Abstract
Taurine chloramine (TauCl) is a major chloramine generated in activated neutrophils as a result of the reaction of highly toxic hypochlorous acid and taurine, the most abundant free amino acid in cytosol. In this study we have tested the influence of TauCl on the properties of murine dendritic cells (DC), the major cell population involved in the initiation of an adaptive immune response against pathogenic organisms. N418+, MHC II+, B7‐2+ dendritic cells, generated from the mouse bone marrow cells cultured in the presence of granulocyte–macrophage colony‐stimulating factor, were stimulated by interferon‐γ and lipopolysaccharide to produce nitric oxide, reactive oxygen species, interleukin‐6 (IL‐6), tumour necrosis factor‐α, and IL‐12, in the presence of different doses of TauCl. TauCl differently inhibited the generation of these inflammatory mediators in a dose‐dependent manner. Furthermore, TauCl selectively modulated the ability of DC to induce the release IL‐2 and IL‐10 from T cells. These results suggest that neutrophil‐derived mediators, such as TauCl, at a site of inflammation, may affect the functions of sentinel DC and macrophages, and play a role in maintaining the balance between the inflammatory response and the induction of an antigen‐specific immune response.
Introduction
Dendritic cells (DC) are bone marrow‐derived cells that function as professional antigen‐presenting cells (APC).1,2 Their strategic distribution in non‐lymphoid tissues (e.g. Langerhans’ cells of the skin), and a high capability for antigen capture and processing indicate their important role in the initiation of immune responses against invading pathogens.2,3 DC are also recruited together with neutrophils (PMN) into acute inflammatory loci.4 On the other hand, inflammatory mediators promote DC migration out of non‐lymphoid tissues into T‐cell areas of lymphoid organs. At this stage the cells (referred to as interdigitating DC) down‐regulate their ability to capture antigen and acquire an increased capacity to stimulate T cells.5,6 This final maturation of DC is under the control of proinflammatory mediators, which are released at a site of inflammation by professional phagocytes (neutrophils and macrophages) as well as by DC themselves (in an autocrine fashion).7,8 Whole bacteria, the microbial cell‐wall components, such as lipopolysaccharide (LPS), and cytokines, including tumour necrosis factor‐α (TNF‐α), granulocyte–macrophage colony‐stimulating factor (GM‐CSF) and interleukin‐1 (IL‐1), all promote DC maturation, whereas it is blocked by IL‐10.5,6,9
PMN, the major cells of acute inflammation, during phagocytosis generate a variety of microbicidal agents, such as reactive nitrogen species (RNS) and reactive oxygen species (ROS), including hypochlorous acid (HOCl), a highly toxic product of the myeloperoxidase–hydrogen peroxide–halide system.10–12 Moreover, activated neutrophils release a number of proinflammatory mediators, including cytokines (TNF‐α, IL‐1, IL‐6, IL‐12) and eicosanoids [prostaglandin E2 (PGE2), leukotriene B4 (LTB4)], which can affect the function of sentinel DC.13,14 The HOCl reacts rapidly with a variety of different molecules to produce less toxic and more long‐lived chloramines.15–18 Because of extremely high concentration of taurine in neutrophils, taurine chloramine (TauCl) is the predominant long‐lived chloramine produced in this way.19 TauCl down‐regulates the production of pro‐inflammatory mediators by macrophages and neutrophil.20–22 However, there are no data as to whether TauCl can modulate the function of DC. In this study, we addressed the question whether TauCl affects the sentinel function of DC by altering their ability to secrete inflammatory mediators and to present antigen. This in vitro system may provide a model for the in vivo scenario, in which neutrophil‐derived TauCl might have an immediate affect on the sentinel DC in situ at the site of inflammation, or a delayed affect on DC which have migrated from the inflammatory site to the draining lymph nodes.
Materials and methods
Preparation of TauCl
Taurine monochloramine (TauCl) was prepared in our laboratory by adding equimolar amounts of NaOCl (Sigma, St. Louis, MO) dropwise to taurine (Sigma) solution in 0·05 m phosphate buffer (pH 7·4–7·5), as described previously.21 Each preparation of TauCl was monitored by ultraviolet absorption spectra (200–400 nm) to confirm the presence of monochloramine (TauCl) and the absence of dichloramine (TauCl2), NH2Cl and unreacted HOCl/OCl–. The concentration of TauCl was determined by absorption at 252 nm (εM = 415).23 Stock solutions of Tau and TauCl (10 mm) were kept at 4° for a maximum period of 4 days before use.
Mice
Male adult 18–22 g BALB/c and CBA/J mice (specific pathogen‐free) were maintained in the animal house at the Departments of Immunology, at Jagiellonian University Medical College, Cracow, Poland or University College London, UK.
