Abstract
Increased serum levels of interferon‐γ (IFN‐γ) have been observed in acute graft‐versus‐host disease (GVHD). Recent in vitro studies have demonstrated that interleukin‐12 (IL‐12) and interleukin‐18 (IL‐18) synergistically up‐regulate IFN‐γ secretion. In this communication, we investigated the factors relevant to IFN‐γ secretion in acute GVHD. A murine model of acute GVHD was established by injecting donor spleen cells into severe combined immunodeficiency (SCID) mice. A series of specimens, including sera, livers and spleens derived from the GVHD mice, were investigated with histological examination, enzyme‐linked immunosorbent assay (ELISA), flow cytometry, and semiquantitative reverse transcription–polymerase chain reaction (RT–PCR). IFN‐γ secretion increased in serum 3 days after spleen cell transfer, peaked on day 7, and then gradually decreased close to the baseline level by day 35. A synchronized increase of activated T cells and mRNA expression of IL‐12, IL‐18 and their respective receptors was observed after spleen cell transfer. However, only the kinetic expression pattern of IL‐12 receptor (IL‐12R) β2 chains was closely correlated with that of IFN‐γ, while IL‐12 dropped to the baseline level earlier than IFN‐γ. Therefore, IFN‐γ expression in the early phase of acute GVHD is a mono‐peak and self‐restricted pattern. Its secretion is closely related with T‐cell activation, the presence of IL‐12, IL‐18 and their respective receptors. However, the limiting factors for IFN‐γ secretion seem to be IL‐12 and IL‐12R β2 chains.
Introduction
Increased expression of interferon‐γ (IFN‐γ) is associated with acute graft‐versus‐host disease (GVHD) in both humans and experimental animal models.1–3 In in vitro experiments, lymphocytes from GVHD animals secrete significantly greater amounts of IFN‐γ than those from the controls.4 IFN‐γ induces major histocompatibility complex (MHC) class II expression and enhances the expression of class I antigens on host target cells, especially keratinocytes and enterocytes, and has also been implicated in the intestinal pathology of GVHD.5,6 On the other hand, administration of exogenous IFN‐γ can markedly inhibit GVHD in murine allogeneic bone marrow transplantation, and this was assumed to be mediated by the action of IFN‐γ on natural suppressor cells that could inhibit GVHD.7,8 These results are obviously conflicting, and further investigation on the IFN‐γ effect in GVHD is needed.
IFN‐γ can be secreted by both T cells and natural killer (NK) cells upon antigen or mitogen stimulation.9 However, in acute GVHD, IFN‐γ production has been shown to be entirely dependent on the presence of donor T cells.10In vitro studies have convincingly demonstrated that IFN‐γ secretion is dependent on the synergistic effect of interleukin‐12 (IL‐12) and interleukin‐18 (IL‐18). Lack of either IL‐12 or IL‐18 would significantly lower or even diminish the production of IFN‐γ.9 IL‐12 receptor (IL‐12R) is composed of a β1 and a β2 chain. While the β2 chain is expressed only on T helper 1 (Th1) cells, the β1 chain is expressed on both Th1 and Th2 cells. However, both the β1 and β2 chains are required for the responsiveness of T cells to IL‐12 and therefore to mediate the Th1 cell differentiation.11,12 IL‐18, designated also as IFN‐γ‐inducing factor, is a more recently cloned cytokine of 18·3 000 MW.9 Similar to IL‐12R, IL‐18 receptor (IL‐18R) is distributed only on Th1 cells, but not on Th2 cells.13
Although the effect of IFN‐γ is not fully established, it definitely plays a role in acute GVHD. Manipulation of IFN‐γ secretion could be useful for the management of acute GVHD. Because the expression of IFN‐γ is regulated by IL‐12 and IL‐18, it should be possible to adjust the IFN‐γ secretion by interfering with the interaction between IL‐12, IL‐18 and their respective receptors on IFN‐γ‐producing cells. A kinetic study on IFN‐γ and its regulatory factors can provide a basis for establishing the regulatory machinery, and for choosing the suitable intervention target as well as the best time point. We initiated such a study in a murine acute GVHD model. The results revealed that IFN‐γ secretion increased in a single‐peak pattern in the first 35 days after the injection of donor spleen cells, and seemed to be self‐restricted. It also suggests that IL‐12 and the expression of IL‐12R β2 chains may be the limiting factors for IFN‐γ secretion.
