Abstract
The capacity of the immune system to respond efficiently to new antigens depends upon a continuous source of naive CD4+ T cells. Such cells exit from the thymus and join the recirculated T‐cell pool. Factors present at the sites of naive CD4+ T‐cell circulation must be responsible for their survival, since upon removal from their host, naive CD4+ T cells die. However, such factors remain unknown. The presence of the cytokine interleukin‐7 (IL‐7) in secondary lymphoid organs and the continuous expression of its receptor on naive CD4+ T cells prompted us to examine the possibility that IL‐7 might be a survival factor for naive CD4+ T cells. Using naive CD4+ T cells isolated from cord blood we show that IL‐7, but not IL‐2, can maintain naive CD4+ T‐cell viability in vitro for at least 15 days. In addition, we find that IL‐7 can induce modest proliferation of naive CD4+ T cells without affecting either their cell surface phenotype or their ability to respond to antigenic stimulation. We also find that after anti‐CD3 stimulation, naive CD4+ T cells lose that ability to respond to IL‐7. However, if cells are primed with IL‐7 prior to antigenic stimulation, their proliferative responses are enhanced. Together, these data suggest a novel and important role for IL‐7 in the maintenance and maturation of naive CD4+ T cells, ensuring that they can respond maximally when they first meet antigen in secondary lymphoid tissue.
Introduction
The size of the recirculating T‐cell pool remains relatively constant throughout adult life, in spite of the dramatic reduction in T‐cell production by the thymus with age.1 In rodents maintained in a specific pathogen‐free environment, the recirculating lymphocyte pool is largely composed of naive T cells which can dominate the immune system for years, even after thymectomy.2 The continuous presence of naive T cells maintains the immune system’s capacity to respond to new antigens. Unlike memory cells, naive T cells can remain in interphase for weeks.3 However, the life span of naive T cells is unlikely to be an intrinsic property since upon removal from their host they rapidly die. This implicates a role for environmental factors in the maintenance of the recirculating naive T‐cell pool. The identity of such factors remains obscure but is of obvious importance.
We hypothesized that interleukin‐7 (IL‐7) might be a survival factor for naive T cells and thereby maintain the recirculating naive T‐cell pool. IL‐7 is produced by stromal cells of the bone marrow and thymus, where it plays an essential role in lymphopoiesis.4–6 The mechanism by which IL‐7 promotes thymocyte development has been investigated.7–9 It was shown that IL‐7 plays a principal role in T‐cell development by enhancing thymocyte viability and inducing thymocyte expansion prior to TCR rearrangement.9 This viability effect of IL‐7 is thought to be mediated via Bcl‐2.9 Both the IL‐7 receptor (IL‐7R) and Bcl‐2 are expressed on double‐negative and single‐positive thymocytes, but not on the double‐positive subset in mice.10 This expression pattern correlates with the ability of only double‐negative and single‐positive thymocytes to respond to IL‐7 stimulation.11 The functional consequences of IL‐7 receptor re‐expression following transition to the single‐positive thymocytes remains obscure.
Mature T cells also show heterogeneity in their expression patterns of the IL‐7R.12 Furthermore, their proliferative responses to IL‐7 are smaller than those to IL‐2.13–15 Previously, we dissected this heterogeneity by showing that it is the naive population of CD4+ T cells that expresses the IL‐7R and responds to IL‐7 by proliferation and differentiation to the T helper type 0 (Th0) subset.16 The responses of naive CD4+ T cells to IL‐7 were greater than those to both IL‐2 and IL‐4. Following antigenic stimulation, we found that naive CD4+ T cells lost detectable expression of IL‐7R and also the ability to respond to this cytokine.16 These results suggested that within a mixed T‐cell population it is the naive and not the memory CD4+ T cells that respond to IL‐7. The responses of naive CD4+ T cells to IL‐7 are in keeping with its in vivo expression. IL‐7 message has been found in secondary lymphoid organs.17,18 Factors present within secondary lymphoid organs are most likely to maintain survival of this recirculating T‐cell pool. Given this information, IL‐7 may be a prime candidate as a survival factor for naive CD4+ T cells.
We have examined the effects of IL‐7 on the in vitro survival of human naive CD4+ T cells. We have used neonatal CD4+ T cells as a source of naive cells. This is because during gestation, the placental barrier minimizes antigenic stimulation of neonatal CD4+ T cells. Thus, CD4+ T cells purified from umbilical cord blood are a rich source of naive T cells and unlike CD45RA+ CD45RO– cells isolated from adult peripheral blood, are unlikely to be revertants from a memory phenotype.19–24 In this study we show that IL‐7 can maintain the survival of these cells for 15 days in culture, without any change in either phenotype or function.