Cells
Bone marrow‐derived DC
DC were prepared as described previously.24 Bone marrow was flushed from the femurs and DC were grown from precursors at a starting concentration of 3 × 105 cells/ml in Iscove’s Modified Dulbecco’s Medium (Gibco, Grand Island, NY) supplemented with apotransferrin, 2‐mercaptoethanol (both Sigma), 10% fetal calf serum (FCS) (Gibco) and GM‐CSF (supernatant from X‐63 cell line, a gift from Dr Brigitta Stockinger, NIMR Mill Hill, London, UK). At 2 days the medium and most of the non‐adherent cells were removed and replaced with fresh medium. After 7 days of culture non‐adherent cells (DC), released spontaneously from proliferating cell clusters, were recovered. The final population was purified by removal of adherent cells (macrophages). The purity of DC was verified by morphological appearance (Giemsa staining) and flow cytometric analysis (91%), as described previously.24
CD4+ T cells
Trinitrophenyl (TNP) ‐specific CD4+ T cells were prepared as follows. CBA mice were sensitized by topical application of 150 µl of 5% trinitrochlorobenzene (Sigma, Poole, Dorset) in ethanol/acetone. Draining lymph nodes were collected 4 days later, and CD4+ T cells were purified as described previously.25 The final T‐cell population, which constituted ≈ 30–35% of the total lymph node population, was 96% positive for CD4+ T cells, as judged by flow cytometry.
Flow cytometry
Cell surface immunophenotypic analysis of DC was performed by cytofluorography using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
DC were incubated with the following primary monoclonal antibodies (mAbs): TIB 93, mouse anti‐mouse I‐Ak (from the American Type Culture Collection, Rockville, MD); N418, hamster anti‐mouse CD11c (a gift from Dr Hal Drakesmith ICRF, London, UK); B7.2, rat anti‐mouse B7.2 (Cambridge Biosciences, Cambridge, UK), or an isotype‐matched control as appropriate. This was followed by fluorescein isothiocyanate (FITC)‐conjugated rabbit anti‐mouse immunoglobulins (Dako, High Wycombe, UK) and FITC‐conjugated rabbit anti‐rat immunoglobulins (PharMingen, San Diego, CA) as appropriate. After staining, the cells were fixed in 1% paraformaldehyde in saline before analysis. For each sample, data from 5000 cells were collected.
Cell culture – activation of cells for production of inflammatory mediators
DC were cultured in 24‐well flat‐bottom cell culture plates at 5 × 105/well in RPMI‐1640 medium (J R Scientific Inc., Woodland, CA) supplemented with 5% FCS, at 37° in an atmosphere of 5% CO2. DC were preincubated with either Tau or TauCl (0·05–0·5 mm) for 1–2 hr and then the cells were activated with interferon‐γ (IFN‐γ) 20 U/ml (Sigma) and LPS 100 ng/ml (Escherichia coli 0111 B:4, Sigma). After 24 hr culture supernatants were collected and were frozen at –20° for NO2–, IL‐6, IL‐10, IL‐12, TNF‐α, and PGE2 assays.
DC‐induced cytokine secretion by T cells
TNP‐system
DC were preincubated for 1 hr with the indicated concentrations of either Tau or TauCl and were washed twice to remove Tau/TauCl before being haptenized with TNP. CD4+ T cells (2 × 105/well), as responder cells, and TNP‐coupled DC (5 × 104/well), as APC cells, were cultured in RPMI‐1640 medium, as described previously.25 After 24 hr, supernatants were collected and frozen at –20° prior to assay for IL‐2.
Primary mixed lymphocyte reaction (MLR) cultures
H‐2d BALB/c responder T cells (2 × 106/well) were cultured with H‐2k CBA/J inducer DC (2 × 105/well) for 3 days. LPS‐pulsed DC were preincubated for 1 hr with either Tau or TauCl. Tau/TauCl and LPS were washed out before DC were added to allogeneic T cells. After 24, 48 and 72 hr supernatants were collected for IL‐2 [T helper type 1 (Th1)] and IL‐10 (Th2) assays.
Measurement of cell viability
In every experiment the viability of cells was routinely monitored by cellular exclusion of trypan blue. In some experiments cell respiration, an indicator of cell viability, was assessed by the mitochondria‐dependent reduction of 3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5 diphenylo tetrazolium bromide (MTT) (Sigma) to formazan.
Chemiluminescence assay: luminol‐dependent chemiluminescence (LCL)
DC were placed in 96‐well flat bottom black plates (Nunc, Roskilde, Denmark), 5 × 105 cells/well in RPMI‐1640 + 10% FCS. After a dark adaptation period of 40 min at 37°, plates were transferred to a Lucy 1 luminometer (Anthos, Salzburg, Austria) immediately after the addition of either zymosan particles opsonized by pretreatment with mouse serum (10 particles/cell) or phorbol myristate acetate (PMA) (200 ng/ml) (both Sigma), and photon emission over 60 min was measured. The following agents were added to cells at the onset of dark adaptation: luminol (Sigma) and Tau or TauCl. Each experiment was run in duplicate.
Measurement of superoxide production
Superoxide production was determined by the cytochrome C reduction assay as described by Pick and Mizel.26 Briefly, DC (2 × 105/well) were resuspended in 160 µm solution of ferricytochrome c (Sigma) in phenol red‐free Hanks’ balanced salt solution (HBSS) and plated in 96‐well flat bottom tissue culture plates (Nunc) in a final volume of 100 µl per well. The cells were stimulated with either opsonized zymosan or PMA (200 ng/ml). Wells supplemented with 300 U/ml of superoxide dismutase (SOD) (Sigma) were used as blanks. Cytochrome C reduction was measured after 2 hr of incubation by measuring absorbance at 550 nm using an ELISA reader (Labsystems Multiscan Plus, Helsinki, Finland) using a 450‐nm reference filter. Results were expressed as nmol/2 × 105 cells/2 hr. Detection limit was about 0·3 nmol/well.