Materials and methods
Induction of acute GVHD
C57BL/6J (H‐2b) mice were used as donors. C.B‐17 severe combined immunodeficiency (SCID) mice (H‐2d) were purchased from the Jackson Laboratory (Bar Harbor, ME), and maintained in a flexible film isolator. These SCID mice were used as recipients at the age of 4 weeks. Spleens were obtained from the donor mice, and homogenized into single cell suspension in RPMI‐1640 medium. Mononuclear cells were harvested from the interface after density centrifugation of the spleen cells on Ficoll–Paque (Pharmacia Biotech AB, Uppsaala, Sweden). After washing twice with RPMI‐1640, 4 × 106 of the spleen cells were injected into each recipient SCID mouse via the tail vein. Thereafter the recipient mice were observed and weighed daily. Recipient mice were killed at various time points. Specimens of serum, spleen, and liver were used freshly, or stored at either –20° or –80° for further analysis.
Haematoxylin and eosin (H & E) staining
Frozen liver specimens were examined with H & E staining. Six micrometre‐thick sections were cut and fixed with 10% formalin for 2 min. After washing, the sections were stained with Gill’s haematoxylin for 2 min, and rinsed with water and then stained with buffered (pH 5·0) eosin solution for 30 s. After rinsing, the sections were dehydrated with ethanol and embedded with mounting medium.
Flow cytometry
Mononuclear cells derived from spleens of both donor and recipient mice were analysed by flow cytometry. Monoclonal antibodies directed at murine CD3 (145‐2C11, fluoroscein isothiocyanate; FITC), CD4 (L3T4, FITC), CD8 (53‐5.8, FITC) and CD25 (7D4, phycoerythrin; PE) were obtained from PharMingen (San Diego, CA), and used at 1 : 40 dilution. Aliquots of 106 mononuclear cells were incubated on ice with anti‐CD3, or a mixture of anti‐CD25 in combination with anti‐CD3, anti‐CD4 and anti‐CD8, respectively, for 30 min. The cells were washed twice with phosphate‐buffered saline (PBS) containing 0·1% bovine serum albumin (BSA), and resuspended in PBS supplemented with 0·1% BSA and 0·5% paraformaldehyde (Sigma, St. Louis, MO). For each preparation, 10 000 cells were counted with a Coulter flow cytometer (Epics Esp, Coulter Corp., Hialeah, FL), and data analysed with the aid of a computer software (WindMDI, University of Massachusetts, Amherst, MA).
Enzyme‐linked immunosorbent assay (ELISA)
Levels of IFN‐γ were established in serum samples obtained at various time points from recipient mice using an ELISA kit (Quantikine M, mouse IFN‐γ, R & D Inc Systems, Minneapolis, MN). The method employed procedures provided by the manufacturer.