Materials and methods
Reagents
T‐cell activation was performed using OKT3, a monoclonal antibody (mAb) directed against CD3 (American Type Tissue Culture Collection, Rockville, MD) and 9.3, a mAb directed against CD28 (donated by Dr J. Ledbetter, Bristol Myers Squibb, Seattle, WA). CTLA‐4Ig was a generous gift of Dr J. Ledbetter (Bristol Myers Squibb). For direct fluorescent staining, phycoerythrin (PE) ‐conjugated anti‐CD4 mAb (Leu‐3a; Becton‐Dickinson, Mountain View, CA), fluorescein isothiocyanate (FITC) ‐conjugated anti‐CD45RA mAb (Leu‐18; Becton‐Dickinson), FITC‐conjugated anti‐CD3 mAb (Leu‐4; Becton‐Dickinson), anti‐FITC‐conjugated anti‐CD19 mAb (Leu‐12; Becton‐Dickinson), FITC‐conjugated anti‐CD8 mAb (Leu‐2a), PE‐conjugated anti‐CD14 mAb (Leu‐M3; Becton‐Dickinson, Mountain View, CA), FITC‐conjugated anti‐CD45RO mAb (UCHL‐1; donated by Prof. P. Beverley, ICRF, London, UK) were used. Recombinant IL‐2 and IL‐7 were kind gifts of Dr P. Lomedico (Hoffmann‐La Roche, Nutley, NJ), and Dr C. Faltynek (Sterling Winthrop Pharmaceuticals, Malvern, PA), respectively. Seven‐amino actinomycin D (7AAD) was purchased from Sigma (Poole, Dorset, UK). Human serum (HS) and fetal calf serum (FCS) were obtained from the North London Blood Transfusion Service (Edgware, UK) and Gibco Laboratories (Paisley, UK), respectively.
Cell purification and assessment
Blood was collected from the cut umbilical cord into heparinized containers following agreement with The Riverside Research Ethics Committee, Charing Cross Hospital, London, UK. CD4+ T cells were isolated from cord blood and their purity was assessed as described. Umbilical cord blood CD4+ T cells isolated by immunomagnetic purification were > 99% CD4+ T, and 95–99% CD45RA+ CD45RO–. To assess cell purity, cells were washed twice in phosphate‐buffered saline (PBS) with 1% FCS and then incubated for 30 min at 4° with FITC‐ or PE‐conjugated mAb to various cell surface antigens. Cells were then washed and analysed by flow cytometry (FACScan; Becton Dickinson).
Cell culture and stimulation
To assess cell survival, 1 × 106 cells were cultured in a 24‐well plate in a final volume of 1 ml with either medium alone (RPMI‐1640 with 10% human serum), IL‐2 or IL‐7 (both at 10 ng/ml). On days 2, 5, 10 and 15, cells were harvested, and permeabilized with 0·1% saponin (Sigma) in PBS with 1% FCS, by incubation for 20 min at 4°. Cells were then washed in PBS and 100 µl of 7AAD at 5 µg/ml was added. Cells were incubated at 4° for a further 30 min prior to addition of 300 µl of PBS and analysis by fluorescence‐activated cell sorter (FACScan). The 7AAD is a DNA stain that, unlike propidium iodide, does not bind double‐stranded RNA, thus negating the need for pretreatment of cells with RNAse.25
For anti‐CD3 stimulation, 96‐well flat‐bottomed plates were coated with 10 µg/ml anti‐CD3 as previously described.25 Cells were plated at a density of 1 × 106/ml in a final volume of 100 µl. For anti‐CD28 costimulation, the mAb 9.3 was added into culture at a concentration of 10 ng/ml. Proliferation was measured by [3H]thymidine incorporation during the final 8 hr of culture.
