Abstract
In previous studies we have shown that histidine‐rich glycoprotein (HRG), a relatively abundant plasma protein, can bind to immunoglobulin G (IgG) and inhibit the insolubilization of IgG‐containing immune complexes (IC). It was of interest, therefore, to determine whether HRG can inhibit the formation of insoluble IC (IIC) resulting from the interaction of rheumatoid factor (RF) with human IgG‐containing IC. Light scattering techniques were used to examine the effect of HRG on the formation of IIC between RF and IC containing human IgG according to three different models. In all three models physiological concentrations of HRG could block the formation of IIC induced by RF. Optical biosensor studies of the RF–IgG interaction also revealed that HRG can mask the epitopes on IgG recognized by RF. Additional studies examined whether HRG can solubilize already formed IIC and demonstrated that HRG can, in fact, partially solubilized IIC. These data indicate that HRG can regulate the formation of IIC induced by RF at three levels: namely by inhibiting the initial recognition of IgG containing IC by RF, by inhibiting the subsequent insolubilization of IgG containing IC by RF and by solubilizing already formed IIC. Collectively, these findings suggest that HRG may be an important inhibitor of the formation of pathogenic IC in diseases such as systemic lupus erythematosus and rheumatoid arthritis.
Introduction
Rheumatoid factors (RF) are autoantibodies (aAb) produced against antigenic determinants on the Fc portion of immunoglobulin G (IgG) molecules and have been detected as IgM, IgA and IgG forms in plasma and tissues.1 Two general types of RF have been described; namely, pathological RFs associated with RA,2 and natural RFs that exist in healthy individuals.3 Both types of RF are known to bind to the Fc portion of IgG, but RFs within each type possess several characteristic differences such as the use of different variable region sequences and different immunoglobulin isotypes.2 Although pathological RF may be present at low levels in normal human plasma, the concentration of this RF is often elevated in the plasma of patients suffering from infections such as infectious mononucleosis and hepatitis C virus infection.4–6 On the other hand, sustained levels of pathological RF are observed in immune complex (IC)‐associated autoimmune diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and vasculitis.4,6–8
Although evidence suggests that natural RF may be involved in the clearance of IC,9,10 the precise function of RF and the mechanism(s) regulating its production in vivo is unclear. Kinetic studies have shown that IgM–RF or IgG–RF each interact with aggregated IgG or IC with much greater affinity (˜100‐fold) than with monomeric IgG, indicating that RF binds specifically to IC containing IgG.4,11 Other studies have shown that human peripheral blood mononuclear leucocytes secrete RF when cultured in the presence of IC, but not when cultured in the presence of monomeric IgG, suggesting that only IC can appropriately display antigenic determinants that activate B cells to secrete RF.12 In addition, only prolonged immunization of mice or rabbits with bacterial antigens has been shown to induce RF production13,14 indicating that the production of pathological RF, particularly of the IgG4 subclass, may be a secondary phenomenon that occurs during chronic infections. These studies collectively suggest that the production of RF may be a physiological phenomenon which potentiates the clearance of primary IC.
Histidine‐rich glycoprotein (HRG) is a ˜80 000 MW plasma glycoprotein, which is relatively abundant (plasma concentration ˜150 µg/ml) and is synthesized by the liver at a relatively high rate (i.e. plasma half life ˜3 days).15 The physiological function of HRG has not yet been established; however, a number of ligands for the molecule have been reported: namely; divalent metal ions,16 heparin,17 heparan sulphate,18 thrombospondin,19 fibrinogen,20 and certain complement proteins.21 These observations suggest that HRG may play an important role in regulating blood clotting and the immune system. Detailed conformational studies also indicate that the molecule has a modular structure, suggesting several independent binding sites which would enable HRG to bring two or more ligands together.22 Recently we identified two previously undescribed ligands for HRG: IgG and C1q. Our additional studies indicated that HRG inhibits the formation of insoluble IC (IIC) by maintaining IC in a soluble form, thus implicating HRG as a key endogenous regulator of the formation of IC.23 In fact, recent studies indicate that when HRG is incorporated in IC the IC more effectively interact with macrophages.24
In the present work experiments were designed to mimic the interaction of RF with human IgG containing IC in order to examine the effect of HRG on the formation of IIC induced by RF. Additional experiments using an IAsys biosensor, studied the binding of RF to immobilized human IgG1κ and the effect of HRG on this interaction. The results show that physiological concentrations of HRG can block the formation of IIC between aggregated human IgG and RF in vitro and also promote solubilization of already formed IIC. Furthermore, the biosensor studies show that the binding of RF to IgG1κ is blocked by HRG, indicating that HRG masks the epitope(s) on IgG recognized by RF. The findings suggest that HRG is a novel regulator of RF induced IIC formation.
