Abstract
Inflammatory response differences between C57BL/6 and BALB/c mice following ovalbumin (OVA) sensitization and a single challenge were investigated. Serum immunoglobulin (Ig)E and IgG1 levels were higher in C57BL/6 mice than in BALB/c mice. In contrast, IgG2a levels in C57BL/6 mice were lower than in BALB/c mice. Furthermore, the number of eosinophils infiltrating into lungs in C57BL/6 mice was significantly higher than in BALB/c mice after OVA challenge. The levels of the T helper 2 (Th2)‐type cytokines interleukin (IL)‐4 and IL‐5, generated in challenged C57BL/6 lung tissue, were also higher than in BALB/c lung tissue. The participation of IL‐4 and IL‐5 in the induction of eosinophil infiltration into the lungs was confirmed in both strains of mice by injection of anti‐IL‐4 and anti‐IL‐5 monoclonal antibodies (mAbs). However, following OVA stimulation, in vitro IL‐4 and IL‐5 production in splenocyte cultures from C57BL/6 mice was lower than in splenocyte cultures from BALB/c mice. These results indicate that C57BL/6 mice induce Th2‐type responses in the lungs, while BALB/c mice induce T helper 1 (Th1)‐type responses in the lungs, despite considerable production of IL‐4 and IL‐5 from splenocytes. Therefore, local immune responses are more important in the induction of allergic inflammation in the lungs and are different from systemic immune responses, which are thought to depend on genetic background.
Introduction
Asthma is a chronic respiratory disease, characterized by reversible dyspnoea, airway hyper‐responsiveness and pulmonary inflammation, accompanied by eosinophil infiltration into the tissue of the airway.1,2 Recent studies using sensitized animals indicate that eosinophil infiltration into the lungs is closely related to late asthmatic response and airway hyper‐responsiveness.3,4 Additional evidence from studies using monoclonal antibodies (mAbs) for the T helper 2 (Th2)‐type cytokines interleukin (IL)‐4 and IL‐5 indicates that these cytokines play essential roles in antigen‐induced eosinophil infiltration into inflammation sites.5,6 IL‐4 induces vascular cell adhesion molecule‐1 (VCAM‐1) expression in endothelial cells. In turn, VCAM‐1 selectively binds to very late antigen‐4 (VLA‐4) on eosinophils and recruits them to the site of inflammation.7 Serum immunoglobulin (Ig)E levels elevated by IL‐4 are also associated with eosinophil infiltration into the airway.5,8 IL‐5 induces eosinophil differentiation, prolongs their survival and acts as an eosinophil chemotactic factor.9,10 Additionally, IL‐5 enhances IL‐4‐induced B‐cell production of IgE.11 In contrast, the T helper 1 (Th1)‐type cytokines interferon‐γ (IFN‐γ) and IL‐12 inhibit eosinophil infiltration,12,13 IgE and IgG1 secretion, and enhance IgG2a secretion in vivo.14 Thus, cross‐talk in the cytokine network regulates the immune system, including allergic responses.
Most animal models for asthma using guinea‐pigs do not properly reflect the complicated mechanism of human pulmonary inflammation, especially the heterogeneous genetic background of humans. Murine asthma models have several advantages. Human and murine immune systems have similar patterns of cytokine and immunoglobulin production. However, humans are different from mice in that they are genetically heterogeneous compared with most congenic mouse strains commonly used. Genetic background in mice is clearly responsible for high or low IgE and IgG secretion after immunization, which reflects the response state in atopic or non‐atopic patients, respectively.15 Consequently, different strains of mice were compared for degree of pulmonary inflammation, resulting from asthmatic induction, to investigate which strain more accurately reflects the response observed in human asthma patients. This study provides useful information for understanding the mechanism of human asthmatic reaction.
Materials and methods
Animals and antigen
Male C57BL/6 Cr and BALB/c Cr mice were purchased from Japan SLC (Hamamatsu, Shizuoka, Japan), maintained in specific pathogen‐free conditions and used at 5–8 weeks of age. Ovalbumin (OVA; Grade V) was purchased from Sigma Chemical Co. (St. Louis, MO).