Determination of H2O2 production
H2O2 detection was based on the horseradish peroxidase (HRPO)‐dependent oxidation of phenol red, as described by Pick and Mizel.26 Briefly, DC were resuspended in assay solution containing 0·56 mm of phenol red (Sigma) and 20 U/ml HRPO (Sigma) in phenol red‐free HBSS and seeded in tissue culture plates in a final volume of 100 µl per well. The cells were stimulated with either opsonized zymosan or PMA, as described above. After incubation for 2 hr the reaction was stopped by addition of 10 µl of 1 m NaOH per well and absorbance was read at 600 nm. Wells with NaOH added at the beginning of testing were used as blanks. Results were expressed as nmol/2 × 105 cells/2 hr. The limit of detection was 0·25 nmol/well.
Nitrite (NO2–) determination
NO, quantified by the accumulation of nitrite as a stable end product, was determined by microplate assay.12 Briefly, 100‐µl samples were removed from supernatants and incubated with an equal volume of Griess reagent (1% sulfanilamide/0·1%, N‐1‐naphthylenediamine dihydrochloride/2·5% H3PO4) (Sigma) at room temperature for 10 min. The absorbance at 550 nm was measured with a microplate reader. Nitrite concentration was calculated from a sodium nitrite standard curve. The limit of detection was 2 µm.
Cytokine assays – measurement of IL‐2, IL‐6, IL‐10, IL‐12 and TNF‐α
Cytokine concentration in culture supernatants was measured using capture ELISA as detailed below. For IL‐2, ‐6, ‐10 and ‐12, microtitre plates (Corning, NY) were coated with rat monoclonal antibody against a mouse cytokine (capture antibody), and biotinylated antibodies against the same cytokine (detecting antibody). The ELISA was developed with HRPO streptavidin (Vector Laboratories, Burlingame, CA), followed by o‐phenylenediamine and H2O2 (both Sigma) as substrates. For TNF‐α, a peroxidase‐conjugated goat anti‐rabbit immunoglobulin G (IgG; Sigma) was used to develop the reaction. The reaction was stopped with 3 m H2SO4 and the optical density of each well at 492 nm was measured in a 96‐well plate reader. The following reagents were used for these assays.
IL‐2
Rat anti‐mouse IL‐2, as a capture mAb, and biotinylated rat anti‐mouse IL‐2, as detecting mAb (both Pharmingen). Recombinant mouse IL‐2 (Pharmingen) was used as a standard. Detection limit was about 60 pg/ml of IL‐2.
IL‐6
Rat anti‐IL‐6 (Genzyme) and biotinylated rat anti‐IL‐6 (Pharmingen) mAbs. Recombinant mouse IL‐6 (PeproTech Rocky Hill, New York) was used as a standard. Detection limit was about 100 pg/ml of IL‐6.
IL‐10
Rat anti‐mouse IL‐10 (Genzyme), and biotinylated rat anti‐mouse IL‐10 (Pharmingen) mAbs. Recombinant mouse IL‐10 (Genzyme) was used as a standard. The limit of detection was 60 pg/ml.
IL‐12
Rat anti‐mouse IL‐12 (p40 subunit), clone C15.6 (Genzyme) and biotinylated rat anti‐mouse IL‐12 (p40/p70 subunits) clone C17.8 (Pharmingen) mAbs were used as capture and detecting antibody, respectively. Recombinant mouse IL‐12 (p70 heterodimer) (Genzyme) was used as a standard. The ELISA measures both p70 and p40. The limit of detection was 100 pg/ml
TNF‐α
Hamster anti‐murine TNF‐α mAb as capture (Genzyme), and rabbit polyclonal anti‐murine TNF‐α (Genzyme) as detector. Recombinant murine TNF‐α (Genzyme) was used as a standard. The limit of detection was 10 U/ml of TNF‐α.
Determination of PGE2
PGE2 was determined by using a PGE2 EIA Kit (Cayman Chemical Co., MI). The limit of detection was 30 pg/ml.
Statistical analysis
Results are expressed as mean ± SD. Statistical significance was determined by Student’s t‐test and the differences were regarded as significant for P < 0·05.
Results
The effect of TauCl on viability of DC
In the previous studies, TauCl at the concentrations of 0·05–1·0 mm was not cytotoxic for macrophages or macrophage cell lines.21,22 In the preliminary experiments, we tested the effect of TauCl on the viability of DC. The viability of DC preincubated with TauCl at a range of 0·05–0·5 mm for 2 hr and measured after 24 hr was not different from that of untreated cells (≈ 87%). The incubation of DC with TauCl at a range of 0·05–0·5 mm for 24 hr, in the presence of GM‐CSF, did not affect the cell viability (84 ± 3% in treated cells versus 88 ± 3% in control DC). However, when DC where cultured for 24 hr with TauCl at the highest concentration of 0·5 mm, in the absence of GM‐CSF, a significant decrease in the cell viability (69 ± 4% versus 88 ± 3% in control DC, P < 0·05) was observed. Thus, all further experiments were performed under conditions at which TauCl was not cytotoxic.