Results
IL‐7 is a survival factor for naive CD4+ T cells
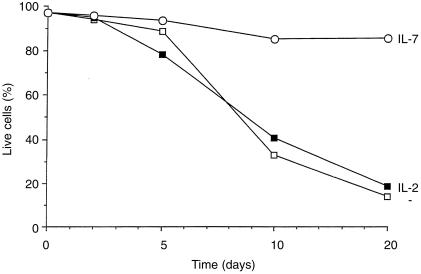
To examine the effect of IL‐7 on naive CD4+ T‐cell survival, CD4+ T cells isolated from human umbilical cord blood were placed in culture with either medium alone, IL‐2, or IL‐7. On days 2, 5, 10 and 15 of culture, cells were harvested and the percentage of viable cells was measured using the DNA stain, 7AAD. Figure 1 shows the percentage of viable cells over this culture period. In the absence of any cytokine, most cells were dead within 10 days of culture. The presence of IL‐2 had no obvious effect on cell survival. This is not surprising, since resting T cells do not express the α‐chain of the IL‐2 receptor. In contrast, the majority of cells cultured in IL‐7 remained viable throughout the 15‐day culture period, showing only a minor decrease in viability (from 98% to 86%; Fig. 1). This effect of IL‐7 was observed in three separate experiments using cord blood from different donors.
Figure 1.
Naive CD4+ T cells were cultured in either medium alone, IL‐2, or IL‐7 for 2, 5, 10 and 15 days. Cells were then harvested, permeabilized, and their DNA was stained using 7AAD. This graph show the percentage of live cells in each culture condition over the 15‐day culture period and is representative of three separate experiments.
Naive CD4+ T cells proliferate modestly to IL‐7 alone
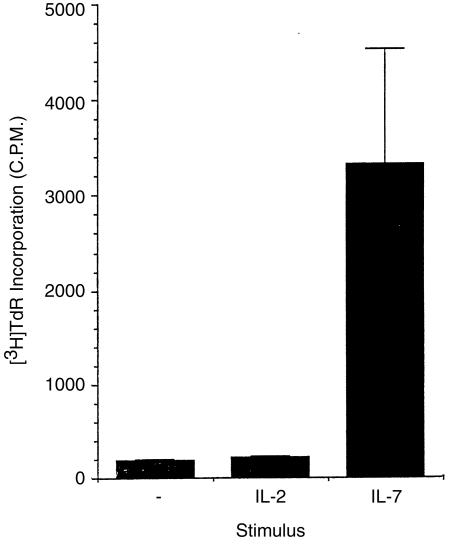
Previously, it has been postulated that a combination of cytokines may be important in maintaining the naive T‐cell repertoire and ensuring that individuals maintain the ability to mount primary responses.26 Unutmaz et al. showed that a combination of tumour necrosis factor‐α (TNF‐α), IL‐2 plus IL‐6 induced the in vitro proliferation of naive T cells isolated from human peripheral blood on the basis of CD45 isoform expression. However, after cytokine treatment the cells expressed surface antigens associated with an activated phenotype.26 To date there is no in vivo data in support of this hypothesis. Furthermore, such a combination of cytokines is more likely to be found in sites of inflammation rather than sites of naive T‐cell circulation or primary activation. In contrast, the cytokine IL‐7 has been detected at sites of naive T‐cell circulation.18 Since IL‐7 appears to prolong the survival of naive CD4+ T cells, we examined the effect of IL‐7 on naive CD4+ T‐cell proliferation in the absence of any other mitogenic signals. To assess the ability of IL‐7 to drive proliferation, 1 × 105 naive CD4 + T cells were placed in 96‐well culture dishes and given either medium, IL‐2, or IL‐7. Proliferation was then measured 3 days later by [3H]thymidine incorporation. As shown in Fig. 2, no proliferation was seen in cultures of either medium alone, or IL‐2. In contrast, a modest, but reproducible, proliferation was seen when cells were given IL‐7.
Figure 2.
Proliferation of naive CD4+ T cells to medium, IL‐2, andIL‐7 was assessed by [3H]thymidine incorporation during the last 8 hr of a 5‐day culture. Results show the mean c.p.m. ± SD and are representative of five separate experiments.
Prolonged culture in IL‐7 does not alter naive CD4+ T‐cell phenotype
If IL‐7 does play a role in maintaining the naive CD4+ T‐cell repertoire, it must do so without affecting the cell phenotype or ability to respond to antigen. To address this question, we cultured naive CD4+ T cells in IL‐7 for a prolonged period and assessed their phenotype. Naive CD4+ T cells were cultured for 15 days in IL‐7 and their expression of various cell surface antigens was assessed. In addition, their ability to respond to antigenic stimulation was also analysed.