Sensitization and challenge
The procedure of sensitization and challenge was modified from the method of Kung et al.16 Briefly, on day 0, mice were sensitized with an intraperitoneal (i.p.) injection of 8 μg of OVA and 2 mg of aluminum hydroxide gel (alum) dissolved in 0·5 ml phosphate‐buffered saline (PBS). The animals received a booster injection of this alum–OVA mixture 5 days later. Twelve days after the first sensitization, the mice were exposed for 1 hr to aerosolized OVA (0·5%) dissolved in 0·9% saline. The aerosolized OVA was produced by an ultrasonic nebulizer (NE‐U12; Omuron, Tokyo, Japan) at a flow rate of 6 l/min. Normal control mice were exposed for 1 hr to aerosolized 0·9% saline vehicle.
Collection and measurement of eosinophils in lung
At various times after OVA challenge, mice were killed by an i.p. injection of sodium pentobarbital. The trachea was cannulated and bronchoalveolar lavage was performed with four 1‐ml aliquots of saline containing 1 U/ml of heparin. The bronchoalveolar lavage fluid (BALF) was centrifuged at 4° and resuspended in 0·3 ml of 0·9% saline. Total cells were counted using a Celltac‐α cell counter (Nihon Kohden, Tokyo, Japan). Differential cell counts were performed on slides prepared using a Cytospin 3™ centrifuge (Shandon Inc., Pittsburgh, PA) and stained using a Diff‐Quik™ Kit (Green Cross Corporation, Osaka, Japan). A total of 300 cells were counted to calculate the eosinophil to neutrophil ratio in BALF.
Measurement of cytokine activity in the lung
Whole lungs were collected from mice at various time‐points after OVA challenge to measure the concentration of different cytokines. Frozen lung tissue was chopped after thawing and was suspended in 1 ml of 50 mm HEPES buffer containing 1 mm EDTA and 50 mm phenylmethylsulphonyl fluoride (PMSF). Samples were homogenized using a Polytron™ tissue disrupter (Kinematica, Lucerne, Switzerland) for 30 seconds and centrifuged (at 15 000 g, for 20 min at 4°). The levels of IL‐4, IL‐5 and IFN‐γ were measured using an enzyme‐linked immunosorbent assay (ELISA) kit purchased from Amersham (Little Chalfont, Bucks, UK).
Measurement of OVA‐specific IgE, IgG1 and IgG2a in sera
Microtitre plates (96‐well) were coated with 1 μg/ml of anti‐IgE mAb (Southern Biotechnology Associates, Birmingham, AL) to detect IgE, or with 200 μg/ml of OVA to detect IgG1 and IgG2a, in sodium bicarbonate buffer (pH 9·6). After overnight incubation at 4°, plates were washed with a PBS‐Tween 20™ (0·05% v/v) solution and blocked for 2 hr at 37° in PBS containing 10% bovine serum albumin (BSA). Serum samples and immunoglobulin standards were appropriately diluted (from 1 : 8 to 1 : 1000) in 1% BSA–PBS. The diluted samples and standards were added to the microtitre plate and incubated for 2 hr at room temperature. After washing three times with PBS–Tween‐20™, biotin‐labelled OVA, anti‐IgG1 or anti‐IgG2a mAb (Zymed Laboratories, Inc. South San Francisco, CA) were added and incubated for 1 hr at room temperature. Plates were then incubated for a further 1 hr in the presence of peroxidase‐labelled streptoavidin. After washing four times with PBS–Tween‐20™, 3, 3′, 5, 5′‐tetramethyl (benzide) (TMB) substrate solution (Sumitomo Bakelite Co., Ltd., Tokyo, Japan) was added. After a 30‐min incubation at room temperature, stop solution (2N sulphuric acid) was added and the plates were read in a plate reader at 450 nm. The amount of immunoglobulin that induced 50% maximum activity of murine IgE, IgG1 or IgG2a in the ELISA system was defined as 1 unit.
Preparation of splenocytes for cytokine assay
Mice were sensitized with the alum–OVA mixture on day 0 and on day 5. On day 12, spleens were removed, suspended and minced in Eagle’s minimum essential medium. Splenocytes in suspension were cultured at 5 × 105 cells/well in 200 μl of RPMI‐1640 (Gibco BRL, Rockville, MD) supplemented with 10% heat‐inactivated fetal bovine serum (FBS; JRH Biosciences, Lenexa, KS), 5 × 10–5m 2‐mercaptoethanol, 100 μg/ml streptomycin and 100 U/ml penicillin, in the presence or absence of 1 mg/ml OVA. The culture supernatants were collected for cytokine assay at different time‐points.
Effects of antibodies on eosinophil infiltration into lung
Anti‐IL‐4 mAb 1D11 was purchased from Endogen (Woburn, MA). Anti‐IL‐5 mAb TRFK‐5 was purchased from Genzyme (Cambridge, MA). Normal rat IgG used as a control antibody was purchased from Biopur AG (Bubendorf, Switzerland). Antibodies (100 μg/kg/10 ml) were injected i.p. 2 hr before OVA challenge.