Reverse transcription–polymerase chain reaction (RT–PCR) and hybridization
Total RNA was isolated from recipient spleens using a high RNA isolation kit from Boehringer Mannheim Corp. (Indianapolis, IN), and quantified with spectrophotometry at UV260. cDNA was synthesized from an equal amount of RNA using oligo‐p(dT)15 primer and avian myeloblastosis virus (AMV) reverse transcriptase. PCR and subsequent hybridization were performed with the oligonucleotides as follows: IL‐12p40: sense, GAGGTGGACTGGACTCCCG; antisense, CAAGT‐TCTTGGGCGGGTCTG; probe, GAGAACTACAGCACCAGCTTCTTC. IL‐12R β1 chain: sense, CTCAGG‐TGTGCTTGGTGACATTTG; antisense, GTGTGTCACCATCTTGGCAGGATC; probe, AGGTGAAGAAACACT‐TGGTGCTGG.14 IL‐12R β2 chain: sense, GGCACAGACTGTTAGAGAATGCTC; antisense, GCAATTCTGACGATTGTCAG; probe, GATCTCAGTTGGTGT‐TGCTCCAGA;14 IL‐18: sense, ACTGTACAACCGC‐AGTAATACGG; antisense, GGCAAGCAAGAAAGT‐GTCCTTCA; probe, AGTGAAGTAAGAGGACTG‐GCTGTG. IL‐18R: sense, CGTGACAAGCAGAGA‐TGTTG; antisense, ATGTTGTCGTCTCCTTCCTG; probe, ACACGGTACAACATCACCAAGACT14. β‐actin: sense, GTGGGCCGCTCTAGGCAG; antisense, CTTTGATGTCACGCACGATTTC. Aliquots (1 µl) of the cDNA mixture were each added into a final volume of 50 µl of PCR buffer (10 mm Tris–HCl, 1·5 mm MgCl2, 50 mm KCl; pH 8·3) containing dinucleoside triphosphate (dNTP) (0·2 mm), sense and antisense primers (1 µm each), and Taq DNA polymerase (1 unit). This mixture was heated to 94° for 5 min, and then amplified 28 cycles, each at 94° for 40 s, 55° for 40 s, and 72° for 60 s, for β‐actin or 36 cycles for others. PCR products were separated in 1% agarose gel. After the gel was denatured for 1 hr in a solution containing 0·5 m of NaOH and 1·5 m of NaCl, it was neutralized for 1 hr in a solution (pH 8·0) containing 1 m of Tris–HCl and 1·5 m NaCl. The DNA was transferred to nylon membrane, and prehybridized in a buffer containing 5× Denhart, 6× SSPE, 0·1% BSA and 0·02% sodium dodecyl sulphate (SDS) at 42° overnight. The internal oligonucleotide probe end‐labelled with digoxigenin‐11‐dUTP (Boehringer Mannheim) was added and allowed to hybridize at 42° for 1 hr. The membrane was washed twice with 2× SSPE containing 0·1% SDS at room temperature (RT) and subsequently at 65°, and twice again with 2× SSPE at RT. The membrane was rinsed with buffer A (0·1 m maleic acid, 0·15 m NaCl; pH 8·5) containing 0·3% Tween 20, and blocked in buffer A supplemented with 1% of the blocking reagents (Boehringer Mannheim) at RT for 1 hr. Anti‐digoxigenin Fab fragment conjugated with alkaline phosphatase (1 : 10 000 dilution; Boehringer Mannheim) was then added and incubated for 30 min, followed by washing twice with buffer A containing 0·3% Tween 20, and incubated with a buffer containing 0·1 m Tris–HCl (pH 9·5) and 0·1 m NaCl for 3 min. After the chemiluminescent alkaline phosphatase substrate Disodium 3‐(4‐methoxyspiro{1,2‐dioxetane‐3,2′‐(5′‐chloro)tricyclo[3.3.1.13,7]decan}‐4‐yl)phenyl phosphate (CSPD)® (Boehringer Mannheim) was added, the signal was detected by exposing the membrane to an X‐ray film, and then analysed with a densitometer (Bio‐RAD, Hercules, CA).
Statistical analysis
Values of serum IFN‐γ were averaged for each time point, and a linear correlation analysis was performed with the Pearson test between the means of IFN‐γ and the adjusted optical density (OD) values (over the OD value of corresponding signals of β‐actin, see also Fig. 3c) of IL‐12, IL‐18 and their respective receptors.