The capacity of DC to release inflammatory mediators
N418+, MHC IIhigh, B7‐2high bone marrow DC generate in vitro a variety of inflammatory/costimulatory mediators. These cells produced TNF‐α, IL‐6, IL‐10, IL‐12 (p40 + p70), nitric oxide (NO), and PGE2 upon stimulation with LPS and IFN‐γ. Unstimulated DC released also substantial amounts of PGE2 (10–15%) and cytokines (2–5%) of the stimulated cell production. In the contrast, the release of NO/NO2– from non‐activated cells was negligible (0–4 µm) (Table 1).
Table 1.
The release of proinflammatory mediators from dendritic cells (DC)*
| Mediator | Stimulated DC | Non‐stimulated DC | Limit of of detection |
|---|---|---|---|
| NO2– (µm) | 53·7 ± 17 | < 4 | 4 |
| TNF‐α (U/ml) | 1510 ± 459 | 20 ± 15 | 10 |
| IL‐6 (ng/ml) | 23·1 ± 9·2 | 0·25 ± 0·2 | 0·1 |
| IL‐10 (pg/ml) | 235 ± 88 | < 60 | 60 |
| IL‐12 (ng/ml)† | 132·7 ± 44 | 2·9 ± 1·1 | 0·1 |
| PGE2 (ng/ml) | 2·8 ± 0·3 | 0·55 ± 0·2 | 0·03 |
5 × 10 5/well DC were cultured in the presence of IFN‐γ (20 U/ml) and LPS (100 ng/ml). After 24 hr supernatants were collected and frozen for assays. Results are the mean ± SD from six separate experiments.
IL‐12 = p40 + p70.
ROS production by the cells was measured by two methods, luminol‐dependent chemiluminescence (LCL) and more specific colorimetric enzymatic assays for the detection of the superoxide anion (O2–) and hydrogen peroxide (H2O2) release. LCL measures both intra‐ and extra‐cellular ROS, but detects a variety of species including O2–, OH· and H2O2.27,28 DC did not produce any detectable O2– either after stimulation with zymosan or with PMA (data not shown). PMA did not stimulate DC effectively for H2O2 production (6·8 ± 0·7 nmol/2 × 105 cells per 2 hr). On the contrary, after the stimulation with zymosan, DC generated the substantial amount of H2O2 (40·1 ± 4·2 nmol/2 hr).
The influence of TauCl on the release of inflammatory mediators from DC
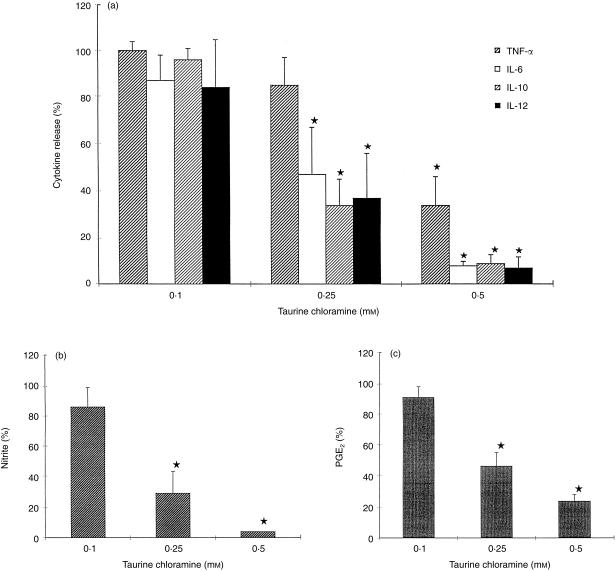
TauCl, preincubated with DC for 2 hr, inhibited the release of TNF‐α, IL‐6, IL‐10, IL‐12, NO and PGE2 in a dose‐dependent manner (Fig. 1). The median inhibitory concentration (IC50) value of TauCl for IL‐6, IL‐10, IL‐12, PGE2, and NO was 200 µm, and 400 µm for TNF‐α. At the highest concentration of 500 µm, TauCl decreased the production of all the mediators (except TNF‐α) to the level of unstimulated cells without affecting the cell viability. Free taurine (0·05–0·5 mm) had no effect on the production of these mediators.
Figure 1.
The effect of TauCl on production of inflammatory mediators by DC. (a) DC (5 × 105/well/ml) were preincubated with TauCl for 2 hr and than the cells were cultured in the presence of LPS (100 ng/ml) and IFN‐γ (20 U/ml). After 24 hr, supernatants were collected and TNF‐α, IL‐6, IL‐10 and IL‐12 were measured by ELISA. (b) DC were cultured as above, and nitrite release was measured by Griess reaction. (c) DC were cultured as above, and PGE2 was measured by ELISA. Results are expressed as the average percentage (± SD) compared to the control group (stimulated DC without TauCl), and are calculated from six independent experiments; * P < 0·05.