Ethical considerations
All experiments were performed in accordance with the regulations of the Animal Ethical Committee of Yamanouchi Pharmaceutical Co., Ltd.
Results
OVA‐specific serum immumoglobulins in C57BL/6 and BALB/c mice sensitized with OVA
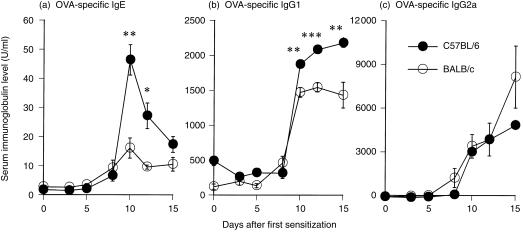
OVA‐specific serum IgE, IgG1 and IgG2a levels were examined to compare humoral immune responses between two genetically different strains of mice. Both strains of mice showed increases in serum IgE and IgG1 levels from day 8 to day 15, and the IgE level of C57BL/6 mice on day 10 was significantly threefold higher than the IgE level of BALB/c mice (Fig. 1a, P < 0·01). Furthermore, on day 10 the serum IgG1 level of C57BL/6 mice was significantly higher than that of the BALB/c mice (Fig. 1b, P < 0·01). However, from days 8–15, C57BL/6 mice generated a lower level of IgG2a than BALB/c mice (Fig. 1c).
Figure 1.
Time course of (a) ovalbumin (OVA)‐specific serum immunoglobulin (Ig)E, (b) IgG1 and (c) IgG2a in C57BL/6 (•) and BALB/c (○) mice sensitized with OVA. Mice were sensitized with aluminium hydroxide gel (alum)–OVA mixture on days 0 and 5 and challenged on day 12. Serum samples were collected 0, 3, 5, 8, 10, 12 and 15 days after the first sensitization. Day 0 data represent non‐sensitized mice, day 5 data represent mice sensitized only once and day 12 data represent non‐challenged mice, respectively. Results are presented as mean ± SEM (n=4 mice per group) and are representative of two separate experiments. Statistical significance between C57BL/6 mice and BALB/c mice was analysed using the Student’s t‐test. *P < 0·05, **P < 0·01 and ***P < 0·001 compared with BALB/c mice.
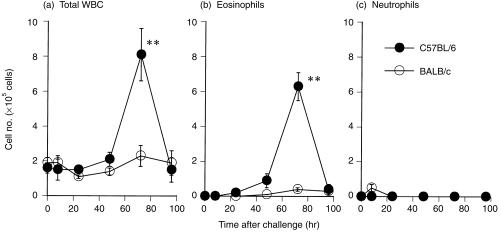
Infiltration of inflammatory cells into lungs after OVA challenge in C57BL/6 and BALB/c mice
C57BL/6 and BALB/c mice were sensitized with alum‐precipitated OVA and challenged with aerosolized OVA to compare the ability of late asthmatic responses to induce accumulation of eosinophils in the lungs. The number of total white blood cells (WBC) and eosinophils in the BALF gradually increased with time, reached a peak 72 hr after challenge and then decreased thereafter in both strains of mice (Fig. 2a, 2b). The number of total WBC and eosinophils in C57BL/6 mice was higher than in BALB/c mice throughout the observation period. A marked 10‐fold difference was evident 72 hr after challenge (P < 0·01). The number of neutrophils reached a small peak 8 hr after challenge in BALB/c mice but no apparent increase was observed in C57BL/6 mice (Fig. 2c).
Figure 2.
Time course of (a) total white blood cell (WBC), (b) eosinophil and (c) neutrophil infiltration into the lungs after ovalbumin (OVA) challenge in different strains of mice. Mice were sensitized with aluminium hydroxide gel (alum)–OVA mixture on days 0 and 5, and challenged on day 12. Bronchoalveolar lavage fluid (BALF) samples from C57BL/6 (•) and BALB/c (○) mice were collected 0, 8, 24, 48, 72 and 96 hr after OVA challenge. The 0‐hr time‐point represents sensitized but non‐challenged mice. Results are presented as mean ± SEM (n = 6–10 mice per group) and are representative of two separate experiments. Statistical significance between C57BL/6 mice and BALB/c mice was analysed using the Student’s t‐test. **P < 0·01 compared with BALB/c mice.