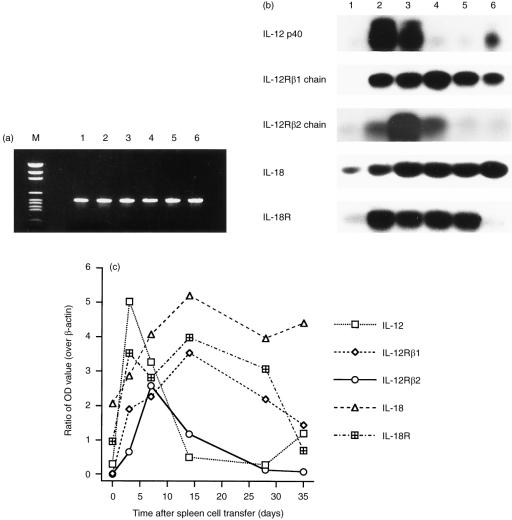
Figure 3.
Representative experiments on the expression of IL‐12p40, IL‐18 and the receptors at the mRNA level in spleens of control and GVHD mice. RNA was isolated and transcribed into cDNA for PCR amplification. PCR product was hybridized with an internal oligonucleotide probe, and the signals were measured with densitometry. (a) PCR products of β‐actin after 28 cycles amplification were separated on an agarose gel stained with ethidium bromide, showing a nearly equal intensity for every sample. M: DNA marker. Lanes 1, 2, 3, 4, 5 and 6 represent specimens obtained, respectively, from control mouse, and experimental mice 3, 7, 14, 28, and 35 days after injection of donor spleen cells. (b) hybridized PCR products of IL‐12p40, IL‐18, β1 and β2 chains of IL‐12R, and IL‐18R. Lanes 1, 2, 3, 4, 5 and 6, see explanation in (a). (c) the optical density of each hybridized PCR signal was determined by densitometry. The OD values were divided by that of the corresponding β‐actin signal, and the adjusted OD values of IL‐12p40, IL‐18 and their respective receptors show a variation with sample and time.
Results
Acute GVHD in recipient mice
A total of 40 SCID mice were included in the experiments; 10 of them were randomly selected as controls, and 30 were each injected with 4 × 106 donor spleen cells. The spleen cells were shown to contain about 50% of T cells by fluorescence‐activated cell sorting (FACS) analysis with an anti‐CD3 monoclonal antibody (data not shown). This dose (4 × 106 cells) was chosen because in preliminary experiments higher doses of donor cells induced more severe GVHD and killed most recipients in 2–3 weeks (data not shown), making it difficult to observe a complete course of the expression of IFN‐γ, IL‐12, IL‐18 and their respective receptors. Even at this dosage of donor spleen cells, five recipient mice died of acute GVHD during the observation period. The remaining 25 recipients were used for analysis. Acute GVHD in these mice started 14 days after the injection. Symptoms included hair loss, red and swelling ears and eyelids, decreased activity, diarrhoea, and decreased body weight. Upon autopsy, recipient mice were found to have enlarged spleens and livers, and in some mice, necrosis could be observed on the surface of the livers.
Microscopic examination of the liver sections with H & E staining revealed that lymphocytic infiltrates started to appear in the peribiliary area 7 days after spleen cell injection. These infiltrates became more intense by day 14, and the necrosis of liver cells adjacent to the lymphocytic infiltrates appeared by day 21 and continued to deteriorate by day 35 (data not shown).
Sustained T‐cell activation in GVHD
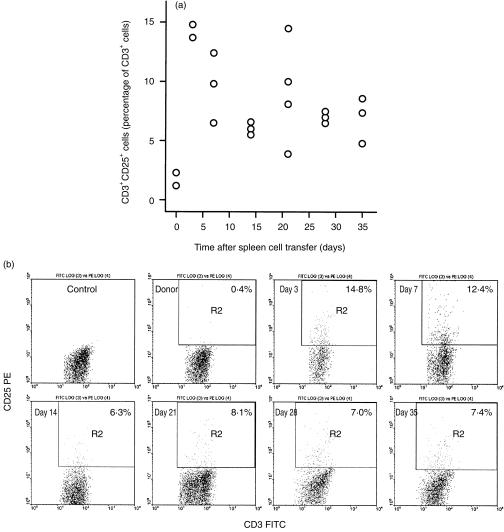
Spleen cells derived from the donor and recipient mice were analysed with flow cytometry, and the IL‐2 receptor expression detected by an anti‐CD25 mAb was used as the parameter to indicate T‐cell activation. Cells were stained with anti‐CD3 or anti‐CD25 in combination respectively with anti‐CD3, CD4, and CD8. Only T cells were included for analysis, as these were gated with the anti‐CD3 staining. The results are presented in Fig. 1(a, b). T cells in the donor spleen had a very small percentage of CD3+ CD25+ cells (less than 2·5%). However, this percentage increased to over 13% 3 days after injecting the donor spleen cells into the recipients, and thereafter gradually decreased to less than 10%, sustaining at 5–8% from day 7 to day 35, in the majority of the mice. Furthermore, CD25+ cells were nearly equally distributed in both CD4+ and CD8+ cell populations (data not shown).