The effect of TauCl on LCL of DC
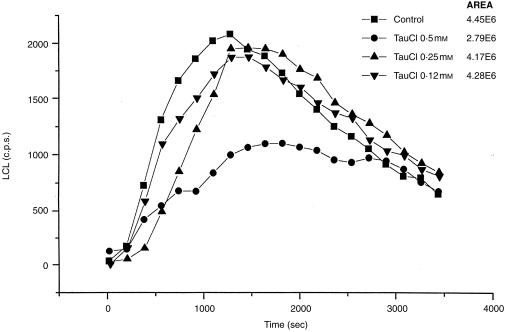
Stimulation of DC with zymosan increased LCL ≈ 100 times over the baseline light emission. It reached a maximum about 12 min after stimulation. When TauCl was added, a dose‐dependent decrease in LCL was observed. (Fig. 2). TauCl, at the highest concentration used (0·5 mm), significantly inhibited LCL (56%, P < 0·05), but did not modify the kinetics of response. In contrast, it had no significant effect on H2O2 generation (the inhibition < 22%). TauCl at concentrations 0·25 mm and below had no effect at all. This suggests that there is selective inhibition of ROS by TauCl. Taurine did not affect either LCL or H2O2 generation.
Figure 2.
The effect of TauCl on luminol‐dependent chemiluminescence (LCL) from DC stimulated with opsonized zymosan. DC (5 × 105/well) were dark adapted in the presence of either luminol alone or luminol and TauCl (0·1–0·5 mm) for 60 min. Chemiluminescence reaction was recorded immediately after the stimulation with zymosan over 3600 seconds. The total area under the curves is shown in the key to the figure. The figure shows the results of one of five separate experiments.
The effect of TauCl on the expression of DC surface markers
LPS‐stimulated DC showed an increased expression of MHC class II and B7‐2 molecules as measured by mean fluorescent intensity. TauCl inhibited this expression of MHC class II and B7‐2 molecules only at the highest tested concentration (0·5 mm) (Table 2). In contrast, TauCl did not affect either the expression of the N418 cell surface DC‐specific marker or the percentage of cells positive for this marker. Free taurine did not affect the expression of any DC surface molecules tested.
Table 2.
The effect of TauCl on the expression of DC surface markers; % (mean)
| DC incubated with†: | |||||
|---|---|---|---|---|---|
| Cell surface markers | ————————— | LPS | LPS + TauCl500 | LPS + TauCl250 | LPS + TauCl100 |
| MHC class II‡ | 62·06 (139·80) | 58·16 (234·98) | 34·58* (36·66*) | 56·28 (187·36) | 61·16 (238·70) |
| B7·2‡ | 56·62 (72·45) | 62·20 (104·56) | 49·46* (82·65*) | 60·12 (140·35) | 60·06 (135·51) |
P < 0·05 versus LPS alone.
DC progenitors from bone marrow were cultured for 7 days as described in the Materials and Methods. Either LPS alone or LPS with TauCl (100–500 µ m) was added for the last 24 hr. Similar effects were observed when DC were pretreated with TauCl for 1 hr.
Expression of DC surface markers was quantified using flow cytometry. DC were incubated with primary monoclonal antibodies as described in the Materials and Methods. The values represent percentage of the markers positive cells and mean fluorescent intensity (MFI) of one of four separate experiments. In all groups non‐specific staining with secondary antibodies was less than 10%.
The cytokine production by T cells stimulated with DC pretreated with TauCl
The effect of pre‐exposure of DC to TauCl on their ability to present antigen was tested in two different systems, TNP‐specific T‐cell‐dependent IL‐2 production and the primary allogeneic MLR. In the first system, TNP‐primed CD4+‐enriched lymph node T cells were stimulated with TNP‐DC pretreated with either Tau or TauCl. Neither Tau nor TauCl affected the capacity of DC to present TNP to TNP specific CD4+ T cells as measured by the production of IL‐2 (data not shown). In the second system, T cells from BALB/c mice (H‐2d) were stimulated with DC from CBA mice (H‐2k). The production of both Th1‐type (IL‐2) and Th2‐type (IL‐10) cytokines was observed in the culture.
T cells cultured in the absence of DC did not produce IL‐2. Neither T cells, in the absence of DC, nor DC alone produced detectable amounts of IL‐10 (data not shown). The differences in the kinetics of IL‐2 and IL‐10 production were observed throughout 96 hr. The production of IL‐2 was not detected after 24 hr, reached a maximum at 48 hr and then declined after 72 hr. In contrast, IL‐10 was characterized by a long (48 hr) lag phase, but after 48 hr the level of IL‐10 rose logarithmically. Pretreatment of DC with TauCl, resulted in a longer lag period of IL‐10 production, but had a minor effect on IL‐2 production (Table 3).
Table 3.
IL‐2 and IL‐10 production by T cells in the primary allogeneic MLR induced by DC pretreated with TauCl
| DC preincubated with†: | 24 hr | 48 hr | 72 hr |
|---|---|---|---|
| Interleukin‐2 (pg/ml)‡ | |||
| Medium | 560 ± 90 | 760 ± 120 | 455 ± 25 |
| LPS | 630 ± 42 | 1440 ± 60 | 1780 ± 120 |
| LPS + TauCl 0·5 mm | 450 ± 60 | 1060 ± 90 | * 870 ± 45 |
| LPS + TauCl 0·25 mm | 610 ± 45 | 1450 ± 30 | 1850 ± 60 |
| LPS + TauCl 0·1 mm | 020 ± 125 | 1790 ± 45 | 605 ± 20 |
| Interleukin‐10 (pg/ml)‡ | |||
| Medium | 59 ± 4 | 60 ± 5 | 102 ± 5 |
| LPS | 51 ± 6 | 171 ± 6 | 290 ± 17 |
| LPS + TauCl 0·5 mm | 40 ± 10 | *40 ± 8 | * 90 ± 6 |
| LPS + TauCl 0·25 mm | 56 ± 5 | *96 ± 8 | *208 ± 12 |
| LPS + TauCl 0·1 mm | 52 ± 4 | 148 ± 9 | 305 ± 25 |
P < 0·05 versus LPS alone.