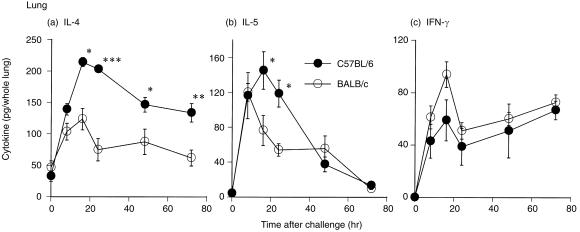
Cytokine generation in lung tissue of C57BL/6 and BALB/c mice after OVA challenge
To examine the cytokine production profile in the lung, the amount of IL‐4, IL‐5 and IFN‐γ was directly measured in lung tissue from both strains of mice after challenge. Compared with BALB/c mice, C57BL/6 mice generated a twofold greater level of IL‐4 in lung tissue throughout the observation period (Fig. 3a). In C57BL/6 mice, a higher level of IL‐5 was observed from 8 hr up to 24 hr after OVA challenge, while in BALB/c mice IL‐5 generation reached a peak at 8 hr and had decreased by ≈ 50% at 24 hr (Fig. 3b). In contrast, the IFN‐γ level in C57BL/6 mice was lower than that in BALB/c mice (Fig. 3c).
Figure 3.
Differences in the time course of cytokine generation in lung tissue between C57BL/6 (•) and BALB/c (○) mice induced by ovalbumin (OVA) challenge. Mice were sensitized with aluminium hydroxide gel (alum)–OVA mixture on days 0 and 5, and challenged on day 12. Whole lungs from C57BL/6 and BALB/c mice were collected 0, 8, 16, 24, 48 and 72 hr after OVA challenge. The 0‐hr time‐point represents sensitized but non‐challenged mice. Results are presented as mean ± SEM (n = 6 mice per group) and are representative of two separate experiments. Statistical significance between C57BL/6 mice and BALB/c mice was analysed using the Student’s t‐test. *P < 0·05, **P < 0·01 and ***P < 0·001 compared with BALB/c mice. IFN‐γ, interferon‐γ; IL, interleukin.
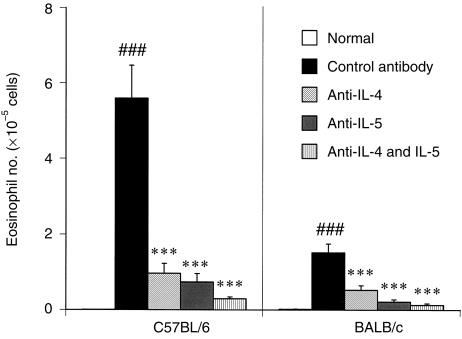
Effects of anti‐IL‐4 and IL‐5 mAbs on eosinophil infiltration in C57BL/6 and BALB/c mice
To confirm participation of IL‐4 and IL‐5 in the induction of eosinophil infiltration into the lungs, the effects of anti‐IL‐4 and anti‐IL‐5 mAbs were examined in both strains of mice. Pretreatment with anti‐IL‐4 mAb or anti‐IL‐5 mAb 2 hr before OVA challenge significantly (P < 0·001) inhibited eosinophil infiltration into the lungs (Fig. 4). Furthermore, pretreatment with a combination of both mAbs completely inhibited infiltration in both strains of mice (P < 0·001).
Figure 4.
Effects of anti‐interleukin (IL)‐4 and IL‐5 monoclonal antibodies on eosinophil infiltration into the lungs of C57BL/6 and BALB/c mice after ovalbumin (OVA) challenge. Mice were sensitized with aluminium hydroxide gel (alum)–OVA mixture on days 0 and 5, and challenged on day 12. Antibodies (100 µg/kg/10 ml) were injected intraperitoneally 2 hr before OVA challenge. Results are presented as mean ± SEM (n = 10–12 mice per group) and are representative of two separate experiments. Statistical significance between the saline‐challenged normal group and the control antibody‐treated group was analysed using the Student’s t‐test. Statistical significance between antibody‐treated groups was analysed using the Dunnett’s multiple range test. ###P < 0·001 compared with the normal group; ***P < 0·001 compared with the control antibody‐treated group.