Figure 1.
Sustained T‐cell activation in the early phase of acute GVHD. Spleen cells from donor and GVHD suffering mice were prepared as single cell suspension, and stained with anti‐CD3 FITC or anti‐CD3 FITC in combination with anti‐CD25 PE. Cells were gated with anti‐CD3 FITC, and therefore, only T cells were included for analysis. (a) CD3+ CD25+ cells increased proportionally in all mice after donor spleen cell transfer, and this increase was maintained in the whole observation period. (b) Representative FACS analysis results showing the increase of CD3+ CD25+ cells in mice after donor spleen cell transfer.
Expression of IFN‐γ in the blood
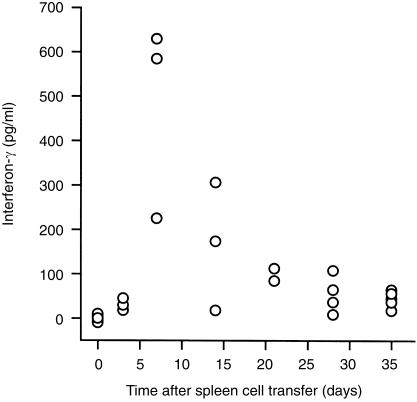
Serum samples from 25 mice were available for the measurement of IFN‐γ with ELISA. As shown in Fig. 2, IFN‐γ was not detectable in the five control mice, but started to appear 3 days after the spleen cell injection. It peaked later on day 7, and thereafter gradually declined between day 14 and day 21, and finally to a low but detectable level on days 28 and 35.
Figure 2.
Mono‐peak pattern increase of IFN‐γ expression in the blood in the early phase of acute GVHD. Serum samples were collected at various time points after donor spleen cell transfer, and IFN‐γ was measured with ELISA. The minimal detectable dose of IFN‐γ by this kit is 3·0 pg/ml.
Expression of IL‐12p40, IL‐18 and their receptors
The expression of IL‐12p40, IL‐18 and their respective receptors was examined on mRNA with RT–PCR and hybridization with an internal oligonucleotide probe. Total RNA isolated from spleen tissues of control and GVHD mice killed at various time points was quantified by spectrophotometry. The same amounts of RNA were used for cDNA synthesis, and subsequently amplified by PCR.
PCR amplification of β‐actin was performed for 28 and 36 cycles, respectively, with both yielding products of nearly equal intensity in ethidium bromide‐stained agarose gel. Results of the 28‐cycle PCR (Fig. 3a) showed that their OD values by densitometry were between 0·17 and 0·22. These values were later used for the adjustment of the OD values of IL‐12, IL‐18 and their respective receptors.
PCR for detection of mRNA encoding IL‐12p40, IL‐18 and the receptors was performed for 36 cycles, because fewer cycles did not generate signals strong enough for analysis. As shown in Fig. 3(b, c), both IL‐12p40 and IL‐18 were detected in all samples, but the amount of both interleukins increased significantly after the spleen cell transfer. IL‐12p40 peaked on day 3, and then gradually decreased to the baseline by day 28, but increased slightly again on day 35. IL‐18 peaked on day 7 and remained at the peak level until day 35. IL‐12R β1 chain was detected with strong signals in the recipient spleen specimen after cell transfer, but not in the control spleen. Signal of IL‐12R β2 chain increased in the recipient spleen 3 days after cell transfer, peaked on day 7, and thereafter gradually decreased to the baseline level. IL‐18R was detected in all spleen samples, but signals intensified significantly in recipient spleens procured between day 3 and day 28 after cell transfer.