H‐2 d T cells were cultured with H‐2k DC for 3 days as described in the Materials and Methods. DC were preincubated with LPS (100 ng/ml) and with TauCl (0·1–0·5 mm) for 1 hr. Supernatants were collected on different days and tested for IL‐2 and IL‐10. T cells cultured in the absence of DC did not produce detectable amounts of IL‐2 and IL‐10. The production of IL‐10 by DC was negligible (data not shown).
Results are expressed as the mean ± SDI and represent one of three similar experiments.
Discussion
Pathogens that enter the body are trapped either by widely distributed resident macrophages (e.g. alveolar macrophages within the respiratory tract, Kupffer cells within the liver),14 or by sentinel DC (e.g. Langerhans’ cells of the skin).1,2,4 The interaction between these cells and pathogens may initiate inflammation and either humoral or cellular antigen‐specific immune reaction.5 At the initial acute phase of inflammation, PMN are the major cells responsible for pathogen killing, but they can also contribute to cause local tissue destruction.13,16 This dual effect of PMN activation may result from the release of the same set of mediators. In addition, PMN can affect the function of other cells, such as macrophages and DC, in the local microenvironment. For example, it is commonly accepted that sentinel DC are under the control of proinflammatory mediators, which induce their final maturation and emigration from the site of inflammation.2,4–6
Many of inflammatory mediators released by PMN are also produced by macrophages and DC themselves.7,14 However, PMN posses the unique ability to generate HOCl, the product of the myeloperoxidase–H2O2–halide system.10,15 HOCl, which is a microbicidal and highly cytotoxic agent, reacts immediately with a variety of targets, but primarily with Tau, the most abundant free amino acid in PMN cytosol, thereby producing the more long‐lived and less toxic TauCl.15,29 We have previously shown that TauCl, at non‐cytotoxic concentrations, decreases the production of tissue‐damaging inflammatory mediators. TauCl inhibits the production of NO, TNF‐α and PGE2 by activated macrophages through the different mechanisms with an IC50 value about 0·3 mm.21 TauCl inhibits transcription of the iNOS gene, suppresses the translation of TNF‐α mRNA and affects the post‐transcriptional regulation of COX‐2 (the inducible form of cyclo‐oxygenase) expression.20,22,30 Recently, it has been shown that TauCl may also regulate the function of activated PMN. TauCl inhibited ROS31 and proinflammatory mediator production by stimulated PMN.32 Since sentinel DC are also present at a site of inflammation where they have contact with both neutrophils and macrophages, it seemed reasonable to explore the possibility of DC regulation by TauCl.
The present study examined the effect of TauCl on murine DC functions. It suggests that TauCl may have an immediate effect on DC secretory properties at a site of inflammation and a delayed effect on the capacity of DC to stimulate T cells in lymphoid organs, at the time unlikely for the presence of TauCl in the DC environment. For this reason, we have examined the effects of TauCl in two types of in vitro model. In the first, paralleling the situation found at the inflammatory site, DC were exposed to a strong activating stimulus (LPS/IFN‐γ) in the presence of various concentrations of TauCl. In the second, DC were pre‐exposed to TauCl, but the TauCl was removed prior to testing their ability to stimulate T cells, assessed by production of IL‐2 or IL‐10. At the highest concentration tested (0·5 m m) TauCl inhibited almost completely the secretion of NO, ROS, PGE2 and cytokines TNF‐α, IL‐6, IL‐10, IL‐12 (p40 + p70) by DC. TauCl (0·5 m m) also inhibited the LPS‐induced expression of MHC class II and B7‐2 molecules. At this concentration, however, TauCl may be toxic for DC when the cells are exposed to TauCl for a long time. On the other hand, at the concentration of 0·25 m m, TauCl had more selective effect, as it inhibited the production of IL‐6, IL‐12, IL‐10, PGE2 and NO but not of TNF‐α or ROS. It remains to be established whether the TNF‐α‐ and ROS‐forming systems are more resistant to TauCl, or whether it is because they are formed non‐uniformly by DC, being at different stages of maturation.5,7 Exposure to TauCl at the lower concentrations (< 0·5 m m) also selectively affected the ability of DC to stimulate T‐cell responses. Thus, TauCl exposure did not affect the production of IL‐2 by DC‐stimulated T cells in either the TNP system or the allogeneic primary MLR. In contrast, TauCl diminished and delayed the subsequent release of IL‐10 by the cells in the primary MLR. These results suggest that pre‐exposure of DC to TauCl may favour the development of a Th1 rather then a Th2 response, although more detailed analysis would be required to validate this conclusion fully.