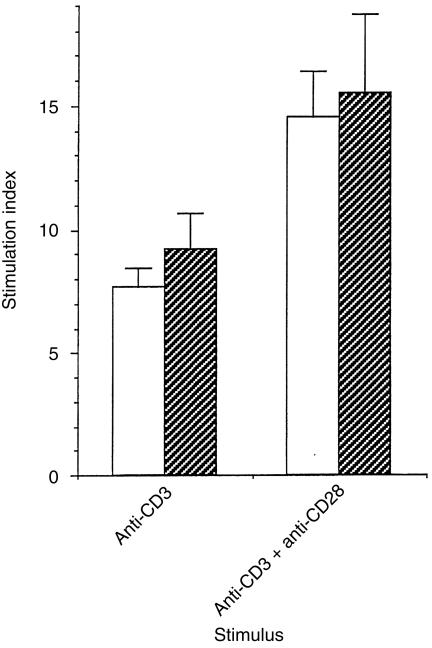
We found that culture in IL‐7 for a maximum of 15 days did not affect expression of CD3 which remained at > 98%, CD4 93%, CD8 6%, CD45RA 90%, or CD45RO 0·5%; this, compared with newly purified cells that were 95% CD4, CD45RA. Moreover, this prolonged culture in IL‐7 did not alter the proliferation of cells to anti‐CD3 or anti‐CD3 plus anti‐CD28 (Fig. 3). Naive CD4+ T cells were stimulated with anti‐CD3 or anti‐CD3 plus anti‐CD28 in the presence of IL‐7 when first isolated and then later, after 15 days in vitro culture in IL‐7. As shown in Fig. 3, the stimulation indices did not alter after 15 days culture in IL‐7 when compared to those of freshly isolated naive CD4+ T cells.
Figure 3.
Naive CD4+ T cells were cultured in IL‐7 for a period of 0 (white bars) or 15 (hatched bars) days prior to stimulation with plate‐immobilized anti‐CD3 in the presence/absence of soluble anti‐CD28 (10 ng/ml). Proliferation was measured 3 days following stimulation by [3H]thymidine incorporation during the last 8 hr of culture. Results show the stimulation indices for triplicate cultures ± SD and are representative of three separate experiments.
Stimulation via CD3 results in unresponsiveness to IL‐7
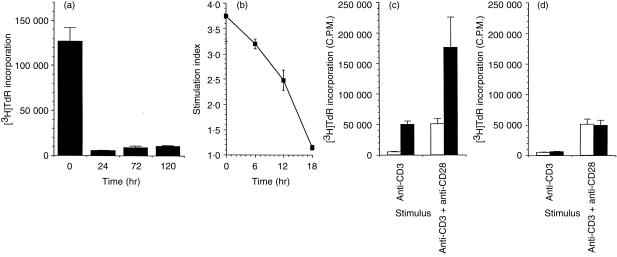
We have previously shown that IL‐7 acts synergistically with anti‐CD3 stimulation for proliferation of naive CD4+ T cells. We wanted to determine if IL‐7 responses were enhanced post anti‐CD3 stimulation, as has previously been shown for primary T cells and T‐cell lines.12 Cells were stimulated with anti‐CD3 for 24, 72 and 120 hr prior to addition of IL‐7 and proliferative responses were measured 3 days after IL‐7 addition. A control culture was set up where cells were stimulated simultaneously with anti‐CD3 and IL‐7. To our surprise, 24 hr after anti‐CD3 stimulation cells had become unresponsive to IL‐7 (Fig. 4a). To further define this induction of anti‐CD3‐induced IL‐7‐unresponsiveness, we stimulated cells for 0, 6, 12 and 18 hr prior to addition of IL‐7 and again measured proliferation 3 days later. As can be seen in Fig. 4(b), there is a gradual decline in IL‐7 responsiveness over the 18‐hr period following anti‐CD3 stimulation. To ensure that this induction of unresponsiveness was not due to anergy induced by anti‐CD3, cells were stimulated with either anti‐CD3 or anti‐CD3 plus anti‐CD28 for 0 and 24 hr prior to addition of IL‐7 and proliferation was measured 3 days later. As shown in Fig. 4(c,d), CD28 costimulation did not reverse anti‐CD3‐induced unresponsiveness.
Figure 4.