Materials and methods
Reagents
Human IgG, human IgG subclasses (myeloma derived), bovine serum albumin (BSA, fraction V), ovalbumin (grade V) and polyoxyethylenesorbitan monolaurate (Tween‐20) were purchased from Sigma Chemical Co., St Louis, MO. RF (60 IU/ml) was supplied by Accurate Chemicals, Westbury, NY. Carboxymethyl‐dextran cuvettes for the IAsys biosensor, 1‐ethyl‐3‐(3‐dimethylaminopropyl)‐carbodiimide (EDC), N‐hydroxysuccinimide (NHS) and ethanolamine were purchased from Affinity Sensors, Cambridge, UK. The homobifunctional reagent bis‐(sulphosuccinimidyl) suberate (BS3), sulphosuccinimidyl 6‐(biotinamido) hexanoate (NHS‐LC‐biotin) and gentle Ag/Ab elution buffer (a cuvette regeneration buffer gentle to the dextran matrix) were purchased from Pierce, Rockford, IL. Streptavidin was purchased from Progen Industries, Brisbane, Australia.
Purification of proteins
Native human HRG of molecular size 80 000 MW was purified from fresh human plasma as previously described25 by equilibrating a phosphocellulose column with loading buffer comprising 10 mm sodium phosphate (pH 6·8) containing 1 mm ethylenediamine tetra‐acetic acid (EDTA) and 0·5 m NaCl (loading buffer). The plasma was mixed with EDTA and NaCl at the final concentrations as in the loading buffer and with 4‐(2‐aminoethyl)‐benzenesulphonyl fluoride hydrochloride (AEBSF) (ICN Pharmaceutical Inc., Costa Mesa, CA) at 100 µg/ml and aprotinin (a trypsin inhibitor) at 2 µg/ml. The plasma was passed through the equilibrated column and unbound protein was removed by extensive washing of the column with the loading buffer. Bound HRG was then eluted from the column using the same buffer containing 2 m NaCl.
Rabbit anti‐ovalbumin IgG was purified from immunized rabbit serum by Na2SO4 precipitation and ion‐exchange chromatography using a diethylaminoethyl (DEAE)–Sephacel column. The IgG was collected from the breakthrough peak as described.26 Human IgG (2 mg/ml) was heat aggregated by incubation at 63° for 40 min in phosphate‐buffered saline (PBS) and then chilling on ice for 2 hr. The aggregated IgG was separated from non‐aggregated IgG by fast‐performance liquid chromatography (FPLC) gel filtration using a Superose 12 column (Pharmacia, Uppsala, Sweden). The aggregated IgG was collected in the exclusion volume peak.
Formation of IIC
The formation of IIC was initiated by the addition of human IgG (from pooled human serum) to solutions of anti‐human IgG antibodies. IIC formation was studied in three different systems: (1) rabbit anti‐human IgG (Fc) antibodies (69 µg/ml) were incubated without or with different concentrations of HRG in 0·01 m phosphate buffer (pH 7·4) containing 0·15 m NaCl and 3 mm NaN3 (PBS‐Az) in a quartz reaction vessel (final volume 1 ml) maintained at 37°. IIC formation was initiated by the addition of human IgG which was either treated or untreated with HRG at different antigen : antibody ratios; (2) RF (8 IU/ml) was incubated in PBS‐Az without or with different concentrations of HRG and then IIC formation was initiated by addition of heat‐aggregated human IgG which was either treated or untreated with HRG at different antigen: antibody ratios; (3) biotinylated human IgG (b‐IgG, from pooled human serum) (30 µg/ml) was incubated without or with different concentrations of HRG for 20 min and then streptavidin (STP) was added to form IC in a quartz reaction cuvette. After 20 min incubation RF (8 IU/ml), which was treated or untreated with HRG, was added to the mixture of STP and b‐IgG to initiate the formation of IIC. Because the absorbance caused by light scattering of a suspension of particles is related to the average size of the particles, in all instances the formation of IIC was monitored by measuring the absorbance of samples at 350 nm using a Varian Cary‐1 spectrophotometer, as described.23
Solubilization of preformed IIC by HRG
Ovalbumin at antigen : antibody ratios between 0·005 and 0·05 was added to a solution of rabbit anti‐ovalbumin IgG (900 µg/ml) in PBS containing 10 mg/ml BSA, 3 mm NaN3, 20 µm Zn2+ (PBS‐BSA‐Zn‐Az) in an Eppendorf tube. Tubes were rotated for 1 hr at 37°, and then the mixtures were further rotated for 12 hr at 37° without or with addition of 150 µg/ml of HRG. In some experiments different concentrations of HRG were incubated with preformed IIC containing anti‐ovalbumin IgG (900 µg/ml) and ovalbumin at equivalence antigen: antibody ratio to assess the effect of different concentrations of HRG on the solubilization of preformed IIC. In all instances, after 12‐hr rotation of IIC with or without HRG, the samples were centrifuged at 104 g for 1 min to collect IIC. The pellets were washed three times in PBS and then dissolved in 0·2 m NaOH. The absorbance of each solution was measured at 280 nm assuming that the extinction coefficient of 0·1% (w/v) solution = 1·4 for IgG. The percent precipitation was calculated for each sample and was plotted against the ovalbumin/IgG ratio (w/w) or plotted against the concentration of HRG.