These results raise the question of what are the levels of TauCl which are likely to occur in vivo during the inflammatory response. In our knowledge, there are no direct data on the local concentration of HOCl/TauCl at a site of inflammation. Previous studies have shown that human PMN, after maximal stimulation in vitro, can generate 200 nmol HOCl/106 cells/2 hr, resulting in a release of ≈ 100 nmol of TauCl.15,16 At a concentration 5 × 106 PMN/ml, this would yield a local concentration of TauCl of about 0·5 m m. However, the production of TauCl is self‐limiting: at 0·5 m m TauCl inhibits the generation of ROS by PMN by > 50%,31 thus controlling the continued production of HOCl. In vivo, a large interstitial inflammatory site may contain as many as 25 × 106 PMN/ml,16 which, in theory, could lead to even higher levels of HOCl/TauCl. However, again the autocrine feedback inhibition of HOCl and TauCl on PMN will limit in vivo concentration of ROS generated by PMN. We can also only speculate about the time of interaction between DC and TauCl in vivo. Due to emigration of fully matured DC from a site of inflammation, it is unlikely that in vivo sentinel DC, which have already endocytosed antigen, will be exposed to TauCl for a long time. For instance, a reduced frequency of Langerhans’ cells in the epidermis local to the site of intradermal exposure to TNF‐α has been observed within 30 min. Subsequent accumulation of DC in draining lymph nodes was observed during 18 hr.33 In contrast, non‐emigrating cells, such as professional phagocytes and immature DC, may be exposed at a site of inflammation to extracellular TauCl for a long period of time.
In summary, this is the first study documenting the effect of TauCl on DC. These results, taken together with our previous studies,34–36 demonstrate that at a site of inflammation, the products of the PMN chlorinating system, HOCl and TauCl, affect the functions of APC and/or react directly with antigens.
HOCl, a highly toxic oxidant, reacts with self and non‐self proteins. Proteins modified by chlorination acquire enhanced susceptibility to degradation by endoproteinases. Degradation of self‐proteins contributes to the damage of surrounding tissue. However, chlorination of non‐self proteins enhances their immunogenicity, probably by facilitating processing and presentation of these proteins by APC.34
Taurine acts simply as a trap to scavenge excess highly reactive HOCl to produce long‐lived and less toxic TauCl. TauCl in turn may exert a negative feedback effect on the production of inflammatory mediators by PMN, macrophages and DC. Thus, TauCl may modulate those functions of DC which are under control of inflammatory mediators. In addition, TauCl may also enhance protein immunogenicity by chlorination, but to a lesser extent then HOCl.37 TauCl may therefore play a role in maintaining the delicate balance between mounting an effective immune response on the one hand, and minimizing the destruction of the tissue by the inflammatory cells, on the other.
Acknowledgments
This work was supported by a grant from the Committee of Scientific Research (Warsaw, Poland) and by a grant from the Wellcome Trust, UK.
Glossary
Abbreviations
- APC
antigen‐presenting cells
- DC
dendritic cells
- LCL
luminol‐dependent chemiluminescence
- MLR
mixed lymphocyte reaction
- Mφ
macrophages
- PMN
neutrophils
- ROS
reactive oxygen species
- Tau
taurine
- TauCl
taurine chloramine
- TNP
trinitrophenyl
References
- 1.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. J Exp Med. 1973;137:1142. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim MM, Chain BM, Katz DR. The injured cell: the role of dendritic cell system as sentinel receptor pathway. Immunol Today. 1995;16:181. doi: 10.1016/0167-5699(95)80118-9. [DOI] [PubMed] [Google Scholar]
- 4.McWilliam AS, Nelson D, Thomas JA, Holt PG. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med. 1994;179:1331. doi: 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony‐stimulating factor. J Exp Med. 1992;176:1693. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romani N, Kampen E, Koch F, Heufler C, Schuler G. Dendritic cell production of cytokines and responses to cytokines. Int Rev Immunol. 1990;6:151. doi: 10.3109/08830189009056626. [DOI] [PubMed] [Google Scholar]
- 8.Kaliñski P, Hilkens CMU, Snijders A, Snijdewint FGM, Kapsenberg ML. IL‐12‐deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28. [PubMed] [Google Scholar]
- 9.Macatonia SE, Doherty TM, Knight SC, O'Garra A. Differential effect of IL‐10 on dendritic cell‐induced T cell proliferation and IFN‐γ production. J Immunol. 1993;150:3755. [PubMed] [Google Scholar]
- 10.Klebanoff SJ, Hamon CB. Role of myeloperoxidase mediated anti‐microbial system in intact leukocytes. J Reticuloendothel Soc. 1992;12:170. [PubMed] [Google Scholar]
- 11.Thomas EL. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: effect of exogenous system on antibacterial action against Escherichia coli. Infect Immun. 1979;25:110. doi: 10.1128/iai.25.1.110-116.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407. [PubMed] [Google Scholar]
