levels and mast cell prevalence is lost but this may be caused by maximal expansion of the dermal mast cell population in C57BL/6 mice of all ages.
Figure 3.
Mast cell expression of Kit in C57BL/6 and BALB/c mice of different ages. Mast cell expression of Kit was evaluated in mice of 6, 8 and 10 weeks of age. Six mice were studied in each group. The integrated optical density (IOD) scales for the different murine strains differ.
Discussion
This is the first report of age‐dependent changes in dermal mast cell prevalence as BALB/c mice mature from 6‐ to 10 weeks of age. That this variation in dermal mast cell prevalence with age can have functional consequences was demonstrated by the differing susceptibilities of BALB/c mice at 6 and 10 weeks of age to the immunosuppressive effects of UV radiation. More generally, these mice may provide a means of exploring a range of mast cell‐dependent responses in a quantitative manner. Previously it was accepted that to determine whether a function was truly mast cell dependent, it was necessary to study the response in mast cell‐deficient mice and then to reconstitute the mice systemically or locally with bone marrow‐derived mast cell precursors. These reconstituted mice have been recently termed as ‘mast‐cell‐knockin mice’.17 The problem with using mast cell‐deficient W/Wv mice in an experimental model is that they have abnormalities other than a mast cell deficiency.12,13 We suggest that an alternative model for studying responses that may be mast cell dependent is to examine the response by BALB/c mice of 6 and 10 weeks of age. In the present study, this approach was used to confirm the importance of mast cell activity in UVB‐induced systemic suppression of CHS responses.
The signal for age‐related changes in dermal mast cell numbers in BALB/c mice is not known. SCF is traditionally recognized as the maintenance factor for mast cells in tissues.9–11 Furthermore, exogenous SCF can increase tissue mast cell numbers; this increase was reversible with numbers of mast cells decreasing owing to apoptosis of the cells with growth factor withdrawal.20 It is not known if there are age‐related changes in SCF production in BALB/c mice. Transforming growth factor‐β1 (TGF‐β1) can decrease SCF mRNA and SCF production in vitro.21 In another study,22 a deficiency of tumour necrosis factor (TNF) or TNF receptors was linked with decreased dermal mast cell prevalence; it is unknown if this was an effect that was manifest in reduced SCF levels. Similarly, mice carrying the Uvs1 gene, a gene for susceptibility to UV suppression, have a dermal mast cell prevalence greater than their BALB/c counterparts.19 These mice were derived by nine generations of successive backcrossing of F1 hybrid BALB/c × C57BL/6 mice to the BALB/c parent with selection for high susceptibility to UV suppression, followed by three generations of intercrossing.23 It is unknown if the product of the Uvs1 gene affects dermal mast cell numbers indirectly via SCF levels.
In this study, as mast cell numbers decreased in BALB/c mice, so too did the expression of Kit, the receptor for SCF, on mast cells. One interpretation of the results is that mast cell numbers and Kit expression on the mast cells are interrelated in BALB/c mice. In a study of Wsh/Wsh mutant mice, such a relationship was demonstrated.24 It was shown that Kit disappeared earlier than the mast cells themselves. The number of mast cells in the skin of Wsh/Wsh embryos of 18 days postcoitum (p.c.) was ≈ 40% of that of normal control +/+ embryos, but the number of mast cells decreased exponentially after birth; the number had dropped to 0·6% that of +/+ mice by day 150 after birth. A weak but apparent signal of c‐kit mRNA was detectable in the skin of 18‐day p.c. Wsh/Wsh embryos by RNase protection assay but not in the skin of 5‐day‐old Wsh/Wsh mice.24 Furthermore, Kit‐protein staining cells were rare in the skin of 5‐day‐old Wsh/Wsh mice.24 In our study of BALB/c mice, there was no apparent decrease in Kit levels on mast cells prior to decreases in the mast cell prevalence. It must be stressed that ours is a study in non‐mutant mice, in or near adulthood, and that the changes in mast cell prevalence and Kit expression occurred much later than those seen in mutant mice. We can only speculate that as both BALB/c and C57BL/6 mice matured from 6 to 10 weeks of age, there may be transcriptional control of the production of Kit and/or of SCF. However, as dermal mast cell numbers were similar for 6‐week‐old BALB/c mice and 6–10‐week‐old C57BL/6 mice, we hypothesize that regardless of the extent of Kit expression, the dermal mast cell populations were maximally expanded in these mice.