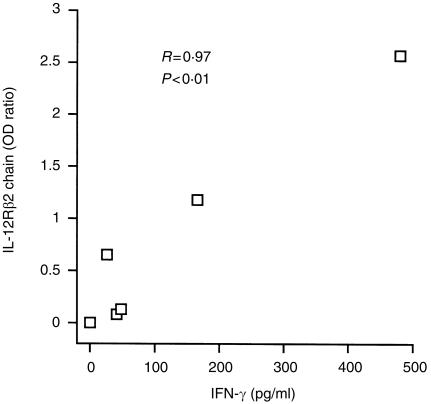
Linear correlation between IFN‐γ and IL‐12R β2 chain
Values of INF‐γ were averaged for each time points, and a Pearson correlation analysis was performed between the IFN‐γ mean concentrations and the adjusted OD values of IL‐12, IL‐18 and the receptors. The correlation coefficient (R) was 0·28 (P = 0·59 > 0·05) between IFN‐γ and IL‐12p40, 0·44 (P = 0·39 > 0·05) between IFN‐γ and IL‐12R β1 chain, 0·97 (P = 0·002 < 0·01) between IFN‐γ and IL‐12R β2 chain (see Fig. 4), 0·39 (P = 0·45 > 0·05) between IFN‐γ and IL‐18, and 0·30 (P = 0·57 > 0·05) between IFN‐γ and IL‐18R.
Figure 4.
Correlation between the secretion of IFN‐γ and the expression of IL‐12R β2 chains. A Pearson linear correlation test was performed between the mean concentration of IFN‐γ at each time point and the corresponding adjusted OD value of IL‐12R β2 chain. A positive correlation was observed (R = 0·97, P = 0·002 < 0·01).
Discussion
SCID mice do not have immunocompetent T and B lymphocytes in peripheral lymphoid organs, and have been used to establish GVHD in various investigations.16 Because T cells are absent in SCID mice, it is most likely that IFN‐γ in acute GVHD recipients is secreted by the activated alloreactive donor T cells and NK cells. However, it is possible that NK cells of the recipient SCID mice are also capable of producing IFN‐γ, although at a much lower level. A previous study demonstrated that in acute GVHD IFN‐γ production was entirely dependent on the presence of donor T cells.10
Several research groups have reported that IFN‐γ secretion was increased in mice suffering from acute GVHD.1–3 In our observation period of the first 35 days after the spleen cell transfer, IFN‐γ secretion was revealed to display a mono‐peak pattern; being detectable on day 3, peaking on day 7, and then gradually decreasing to low levels during the observation period. Interestingly, this IFN‐γ secretion pattern did not completely correlate with the activation status of the spleen T cells, which had peaked proportionally 3 days after donor cell transfer, and then gradually reduced to a sustained level higher than that observed in the controls. It is known that activated T cells, both Th1 and type 1 cytotoxic T cells (Tc1), are the main source of IFN‐γ in acute GVHD.10 Therefore, this incomplete correlation between IFN‐γ secretion and T‐cell activation indicated that the activated T cells actively secreted IFN‐γ at first; thereafter even though T‐cell activation sustained phenotypically, these seemingly activated T cells did not maintain the IFN‐γ secretion. This suggests that IFN‐γ secretion is a self‐restricted process, dependent on T‐cell activation, but also having its own regulatory machinery.