Naive CD4+ T cells were stimulated with plate immobilized anti‐CD3 for 0–120 hr in the presence/absence of soluble anti‐CD28 (10 ng/ml). Cells were then placed in 96‐well flat‐bottomed plates in the presence/absence of IL‐7 and proliferation was measured 3 days later by [3H]thymidine incorporation. (a) shows proliferative responses to IL‐7 0, 24, 72 and 120 hr after anti‐CD3 stimulation. (b) shows proliferative responses to IL‐7 0, 6, 12, and 18 hr after anti‐CD3 stimulation. (c) shows proliferation to anti‐CD3 or anti‐CD3 plus anti‐CD28 in the presence (filled bars) or absence (open bars) of IL‐7. (d) shows proliferation to medium (open bars) and IL‐7 (filled bars) following 24 hr of stimulation with anti‐CD3 or anti‐CD3 plus anti‐CD28.
Discussion
IL‐7 was identified and cloned nearly a decade ago by virtue of its ability to induce short‐term proliferation of B‐cell progenitors in the absence of stromal cells.17 Since then, the in vitro and in vivo properties of IL‐7 have implicated this cytokine in both B‐ and T‐cell development and function.4–12 Characterization of mice deficient in IL‐7 or either of its receptors have shown that IL‐7 is crucial for normal lymphopoiesis.4–6 Recently, the block in T‐cell development in IL‐7–/– mice has been attributed to defects in Bcl‐2 regulation. In the multipotential thymocyte subset, IL‐7–/– mice show normal levels of Bcl‐2 expression.7–9 However, after the transition to the T‐cell‐restricted lineage, Bcl‐2 expression becomes dependent upon IL‐7. These cells show reduced Bcl‐2 expression and increased apoptosis in IL‐7–/– mice. Overexpression of Bcl‐2 results in restoration of lymphocyte numbers in IL‐7R–/– mice. Thus, in T‐cell development there appears to be a requirement for IL‐7, prior to T‐cell receptor rearrangement, that is linked to the cell cycle and to the prevention of apoptosis. The lack of normal, mature T cells in IL‐7 or IL‐7R mutant mice has impeded analysis of in vivo T cell function in the absence of IL‐7.
The effects of IL‐7 on mature T cells have largely been investigated using peripheral T cells.12–15 Such cells are a mixture of memory and naive cells. Previously, we examined the effects of IL‐7 on naive CD4+ T cells purified from human umbilical cord blood.16 Using this population of cells we were able to demonstrate that IL‐7 is an important modulator of T‐cell function. We showed that: > 95% of naive CD4+ T cells express the IL‐7R; naive CD4+ T cells show greater responses to IL‐7 than to IL‐2 following antigenic stimulation; IL‐7 stimulation enhances IL‐2 receptor expression and increases IL‐2 responsiveness; and following antigenic stimulation, cells lose detectable expression of the IL‐7R and the ability to respond to IL‐7.16 These properties of IL‐7 on mature T cells had been previously underestimated, probably due to the presence of memory T cells whose responsiveness to IL‐7 is attenuated.16 Our observation that expression of IL‐7R and responsiveness were lost following antigenic stimulation led us to speculate that there is a narrow window during mature T‐cell differentiation when T cells can respond to IL‐7. The anti‐apoptotic effects of IL‐7 during the transition from the triple negative (CD3– CD4– CD8–) CD44+ CD25– to the CD44+ CD25+ subset during thymocyte development, suggested a similar role for IL‐7 in naive CD4+ T cells.