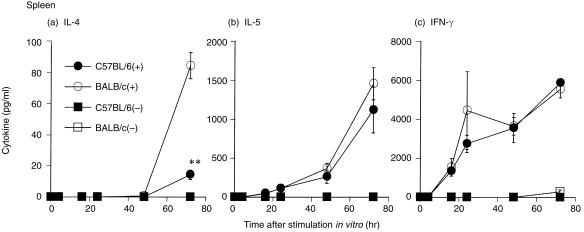
Cytokine production in splenocytes from sensitized C57BL/6 and BALB/c mice after OVA stimulation in vitro
It is likely that there are differences in cytokine production between the lungs and other organs. Therefore, the ability of splenocytes from both strains to produce antigen‐specific Th1/Th2 cytokines in vitro was examined. IL‐4 production by splenocytes from BALB/c mice 72 hr after stimulation was significantly higher than production from C57BL/6 mouse splenocytes at the same time‐point (Fig. 5a, P < 0·01). IL‐5 production in splenocytes from BALB/c mice was slightly higher than in splenocytes from C57BL/6 mice (Fig. 5b). IFN‐γ production in C57BL/6 mice was almost the same as that in BALB/c mice 72 hr after stimulation (Fig. 5c).
Figure 5.
Time course of (a) interleukin (IL)‐4, (b) IL‐5 and (c) interferon‐γ (IFN‐γ) production by cultured C57BL/6 and BALB/c splenocytes stimulated with ovalbumin (OVA). Mice were sensitized with an aluminium hydroxide gel (alum)–OVA mixture on days 0 and 5. C57BL/6 splenocytes were cultured in the presence (•) or absence (▪) of 1 mg/ml OVA. BALB/c splenocytes were cultured in the presence (○) or absence (□) of 1 mg/ml OVA. The culture supernatants were collected 0, 4, 16, 24, 48 and 72 hr after stimulation of OVA in vitro. Results are presented as mean ± SEM (n = 3 mice per group) and are representative of two separate experiments. Statistical significance between C57BL/6 mice and BALB/c mice was analysed using the Student’s t‐test. **P < 0·01 compared with BALB/c mice.
Discussion
In this study, sensitized C57BL/6 mice showed dramatically elevated levels of antigen‐specific serum IgE compared with sensitized BALB/c mice. This finding seems contradictory to the fact that BALB/c mice are IgE high‐responders, while C57BL/6 mice are low‐responders.17,18 BALB/c mice have also been reported to exhibit Th2‐dominant responses to parasite infection or exogenous antigen challenge, while C57BL/6 mice exhibit Th1‐dominant responses to the same stimuli.8,19,20 These phenomena are believed to be attributable to the genetic control of IL‐4 production by BALB/c mice. T cells in BALB/c mice are more likely to exhibit a Th2‐like phenotype and lose IL‐12 responsiveness than those in other strains of mice.21,22 However, BALB/c mice do not always exhibit Th2‐type responses and C57BL/6 mice are not always committed to Th1‐type responses. For example, BALB/c mice immunized with a low dose of Leishmania major exhibited Th1‐type responses, including high IFN‐γ production, and successfully countered the infection.23 When C57BL/6 and BALB/c mice are intratracheally inoculated with Cryptococcus neoformas 52, C57BL/6 mice produce a high level of IL‐5 and exhibit high pulmonary eosinophilia compared with BALB/c mice.24 BALB/c mice with murine candidasis infection exhibit Th1‐type responses and produce lower IgE, IgG1 and higher IgG2a levels compared with Th1‐dominant responder DBA/2 mice.25 Constant & Bottomly have recently revealed that immunization with low‐dose antigen elicits Th2‐type immune responses, including high IgE secretion, while immunization with high‐dose antigen elicits Th1‐type immune responses.26 Indeed, the dose of OVA antigen for immunization in the present study (8 µg) was five to 10 times lower than doses used in conventional sensitization.18,27 The number of immunizations and challenges were also fewer than reported by others.17,18 Mice were sensitized only twice in a short period and singly challenged with aerosolized OVA. We examined the effect of OVA sensitization, using different dose levels, on serum IgE production (data not shown). BALB/c mice that received two sensitizations with a very low dose of OVA (100 ng), using the same protocol described in Fig. 1, failed to produce IgE, although C57BL/6 mice sensitized with the very low dose of OVA induced IgE secretion. On the other hand, BALB/c mice sensitized with a very high dose of OVA (1 mg) secreted a high level of IgE, as did C57BL/6 mice sensitized with the low dose of OVA (8 µg) used throughout this study (T. Morokata, unpublished). It is probable that a low dose of OVA may induce different rates of IgE secretion in both strains owing to differences in genetic background. These results indicate that, under these conditions, OVA‐sensitized C57BL/6 mice preferentially produce Th2‐type immunoglobulins, whereas BALB/c mice produce Th1‐type immunoglobulins, in the peripheral humoral immune system.