IL‐12 and IL‐18 have been shown to be essential factors in regulating IFN‐γ expression in in vitro studies.9 However, IL‐12 or IL‐18 alone induces only a trace amount of IFN‐γ in T cells. IFN‐γ expression could be increased significantly by the synergistic effect of IL‐12 and IL‐18.14 Both IL‐12 and IL‐18 are produced by activated macrophages, while IL‐18 could also be secreted by keratinocytes, osteoblast cells, adrenal cortical cells, and intestinal epithelial cells as well.9,15In vitro data indicate that the IL‐12p40 is an inducible subunit, which rapidly increases at the mRNA level upon stimulation, and decreases in a few hours in the absence of stimulation.14 In contrast, the mRNA encoding IL‐18 is usually constitutively expressed.9 This correlates well with the IL‐12p40 and IL‐18 mRNA expression in mouse spleens of the present study. Both IL‐12p40 and IL‐18 were detectable, but the expression was obviously increased after the spleen cell transfer, indicating that the IL‐12 and IL‐18 expressing cells, possibly mainly macrophages, were functionally activated. While this increase of both IL‐12p40 and IL‐18 mRNA synchronized with the serum IFN‐γ increment, they do not seem to completely correlate with the IFN‐γ secretion pattern. While IL‐18 mRNA remained at a high level for the whole observation period, IL‐12p40 decreased to a low level earlier than IFN‐γ, indicating that IL‐12 may act as a limiting factor for IFN‐γ secretion.
It is known that receptors for both IL‐12 and IL‐18 are distributed only on Th1 cells but not on Th2 cells.11–13 Furthermore, IL‐12 induces the expression of IL‐18R on T cells.9,14 The analysis of IL‐12R mRNA in the present study revealed that the β1 chain of IL‐12R was increased in all spleen specimens obtained after spleen cell transfer. Surprisingly, the β2 chain showed exactly the same pattern as that of IFN‐γ levels in the blood, and the correlation coefficient between the β2 chain and IFN‐γ was 0·97, a value very close to a perfect positive correlation (R = 1). The IL‐18R was detected in all spleen samples, with signals intensifying in the recipient spleens procured between day 3 and day 28 after the cell transfer. Although the early pattern of increase was similar to IFN‐γ, the level of IL‐18R remained high for a much longer period before decreasing after day 28.
It is rational to suggest that IFN‐γ expression is indeed closely related with T‐cell activation, the presence of IL‐12 and IL‐18, and the expression of IL‐12 and IL‐18 receptors. If IL‐12 and IL‐18 are the only cytokines regulating IFN‐γ, it seems possible that the kinetic secretion pattern of IFN‐γ is largely determined by IL‐12 and the expression of IL‐12R β2 chains. IFN‐γ is a very important cytokine in the immune response because it is capable of regulating hundreds of genes.17 Although its exact effect in acute GVHD is unclear, IFN‐γ surely plays a critical role in this clinical syndrome, which is still a major problem for successful bone marrow transplantation. A delicate adjustment of IFN‐γ secretion could become a way of managing GVHD in the clinic,18 and this adjustment is achievable by manipulating IL‐12, IL‐18 and their respective receptors.9 This is supported by a recent study in which blocking IL‐18R with specific antibodies successfully down‐regulated IFN‐γ secretion.13 However, data in the present study suggest that regulating the expression of IL‐12R β2 chain on IFN‐γ‐producing cells might be more effective, although at this moment it is not known how the β2 chain itself is regulated and neutralizing antibodies are not yet available.9,11–13 Because IL‐12 seems to be another limiting factor, it should also be possible to regulate IFN‐γ secretion by providing or depriving IL‐12. Furthermore, our data also suggest that the manipulation of IL‐12 and/or IL‐18 receptors should be performed within the first 3 days after cell transfer.
In summary, we have studied the kinetics of circulatory IFN‐γ and its regulatory factors in murine acute GVHD. It is found that IFN‐γ expression in the early phase of acute GVHD is a mono‐peak and self‐restricted pattern. Its secretion is closely related with T‐cell activation, the presence of IL‐12, IL‐18 and their respective receptors. But the major limiting factors appear to be IL‐12 and IL‐12R β2 chains.
Acknowledgments
We thank Mr Kin Fai Tang, Ms Ping Feng, and staffs at the Laboratory Animals Centre, National University of Singapore, for their expert technical assistance. This study is supported by a seed grant to HHZ (#30030) from MFRC, National University of Singapore.