To test these hypotheses, we first looked at the effect on IL‐7 on in vitro survival of naive CD4+ T cells. We know that the majority of these cells (> 95%) express the IL‐7R.16 We found that cells cultured in IL‐7 survived for at least 15 days, and that this prolonged culture did not cause any obvious change in cell phenotype. These cells remained naive (as defined by CD45 isoform expression) and maintained the ability to respond to anti‐CD3 stimulation. In mice deficient for the γc receptor chain (through which IL‐7 signals), the few mature T cells that circulate in the periphery are typical of memory/activated cells and there is no evidence of T cells remaining in their naive state and surviving,29,30 Thus, it appears that IL‐7 may act as a survival factor not only for thymocytes but also for naive CD4+ T cells. This has recently been shown by another group using T‐cell receptor transgenic mice.31 They also observed that following isolation of T cells from mice, levels of Bcl‐2 and Bcl‐x1 rapidly fall. This implies that a factor produced at sites where T cells recirculate must maintain Bcl‐2 and Bcl‐x1 levels in resting T cells and thereby prevent cell death. Given that IL‐7 is important in Bcl‐2 regulation during thymocyte development, we suggest that IL‐7 may also maintain naive CD4+ T‐cell survival in vivo by a similar mechanism. The lack of naive CD4+ T cells, but the predominence of T cells of activated phenotype in mice deficient for the γc, would support this. Also, it is worth noting that unlike the receptors for other T‐cell growth factors (e.g. IL‐2, IL‐4), the IL‐7R is more highly expressed on resting rather than activated cells.12
A role for JAK3 in the maintenance of mature peripheral T cells has recently been postulated,32,33 IL‐7 signals through the γc cytokine receptor chain which is associated with JAK3. The similarities between mice deficient for JAK3 and IL‐7 or its receptors suggest that many of the biological effects of IL‐7 are mediated via JAK3.4–6,33 Like IL‐7–/– mice, JAK3–/– mice also show a profound block in T‐cell development, hindering analysis of the role of either JAK3 or IL‐7 in mature T‐cell responses.4–6,33 However, when a JAK3 transgene driven by the lck proximal promoter is introduced into JAK3–/– mice the block in T‐cell development is overcome.32 This allows analysis of the role of JAK3 in mature T cells where the lck proximal promoter is inactive and so the T cells remain JAK3–/–. T cells from such mice are impaired in their responses to antigenic stimulation. Although there is no loss of T cells from the periphery, as would be predicted from the data presented in this manuscript, there is an accumulation of T cells manifesting an activated phenotype. This implies that JAK3 plays a role in maintenance of the peripheral T‐cell compartment.
Other cytokines have been postulated to promote naive T‐cell survival. TNF‐α and IL‐6 in combination with either IL‐2 or IL‐15 have been shown to induce naive T cells to proliferation and to differentiate to a split phenotype with functional features intermediate between naive and memory T cells.26,27 We also found that IL‐7 caused some proliferation of naive CD4+ T cells. However, the levels of proliferation were very minor when compared to those induced by TNF‐α, IL‐6 and IL‐2 and we found no evidence of a ‘split phenotype’ phenotype described following culture in TNF‐α, IL‐6 and IL‐2. Unlike IL‐7, this combination of cytokines is unlikely to be present in secondary lymphoid organs, but is more likely to be found at sites of chronic inflammation such as the inflamed joint in rheumatoid arthritis.34 It has been postulated that T cells stimulated with TNF‐α, IL‐6 and IL‐2 or IL‐15 might play a role in the pathogenesis of rheumatoid arthritis by interacting with macrophages resulting in excessive production of TNF‐α.27
Given that IL‐7 can act on naive CD4+ T cells prior to antigenic stimulation, we wished to ascertain whether the effects of IL‐7 costimulation on cell proliferation and cytokine production were due to IL‐7 acting on cells prior to TCR engagement. Such a result would imply that IL‐7 acts in vivo not only by maintaining survival of naive CD4+ T cells but also by priming them for stimulation. To do this we first stimulated cells with anti‐CD3, added IL‐7 at various time‐points following anti‐CD3 stimulation, and measured responses by proliferation. We found that responses to IL‐7 were ablated within 18 hr of antigenic stimulation and did not return by 5 days after stimulation. This induction of IL‐7‐unresponsiveness could not be overcome by CD28 co‐stimulation, indicating that it was not due to some sort of ‘classical’ anergy induction. Previously, we showed that responses to IL‐7 were lost after anti‐CD3 stimulation in peripheral T cells.12 This correlated with loss of IL‐7R expression.12 However, we now show that responses to IL‐7 are lost prior to down‐regulation of the IL‐7R that are still present although diminished at 24 hr.16 The molecular events regulating IL‐7 unresponsiveness remain unknown. However, studies by Noguchi et al. have shown that γc may be degraded following antigenic stimulation by calpain, a calcium‐dependent cysteine protease.30 These data could also explain how antigenic stimulation of naive CD4+ T cells attenuates IL‐7 responses.
The essential role of IL‐7 in lymphopoiesis is undisputed. However, given the above data, we propose that IL‐7 may also play an important role in the maintenance of the recirculating naive CD4+ T‐cell pool. We know that extrinsic factors must act upon naive T cells to maintain their viability since upon in vitro culture in the absence of cytokines/growth factor they die. As yet, no candidate survival factors for naive CD4+ cells have been proposed. The combination of IL‐2, IL‐7 and IL‐4 has been shown to promote the survival of resting peripheral blood T cells (a mixture of naive and memory cells) following gamma irradiation.31 Murine thymocytes lose expression of the IL‐7R during transition from the double‐negative to the double‐positive stage of development. However, the functional significance of re‐expression of the IL‐7R on single‐positive thymocytes remains obscure. We propose that expression of the IL‐7R and maintenance of expression throughout subsequent maturation steps is required so that mature naive T cells can functionally respond to IL‐7. These responses to IL‐7 include survival and priming for antigenic stimulation. The expression of IL‐7 within secondary lymphoid organs is ideal for such a role.17,18 We have shown that IL‐7 can promote the in vitro survival of naive CD4+ T cells and in doing so, primes T cells for antigenic stimulation. We propose that in addition to its essential role in lymphopoiesis, IL‐7 plays a key role in maintaining the pool of recirculating naive CD4+ T cells and ensuring that, should they encounter antigen, their responses are maximized within the areas of primary T‐cell activation.