The finding that changes in Kit expression on dermal mast cells parallel dermal mast cell prevalence in BALB/c mice suggests that, in these mice, there may be exponential differences in the response to SCF. In 6‐week‐old mice, not only are there a greater number of cells to respond to SCF but also, potentially, a greater response per cell. This is a regulatory point for responses to SCF that is not generally recognized.
This study suggests that in BALB/c mice, as they age from 6 to 10 weeks, there are signals to reduce both mast cell prevalence and Kit expression on the remaining mast cells. We hypothesize that similar signals may occur in C57BL/6 mice but that Kit remains at sufficient levels on adult mice for maintenance of a high dermal mast cell prevalence. We advocate the study of potential mast cell‐dependent responses in BALB/c mice of different ages. Furthermore, if one wishes to study the effect of SCF on mast cells, ageing BALB/c mice provide a unique opportunity to study the greater extremes of mast cell activity as the responses will be governed by not only mast cell number but also by a greater frequency of ligand–receptor interaction on a per cell basis.
Acknowledgments
This work was supported by the Anti‐Cancer Foundation of South Australia, the National Health and Medical Research Council of Australia (Project 34273) and the Flinders Medical Centre Foundation (to P. H. H. and J. J. F.-J.) and in part by NIH RO1 CA53765 (to F. P. N.).
Glossary
Abbreviations
- CHS
contact hypersensitivity
- IOD
integrated optical density
- SCF
stem cell factor
- TNCB
2,4,6‐trinitrochlorobenzene
- UVB
UV radiation of wavelength 280–320 nm
References
- 1.Benyon RC, Lowman MA, Church MK. Human skin mast cells: their dispersion, purification, and secretory characterization. J Immunol. 1987;138:861. [PubMed] [Google Scholar]
- 2.He D, Esquenazi‐Behar S, Soter NA, Lim HW. Mast‐cell heterogeneity: functional comparison of purified mouse cutaneous and peritoneal mast cells. J Invest Dermatol. 1990;94:178. doi: 10.1111/1523-1747.ep12477951. [DOI] [PubMed] [Google Scholar]
- 3.Galli SJ. Biology of disease. New insights into ‘The riddle of the mast cells’: microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab Invest. 1990;62:5. [PubMed] [Google Scholar]
- 4.Dvorak AM, Seder RA, Paul WE, Morgan ES, Galli SJ. Effects of interleukin‐3 with or without the c‐kit ligand, stem cell factor, on the survival and cytoplasmic granule formation of mouse basophils and mast cells in vitro. Am J Pathol. 1994;144:160. [PMC free article] [PubMed] [Google Scholar]
- 5.Gurish MF, Ghildyal N, Mcneil HP, Austen KF, Gillis S, Stevens RL. Differential expression of secretory granule proteases in mouse mast cells exposed to interleukin 3 and c‐kit ligand. J Exp Med. 1992;175:1003. doi: 10.1084/jem.175.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuda H, Kannan Y, Ushio H, et al. Nerve growth factor induces development of connective tissue‐type mast cell in vitro from murine bone marrow cells. J Exp Med. 1991;174:7. doi: 10.1084/jem.174.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rennick D, Hunte B, Holland G, Thompson‐Snipes L. Cofactors are essential for stem cell factor‐dependent growth and maturation of mast cell progenitors: comparative effects of interleukin‐3 (IL‐3), IL‐4, IL‐10, and fibroblasts. Blood. 1995;85:57. [PubMed] [Google Scholar]