References
- 1.Szebeni J, Wang M‐g, Pearson DA, Szot GL, Sykes M. IL‐2 inhibits early increases in serum gamma interferon levels associated with graft‐versus‐host‐disease. Transplantation. 1994;58:1385. [PubMed] [Google Scholar]
- 2.Dickinson AM, Sviland L, Hamilton PJ, et al. Cytokine involvement in predicting clinical graft‐versus‐host disease in allogeneic bone marrow transplant recipients. Bone Marrow Transpl. 1994;13:65. [PubMed] [Google Scholar]
- 3.Allen RD, Staley TA, Sidman CL. Differential cytokine expression in acute and chronic murine graft‐versus‐host disease. Eur J Immunol. 1993;23:333. doi: 10.1002/eji.1830230205. [DOI] [PubMed] [Google Scholar]
- 4.Troutt AB, Maraskovsky E, Rogers LA, Pech MH, Kelso A. Quantitative analysis of lymphokine expression in vivo and in vitro. Immunol Cell Biol. 1991;70:51. doi: 10.1038/icb.1992.8. [DOI] [PubMed] [Google Scholar]
- 5.Niederwieser D, Herold M, Woloszczuk W, et al. Endogenous IFN‐γ during human bone marrow transplantation: analysis of serum levels of interferon and interferon‐dependent secondary messages. Transplantation. 1990;50:620. doi: 10.1097/00007890-199010000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Guy‐Grand D, Vassalli P. Gut injury in mouse graft‐vs.‐host reaction: study of its occurrence and mechanism. J Clin Invest. 1986;77:1584. doi: 10.1172/JCI112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brok HPM, Heidt PJ, Van der Meide PH, Zurcher C, Vossen JM. Interferon‐γ prevents graft‐versus‐host disease after allogeneic bone marrow transplantation in mice. J Immunol. 1993;151:6451. [PubMed] [Google Scholar]
- 8.Holda JH, Maier T, Claman HN. IL‐3, IL‐4, and IL‐6 enhance IFN‐gamma‐dependent bone marrow natural supressor activity. Cell Immunol. 1990;125:459. doi: 10.1016/0008-8749(90)90099-d. [DOI] [PubMed] [Google Scholar]
- 9.Okamura H, Kashiwamura S‐i, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon‐γ production by IL‐12 and IL‐18. Curr Opin Immunol. 1998;10:259. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 10.Rus V, Svetic A, Nguyen P, Gause WC, Via CS. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft‐versus‐host disease: regulatory role of donor CD8+ T cells. J Immunol. 1995;155:2396. [PubMed] [Google Scholar]
- 11.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL) ‐12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogge L, Barberis‐Maino L, Biffi M, et al. Selective expression of an interleukin‐12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu DM, Chan WL, Leung BP, et al. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J Exp Med. 1998;188:1485. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimoto T, Takeda K, Tanaka T, et al. IL‐12 up‐regulates IL‐18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL‐18 for IFN‐γ production. J Immunol. 1998;161:3400. [PubMed] [Google Scholar]
- 15.Stoll S, Muller G, Kurimoto M, et al. Production of IL‐18 (IFN‐gamma‐inducing factor) messenger RNA and functional protein by murine keratinocytes. J Immunol. 1997;159:298.. [PubMed] [Google Scholar]
- 16.Phillips RA, Spaner DE. The scid mouse: mutation in a DNA repair gene creates recipients useful on stem cells, lymphocyte development and graft‐versus‐host disease. Immunol Rev. 1991;124:63. doi: 10.1111/j.1600-065x.1991.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 17.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon‐γ. Annu Rev Immunol. 1997;15:749. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara JLM, Cooke KR, Pan LY, Krenger W. The immunopatho‐physiology of acute graft‐versus‐host disease. Stem Cells. 1996;14:473. doi: 10.1002/stem.140473. [DOI] [PubMed] [Google Scholar]