Acknowledgments
This work was funded by Wellcome Trust, Medical Research Council and Arthritis Research Campaign. We thank Drs Ledbetter, Beverley, Lomedico and Faltynek for provision of reagents, and Janet North and the staff of the Chelsea and Westminster Labour ward for collection of cord blood.
Glossary
Abbreviations
- γc
common γ‐chain
- IL
interleukin
- R
receptor
- TNF
tumour necrosis factor
References
- 1.Sprent J, Tough DF. Lymphocyte life‐span and memory. Science. 1994;265:1395. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 2.Miller JFAP, Mitchell GF. Thymus and antigen‐reactive cells. Transplantation Rev. 1969;1:3. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 3.Tough DF, Sprent J. Turnover of naive and memory‐phenotype T cells. J Exp Med. 1994;174:1127. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao W, Shores EW, Hu‐Li J, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 5.Peschon JJ, Morrissey PJ, Grabstein KH, et al. Early lymphocyte expansion is severely impaired in interleukin‐7 deficient mice. J Exp Med. 1994;180:1955. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Freeden‐Jeffry U, Vieira P, Lucian L, McNeil T, Burdach S, Murray R. Lymphopenia in interleukin‐7 gene‐deleted mice identifies IL‐7 as a nonredundant cytokine. J Exp Med. 1995;181:1519. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondon M, Akashi K, Domen J, Sugamura K, Weissman IL. Bcl‐2 rescues T lymphopoiesis, but not B or NK cell development, in common gamma chain‐deficient mice. Immunity. 1997;7:155. doi: 10.1016/s1074-7613(00)80518-x. [DOI] [PubMed] [Google Scholar]
- 8.von Freeden‐Jeffry U, Solvason N, Howard M, Murray R. The earliest T lineage‐committed cells depend on IL‐7 for Bcl‐2 expression and normal cell cycle progression. Immunity. 1997;7:147. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- 9.Akashi K, Kondon M, von Freeden‐Jeffry U, Murray R, Weissman IL. Bcl‐2 rescues T lymphopoiesis in interleukin‐7 receptor deficient mice. Cell. 1997;89:1033. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 10.Sudo T, Nishikawa S, Ohno N, et al. Expression and function of the interleukin‐7 receptor in murine lymphocytes. Proc Natl Acad Sci USA. 1993;90:9125. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray R, Suda T, Wrighton N, Lee F, Zlotnik A. IL‐7 is a growth and maintenance factor for mature and immature thymocyte subsets. Int Immunol. 1989;1:526. doi: 10.1093/intimm/1.5.526. [DOI] [PubMed] [Google Scholar]
- 12.Page TH, Wilcocks JL, Taylor‐Fishwick DA, Foxwell BMJ. Characterisation of a novel high affinity human IL‐7R. Expression on T cells and association with IL‐7 driven proliferation. J Immunol. 1993;151:4753. [PubMed] [Google Scholar]
- 13.Costello R, Brailly H, Mallet F, Mawas C, Olive D. Interleukin‐7, in association with CD2 and CD28 adhesion molecules stimulation, is a potent costimulus of T cell proliferation and cytokine secretion. Immunology. 1993;80:451. [PMC free article] [PubMed] [Google Scholar]
- 14.Armitage RJ, Namen AE, Sassenfeld HM, Grabstein KH. Regulation of human T cell proliferation by IL‐7. J Immunol. 1990;144:938. [PubMed] [Google Scholar]
- 15.Londei M, Verhoef A, Hawrylowicz C, Groves J, De Berardinis P, Feldmann M. Interleukin‐7 is a growth factor for mature human T cells. Europ J Immunol. 1990;20:425. doi: 10.1002/eji.1830200228. [DOI] [PubMed] [Google Scholar]
- 16.Webb LM, Foxwell BM, Feldmann M. Interleukin‐7 activates human naive CD4+ T cells and primes for interleukin‐4 production. Europ J Immunol. 1997;27:633. doi: 10.1002/eji.1830270309. [DOI] [PubMed] [Google Scholar]