- 8.Renauld J‐c, Kermouni A, Vink A, Louahed J, Van Snick J. Interleukin‐9 and its receptor: involvement in mast cell differentiation and T cell oncogenesis. J Leuk Biol. 1995;57:353. doi: 10.1002/jlb.57.3.353. [DOI] [PubMed] [Google Scholar]
- 9.Galli SJ, Tsai M, Wershil BK. The c‐kit receptor, stem cell factor, and mast cells. What each is teaching us about the others. Am J Pathol. 1993;142:965. [PMC free article] [PubMed] [Google Scholar]
- 10.Metcalfe DD, Mekori JA, Rottem M. Mast cell ontogeny and apoptosis. Exp Dermatol. 1995;4:227. doi: 10.1111/j.1600-0625.1995.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 11.Mcniece IK, Brodde; RA. Stem cell factor. J Leuk Biol. 1995;57:14. doi: 10.1002/jlb.58.1.14. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura Y, Hanataka S. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447. [PubMed] [Google Scholar]
- 13.Kitamura Y, GOS Decreased production of mast cells in Si/sid anemic micE. Blood. 1979;53:492. [PubMed] [Google Scholar]
- 14.Tharp MD. Understanding mast cells and mastocytosis. J Invest Dermatol. 1997;108:698. doi: 10.1111/1523-1747.ep12292075. [DOI] [PubMed] [Google Scholar]
- 15.Marshall JS, Bienenstock J. The role of mast cells in inflammatory reactions of the airways, skin and intestine. Curr Opin Immunol. 1994;6:853. doi: 10.1016/0952-7915(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 16.Galli SJ, Wershil BK. The two faces of the mast cell. Nature. 1996;381:21. doi: 10.1038/381021a0. [DOI] [PubMed] [Google Scholar]
- 17.Galli SJ, Maurer M, Lantz CS. Mast cells as sentinels of innate immunity. Curr Opin Immunol. 1999;11:53. doi: 10.1016/s0952-7915(99)80010-7. [DOI] [PubMed] [Google Scholar]
- 18.Thomas WR, Schrader JW. Delayed hypersensitivity in mast‐cell‐deficient mice. J Immunol. 1983;130:2565. [PubMed] [Google Scholar]
- 19.Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay‐Jones JJ. Dermal mast cells determine susceptibility to ultraviolet B‐induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med. 1998;187:2045. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galli SJ, Iemura A, Garlick DS, Gamba‐Vitalo C, Zsebo KM, Andrews RG. Reversible expansion of primate mast cell populations in vivo by stem cell factor. J Clin Invest. 1993;91:148. doi: 10.1172/JCI116164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubois CM, Ruscetti FW, Stankova J, Keller JR. TGF‐beta regulates c‐kit message stability and cell‐surface protein expression in hemopoietic progenitors. Blood. 1994;83:3138. [PubMed] [Google Scholar]
- 22.Hart PH, Grimbaldeston MA, Swift GJ, Sedgwick JD, Korner H, Finlay‐Jones JJ. TNF modulates susceptibility to UVB‐induced systemic immunomodulation in mice by effects on dermal mast cell prevalence. Eur J Immunol. 1998;28:2893. doi: 10.1002/(SICI)1521-4141(199809)28:09<2893::AID-IMMU2893>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 23.Noonan FP, Hoffman HA. Control of UVB immunosuppression in the mouse by autosomal and sex‐linked genes. Immunogenetics. 1994;40:247. doi: 10.1007/BF00189969. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki M, Tsujimura T, Morii E, et al. C‐kit gene is expressed by skin mast cells in embryos but not in puppies of Wsh/Wsh mice: age‐dependent abolishment of c‐kit gene expression. Blood. 1994;83:3509. [PubMed] [Google Scholar]