- 13.Smith J. Neutrophils, host defence, and inflammation: a double‐edged sword. J Leukoc Biol. 1994;56:672. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 14.Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993;54:171. [PubMed] [Google Scholar]
- 15.Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils: evidence for hypochlorus acid generation. J Clin Invest. 1982;70:598. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 17.Zgliczynski MJ, Stelmaszyñska T, Domanski J, Ostrowski W. Chloramines as intermediates of oxidation reaction of amino acids by myeloperoxidase. Biochim Biophys Acta. 1971;253:419. doi: 10.1016/0005-2744(71)90281-6. [DOI] [PubMed] [Google Scholar]
- 18.Wright CE, Tallan HH, Lin YY. Taurine, biological update. Annu Rev Biochem. 1986;55:427. doi: 10.1146/annurev.bi.55.070186.002235. [DOI] [PubMed] [Google Scholar]
- 19.Fukkuda K, Hirai Y, Yoshida H, Hakaijma T, Usii T. Free amino acids content of lymphocytes and granulocytes compared. Clin Chem. 1982;28:1758. [PubMed] [Google Scholar]
- 20.Park E, Quinn MR, Wright CE, Schuller‐Levis G. Taurine chloramine inhibits the synthesis of nitric oxide and the release of tumor necrosis factor in activated RAW 264.7 cells. J Leukoc Biol. 1993;54:119. doi: 10.1002/jlb.54.2.119. [DOI] [PubMed] [Google Scholar]
- 21.Marcinkiewicz J, Grabowska A, Bereta J, Stelmaszyñska T. Taurine chloramine, a product of activated neutrophils, inhibits in vitro the generation of nitric oxide and other macrophage inflammatory mediators. J Leukoc Biol. 1995;58:667. doi: 10.1002/jlb.58.6.667. [DOI] [PubMed] [Google Scholar]
- 22.Park E, Schuller‐Levis G, Quinn MR. Taurine chloramine inhibits production of nitric oxide and TNF‐α in activated RAW 264.7 cells by mechanisms that involve transcriptional and translational events. J Immunol. 1995;154:4778. [PubMed] [Google Scholar]
- 23.Thomas EL, Grisham MB, Jefferson MM. Preparation and characterisation of chloramines. Methods Enzymol. 1986;132:569. doi: 10.1016/s0076-6879(86)32042-1. [DOI] [PubMed] [Google Scholar]
- 24.Drakesmith H, O'Neil D, Schneider S, et al. Direct priming of T cells against cryptic determinants by dendritic cells exposed to IL‐6 and native antigen. Proc Natl Acad Sci USA. 1998;95 (25):14 903. doi: 10.1073/pnas.95.25.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcinkiewicz J, Grabowska A, Chain BM. Is there a role for nitric oxide in regulation of T cell secretion of IL‐2. J Immunol. 1996;156:4617. [PubMed] [Google Scholar]
- 26.Pick E, Mitzel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Meth. 1981;46:211. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- 27.Albrecht D, Jungi TW. Luminol‐enhanced chemiluminescence induced peripheral blood‐derived human phagocytes: obligatory requirement of myeloperoxidase endocytosis by monocytes. J Leukoc Biol. 1993;54:300. doi: 10.1002/jlb.54.4.300. [DOI] [PubMed] [Google Scholar]
- 28.Allen RC, Loose L. Phagocytic activation of a luminol dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Comm. 1976;69:245. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- 29.Learn DB, Fried VA, Thomas EL. Taurine and hypotaurine content of human leukocytes. J Leukoc Biol. 1990;48:174. [PubMed] [Google Scholar]
- 30.Quinn MR, Park E, Schuller‐Levis G. Taurine chloramine inhibits prostaglandin E2 production in activated RAW 264.7 cells by post‐transcriptional effects on inducible cyclooxygenase expression. Immunol Lett. 1996;50:185. doi: 10.1016/0165-2478(96)02542-4. [DOI] [PubMed] [Google Scholar]
- 31.Kim Ch, Park E, Quinn MR, Schuller‐Levis G. The production of superoxide anion and nitric oxide by cultured murine leukocytes and the accumulation of TNF‐α in the conditioned media is inhibited by taurine chloramine. Immunopharmacology. 1996;34:89. doi: 10.1016/0162-3109(96)00113-0. [DOI] [PubMed] [Google Scholar]
- 32.Marcinkiewicz J, Grabowska A, Bereta J, Bryniarski K, Nowak B. Taurine chloramine down‐regulates the generation of murine neutrophil inflammatory mediators. Immunopharmacology. 1998;40:27. doi: 10.1016/s0162-3109(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 33.Cumberbatch M, Dearman RJ, Kimber I. Langerhans cells reguire signals from both tumour necrosis factor‐α and interleukin‐1β for migration. Immunology. 1997;92:388. doi: 10.1046/j.1365-2567.1997.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcinkiewicz J, Chain BM, Olszowska E, Olszowski S, Zgliczyñski JM. Enhancement of immunogenic properties of ovalbumin as a result of its chlorination. Int J Biochem. 1991;23:1393. doi: 10.1016/0020-711x(91)90280-z. [DOI] [PubMed] [Google Scholar]
- 35.Marcinkiewicz J, Olszowska E, Olszowski S, Zgliczyñski JM. Enhancement of trinitrophenyl‐specific humoral response to TNP proteins as the result of carrier chlorination. Immunology. 1992;76:385. [PMC free article] [PubMed] [Google Scholar]
- 36.Marcinkiewicz J. Neutrophils chloramines – missing link between innate and acquired immunity. Immunol Today. 1997;18:577. doi: 10.1016/s0167-5699(97)01161-4. [DOI] [PubMed] [Google Scholar]
- 37.Marcinkiewicz J, Grabowska A, Chain BM. Modulation of antigen‐specific T‐cell activation in vitro by taurine chloramine. Immunology. 1998;94:325. doi: 10.1046/j.1365-2567.1998.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]