- 17.Namen AE, Lupton S, Hjerrild K, et al. Stimulation of B‐cell progenitors by clones murine interleukin‐7. Nature. 1989;333:571. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 18.Kroncke R, Loppnow H, Flad HD, Gerdes J. Human follicular dendritic cells and vascular cells produce interleukin‐7: a potential role for interleukin‐7 in the germinal center reaction. Europ J Immunol. 1996;26:2541. doi: 10.1002/eji.1830261040. [DOI] [PubMed] [Google Scholar]
- 19.Ehlers S, Smith KA. Differentiation of T cell lymphokine gene expression: The in vitro acquisition of T cell memory. J Exp Med. 1991;173:25. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell EB, Sparshott SM. Interconvertion of CD45R subsets of CD4 T cells in vivo. Nature. 1990;348:163. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- 21.Michie CA, McLean A, Alcock C, Beverley PC. Life span of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:254. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 22.Brod SA, Rudd CE, Purvee M, Hasler DA. Lymphokine regulation of CD45R expression on human T cell clones. J Exp Med. 1989;170:2147. doi: 10.1084/jem.170.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joyce DA, Gibbons DL, Green P, Steer JH, Feldmann M, Brennan FM. Two inhibitors of pro‐inflammatory cytokine release, interleukin‐10 and interleukin‐4 have contrasting effects on release of soluble p75 tumor necrosis factor receptor by cultured monocytes. Europ J Immunol. 1994;24:2699. doi: 10.1002/eji.1830241119. [DOI] [PubMed] [Google Scholar]
- 24.Webb LM, Feldmann M. Critical role of CD28/B7 costimulation in the development of human Th2 cytokine‐producing cells. Blood. 1995;86:3479. [PubMed] [Google Scholar]
- 25.Schmid I, Krall WJ, Uittenboggart CH, Braun J, Giorgi JV. Dead cell discrimination with 7‐amino‐actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry. 1992;13:204. doi: 10.1002/cyto.990130216. [DOI] [PubMed] [Google Scholar]
- 26.Unutmaz D, Baldoni F, Abrignani S. Human naive T cells activated by cytokines differentiate into a split phenotype with functional features intermediate between naive and memory T cells. Int Immunol. 1995;7:1417. doi: 10.1093/intimm/7.9.1417. [DOI] [PubMed] [Google Scholar]
- 27.Sebbag M, Parry SL, Brennan FM, Feldmann M. Cytokine stimulation of T lymphocytes regulated their capacity to induce monocyte production of tumor necrosis factor‐alpha, but not interleukin‐10: possible relevance to pathophysiology of rheumatoid arthritis. Europ J Immunol. 1997;27:624. doi: 10.1002/eji.1830270308. [DOI] [PubMed] [Google Scholar]
- 28.Creery WD, Diaz‐Mitoma F, Filion L, Kumar A. Differential modulation of B7 and B7 isoform expression on human monocytes by cytokines which influence the development of T helper cell phenotype. Europ J Immunol. 1996;26:1273. doi: 10.1002/eji.1830260614. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima H, Shores EW, Noguchi M, Leonard WJ. The Common Cytokine Receptor γ Chain Plays an Essential Role in Regulating Lymphoid Homeostasis. J Exp Med. 1997;185:189. doi: 10.1084/jem.185.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noguchi M, Sarin A, Javad Aman M, et al. Functional cleavage of the common cytokine receptor γ chain (γc) by calpain. Proc Natl Acad Sci USA. 1997;94:11534. doi: 10.1073/pnas.94.21.11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrence BH, Minn AJ, June CH, Lindsten T, Thompson CB. Growth factors can enhance lymphocyte survival without committing the cell to undergo cell division. Proc Natl Acad Sci USA. 1995;92:5491. doi: 10.1073/pnas.92.12.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sohn SJ, Forbush KA, Nguyen N, et al. Requirement for Jak3 in Mature T cells: Its role in Regulation of T cell Homeostasis. J Immunol. 1998;160:2130. [PubMed] [Google Scholar]
- 33.Thomas DC, Gurniak CB, Tivol E, Sharpe AH, Berg LJ. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995;270:800. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- 34.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]