C57BL/6 mice exhibited a higher eosinophil infiltration into the lungs than BALB/c mice. Additionally, C57BL/6 mice produced higher levels of IL‐4 and IL‐5 in lung tissue than BALB/c mice. It is well known that IL‐4 and IL‐5 play important roles in eosinophil infiltration into the lungs. IL‐4 evokes transendothelial migration of eosinophils by inducing VCAM‐1 expression.7 IL‐5 strongly promotes the maturation, adhesion and activation of eosinophils.9,10 Eosinophil infiltration was completely inhibited in both strains following administration of mAbs to IL‐4 and IL‐5, although the quantity of Th2‐type cytokine was different between strains. Furthermore, it is probable that the chemoattraction and activation of eosinophils by these Th2 cytokines result in the local release of eosinophil‐associated granule proteins, such as major basic protein (MBP), eosinophil cationic protein (ECP) and eosinophil‐derived neurotoxin (EDN), followed by local propagation of the inflammatory reaction and tissue damage,28,29 although histological study remains to be performed. These results indicate that eosinophil infiltration is caused by IL‐4 and IL‐5 and that the higher eosinophil infiltration observed in C57BL/6 mice, compared with BALB/c mice, is probably caused by greater IL‐4 and IL‐5 generation in local lung tissue.
Interestingly, there are apparent differences in the pattern of cytokine production between spleen and lung tissue. In vitro cytokine assay showed that IL‐4 production by splenocytes from C57BL/6 mice was lower than that from BALB/c mice. C57BL/6 mice seemed to exhibit Th1‐type responses in splenic tissue while BALB/c mice appeared to exhibit Th2‐type responses. Within 12 days after the first OVA sensitization, serum IgE secretion of C57BL/6 mice had peaked and started to decrease, while IgG2a production had started to increase. It is possible that differentiation of naive T cells into Th2‐type cells was induced by IL‐4 during the early‐stage immune responses. Therefore, IL‐4 production in C57BL/6 mice, which is supposed to regulate serum IgE secretion and Th2‐type responses in the lungs, could not be detected in the supernatant of splenocytes on the 12th day after sensitization.
Furthermore, there may be intrinsic differences in local cytokine production between spleen and lung tissue. C57BL/6 mice were reported to exhibit Th2‐dominant responses, while BALB/c mice exhibited Th1‐dominant responses to inhaled OVA antigen. This finding might be explained by the observation that IL‐12 mRNA expression in lung‐derived cells from BALB/c mice stimulated with lipopolysaccharide is higher than the corresponding response in cells from C57BL/6 mice.27 Consequently, generation of IL‐12 in BALB/c mice may be higher than that in C57BL/6 mice in local lung tissue where inflammatory responses are developing. Indeed, in this study, generation of IFN‐γ, potently induced by IL‐12, in the lungs of BALB/c mice was higher than that in C57BL/6 mice. Furthermore, IFN‐γ may inhibit proliferation of Th2 cells30,31 and result in the inhibition of eosinophil infiltration into the lungs.12 Therefore, high levels of IL‐4 in the lungs of C57BL/6 mice might induce eosinophil infiltration into the lung, while high levels of IFN‐γ in the lungs of BALB/c mice might suppress eosinophil infiltration into the lungs. It is evident that an imbalance in the Th1/Th2 response and genetic background strongly influence the triggering of asthmatic reactions. There are some reports that directly compared the pulmonary eosinophilia between C57BL/6 mice and BALB/c mice; however, conflicting views exist. Some groups reported that in BALB/c mice which received a repeated aerosolized OVA, a high level of serum IgE and airway hyper‐responsiveness was induced without pulmonary eosinophilia27,32 and that in C57BL/6 mice only a weak airway hyper‐responsiveness was induced compared with BALB/c mice, despite marked pulmonary eosinophilia.18 Another group reported that C57BL/6 mice decreased pulmonary eosinophilia compared with BALB/c mice, despite a similar degree of airway hyper‐responsiveness.17 These reports have found no definitive relationships between local and systemic immune responses between the two strains of mice. Our results suggest that local immune responses are more important in inducing accumulation of eosinophils in the lungs, and are different from systemic immune responses that are though to depend on genetic background.
Haematological and histological characteristics of asthma are: eosinophil infiltration into the lungs, Th2‐type cytokine production and high levels of serum IgE. C57BL/6 mice used during this study satisfy these three salient asthmatic features. In contrast, there are some disadvantages in guinea‐pig models: lack of proper genetics, lack of immunological and biochemical tools, and unestablished concept of Th1/Th2,33 although guinea‐pigs have been widely used for the asthma model because of the high sensitivity of bronchial smooth muscle to allergen and to histamine and lipid mediators, which are considered to be major mediators for immediate asthmatic response.34 The model, using C57BL/6 mice, offers many advantages over guinea‐pigs. C57BL/6 mice are congenic, which limits variability in response, and exhibit Th2‐type reactions in local lung tissue when sensitized and challenged with OVA.
In conclusion, C57BL/6 mice may be useful in elucidating the mechanisms of asthmatic reactions because of the severity of local pulmonary inflammation in this strain.
Acknowledgments
The authors wish to thank Dr K. Honda for his invaluable advice and Dr K. Miyata for reviewing the manuscript. The authors would also like to thank Mr Steven Johnson for editing the manuscript.
Glossary
Abbreviations
- alum
aluminum hydroxide gel
- BALF
bronchoalveolar lavage fluid
- IFN‐γ
interferon‐γ
- IL
interleukin
- mAb
monoclonal antibody
- OVA
ovalbumin
- VCAM‐1
vascular cell adhesion molecule‐1
- VLA‐4
very late activation antigen‐4
- WBC
white blood cells
References
- 1.Durham SR, Kay AB. Eosinophils, bronchial hyperreactivity and late‐phase asthmatic reactions. Clin Allergy. 1985;15:411. doi: 10.1111/j.1365-2222.1985.tb02290.x. [DOI] [PubMed] [Google Scholar]
- 2.Gleich GJ. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol. 1990;85:422. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- 3.Elwood W, Barnes PJ, Chung KF. Airway hyperresponsiveness is associated with inflammatory cell infiltration in allergic Brown–Norway rats. Int Arch Allergy Immunol. 1992;99:91. doi: 10.1159/000236340. [DOI] [PubMed] [Google Scholar]
- 4.Iijima H, Ishii M, Yamauchi K, et al. Bronchoalveolar lavage and histologic characterization of late asthmatic response in guinea pigs. Am Rev Respir Dis. 1987;136:922. doi: 10.1164/ajrccm/136.4.922. [DOI] [PubMed] [Google Scholar]
- 5.Coyle AJ, Le Gros G, Bertrand C, et al. Interleukin‐4 is required for the induction of lung Th2 mucosal immunity. Am J Respir Cell Mol Biol. 1995;13:54. doi: 10.1165/ajrcmb.13.1.7598937. [DOI] [PubMed] [Google Scholar]
- 6.Nakajima H, Iwamoto I, Tomoe S, et al. CD4+ T‐lymphocytes and interleukin‐5 mediate antigen‐induced eosinophil infiltration into the mouse trachea. Am Rev Respir Dis. 1992;146:374. doi: 10.1164/ajrccm/146.2.374. [DOI] [PubMed] [Google Scholar]
- 7.Schleimer RP, Sterbinsky SA, Kaiser J, et al. IL‐4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium. Association with expression of VCAM‐1. J Immunol. 1992;148:1086. [PubMed] [Google Scholar]
- 8.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different function properties. Annu Rev Immunol. 1989;7:145. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 9.Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988;167:219. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi Y, Hayashi Y, Sugama Y, et al. Highly purified murine interleukin 5 (IL‐5) stimulates eosinophil function and prolongs in vitro survival. IL‐5 as an eosinophil chemotactic factor. J Exp Med. 1988;167:1737. doi: 10.1084/jem.167.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pene J, Chretien I, Rousset F, Briere F, Bonnefoy JY, De Vries JE. Modulation of IL‐4‐induced human IgE production in vitro by IFN‐γ and IL‐5: the role of soluble CD23 (s‐CD23) J Cell Biochem. 1989;39:253. doi: 10.1002/jcb.240390305. [DOI] [PubMed] [Google Scholar]
- 12.Iwamoto I, Nakajima H, Endo H, Yoshida S. Interferon‐γ regulates antigen‐induced eosinophil recruitment into mouse airways by inhibiting the infiltration of CD4+ T cells. J Exp Med. 1993;177:573. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwamoto I, Kumano K, Kasai M, Kurasawa K, Nakao A. Interleukin‐12 prevents antigen‐induced eosinophil recruitment into mouse airways. Am J Respir Crit Care Med. 1996;154:1257. doi: 10.1164/ajrccm.154.5.8912732. [DOI] [PubMed] [Google Scholar]
- 14.Finkelman FD, Katona IM, Mosmann TR, Coffman RL. IFN‐γ regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988;140:1022. [PubMed] [Google Scholar]
- 15.Mancino D, Ovary Z. Adjuvant effects of amorphous silica and of aluminium hydroxide on IgE and IgG1 antibody production in different inbred mouse strains. Int Arch Allergy Appl Immunol. 1980;61:253. doi: 10.1159/000232443. [DOI] [PubMed] [Google Scholar]
- 16.Kung TT, Jones H, Adams GK, et al. Characterization of a murine model of allergic pulmonary inflammation. Int Allergy Immunol. 1994;105:83. doi: 10.1159/000236807. [DOI] [PubMed] [Google Scholar]
- 17.Herz U, Braun A, Ruckert R, Renz H. Various immunological phenotypes are associated with increased airway responsiveness. Clin Exp Allergy. 1998;28:625. doi: 10.1046/j.1365-2222.1998.00280.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Lamm WJ, Albert RK, Chi EY, Henderson WR, Jr, Lewis DB. Influence of the route of allergen administration and genetic background on the murine allergic pulmonary response. Am J Respir Crit Care Med. 1997;155:661. doi: 10.1164/ajrccm.155.2.9032210. [DOI] [PubMed] [Google Scholar]
- 19.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 20.Guler ML, Gorham JD, Hsieh CS, et al. Genetic susceptibility to Leishmania: IL‐12 responsiveness in TH1 cell development. Science. 1996;271:984. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh CS, Macatonia SE, O’Garra A, Murphy KM. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181:713. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 23.Menon JN, Bretscher PA. Characterization of the immunological memory state generated in mice susceptible to Leishmania major following exposure to low doses of L. major and resulting in resistance to a normally pathogenic challenge. Eur J Immunol. 1996;26:243. doi: 10.1002/eji.1830260138. [DOI] [PubMed] [Google Scholar]
- 24.Huffnagle GB, Boyd MB, Street NE, Lipscomb MF. IL‐5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6) J Immunol. 1998;160:2393. [PubMed] [Google Scholar]
- 25.Romani L, Mencacci A, Cenci E, et al. CD4+ subset expression in murine candidiasis. Th responses correlate directly with genetically determined susceptibility or vaccine‐induced resistance. J Immunol. 1993;150:925. [PubMed] [Google Scholar]
- 26.Constant SL, Bottomly K. Induction of Th1 and Th2, CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 27.Lin JY, Wang LF, Lin RH. The association between lung innate immunity and differential airway antigen‐specific immune responses. Int Immunol. 1995;8:499. doi: 10.1093/intimm/8.4.499. [DOI] [PubMed] [Google Scholar]
- 28.Wardlaw AJ. Eosinophils in the 1990s: new perspectives on their role in health and disease. Postgrad Med J. 1994;70:536. doi: 10.1136/pgmj.70.826.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sosman JA, Bartemes K, Offord KP, et al. Evidence for eosinophil activation in cancer patients receiving recombinant interleukin‐4: effects of interleukin‐4 alone and following interleukin‐2 administration. Clin Cancer Res. 1995;1:805. [PubMed] [Google Scholar]
- 30.Gajewski TF, Fitch FW. Anti‐proliferative effect of IFN‐γ in immune regulation. I. IFN‐γ inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988;140:4245. [PubMed] [Google Scholar]
- 31.Fernandez‐Botran R, Sanders VM, Mosmann TR, Vitetta ES. Lymphokine‐mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J Exp Med. 1988;168:543. doi: 10.1084/jem.168.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renz H, Smith HR, Henson JE, Ray BS, Irvin CG, Gelfand EW. Aerosolized antigen exposure without adjuvant causes increased IgE production and increased airway responsiveness in the mouse. J Allergy Clin Immunol. 1992;89:1127. doi: 10.1016/0091-6749(92)90296-e. [DOI] [PubMed] [Google Scholar]
- 33.Pretolani M, Vargaftig BB. From lung hypersensitivity to bronchial hyperreactivity. What can we learn from studies on animal models? Biochem Pharmacol. 1993;45:791. doi: 10.1016/0006-2952(93)90161-o. [DOI] [PubMed] [Google Scholar]
- 34.Vargaftig BB. Modifications of experimental bronchopulmonary hyperresponsiveness. Am J Respir Crit Care Med. 1997;156:S97. doi: 10.1164/ajrccm.156.4.12-tac-4. [DOI] [PubMed] [Google Scholar]