Coupling of streptavidin to the dextran matrix
An IAsys resonant mirror biosensor (Affinity Sensors, Cambridge, UK)27,28 with a carboxymethyl‐dextran sensing cuvette was used to determine the kinetic constants and affinities of the binding of RF to immobilized IgG1κ. STP was coupled via ɛ‐amino groups to the carboxymethyl‐dextran sensing surface of the IAsys biosensor cuvette using EDC and NHS.27,28 This was done by equilibrating the cuvette in PBS buffer containing 0·05% Tween‐20 (PBS‐T) and then reacting the cuvette with a mixture of EDC/NHS for 7 min. Unreacted EDC/NHS was washed away with PBS‐T followed by three washes with 0·01 m sodium acetate buffer (pH 4·5). STP (50 µg/ml) in acetate buffer was added to the cuvette and allowed to react with the activated carboxyl groups for 5 min. Uncoupled STP was removed by washing with acetate buffer, and unreacted succinimidyl groups were blocked by incubating with ethanolamine (1 m, pH 8·5) for 2 min. The cuvette was washed three times with acetate buffer, and then washed with PBS‐T followed by a wash with 10 mm HCl to remove any non‐covalently bound protein. Consistent with studies using other proteins29 a biosensor response of ˜500 arc seconds was observed when STP was immobilized to the sensing surface. According to data provided by the manufacturer this response represents the coupling of 3 ng of STP per mm2 of sensing surface.
Biotinylation of proteins and their binding to the biosensor surface
The proteins to be biotinylated (e.g. human IgG and IgG1κ) were dissolved in PBS (pH 7·2) at a concentration of 1 mg/ml and then reacted with NHS‐LC‐biotin (1 mg/ml) (Pierce, Rockford, IL) for 30 min at room temperature. The reaction was stopped by the addition of Tris–HCl buffer (pH 8·0) to a final concentration of 100 mm. Unreacted biotin was removed by washing the sample extensively (five cycles of concentration and dilution) in a Centricon 10 microconcentrator (Amicon Inc, Danvers, MA), before storing the biotinylated proteins at –20° in small aliquots until use.
Biotinylated IgG1κ was coupled to the immobilized STP as follows. Biotinylated IgG1κ (50 µg/ml) in PBS‐T was added to the STP‐coupled dextran cuvette equilibrated in PBS‐T, and allowed to bind to the immobilized STP for 5 min. Non‐specifically bound biotinylated protein was removed by washing three times with PBS‐T, followed by three washes with 10 mm HCl for 2 min (for three cycles).
Binding of protein ligands to proteins on the biosensor surface
Except where indicated, all experiments were performed in PBS containing 0·05% Tween‐20 and 10 mg/ml BSA (PBS‐BSA‐T) and at a temperature of 25°. The BSA (1%, w/v) was included in the buffer to reduce non‐specific binding. The reaction vessel was stirred continuously by the aid of a propeller. Binding was measured at 2‐s intervals, and the readout from the biosensor was in units of arc‐sec. Each binding reaction was routinely followed for 5 or 10 min. All binding experiments were performed at least in duplicate. The ‘Fast Fit’ program supplied by Fisons (Cambridge, UK) was used to evaluate the kinetic constants.23
Before immobilization of the biotinylated proteins on the STP‐dextran, non‐specific binding between the protein ligands and the STP‐dextran was assessed by the addition of different amounts of the non‐biotinylated protein ligands in BSA‐PBS‐T to the cuvette. Under these conditions no significant level of non‐specific binding of any of the protein ligands used in this study could be detected.
Preliminary experiments were performed to establish the concentration range of RF suitable for kinetic analysis. PBS‐T was added to the protein‐coupled cuvette to establish a baseline (5 min) and RF was then added in PBS‐T at different concentrations. Binding of the RF was studied by monitoring the association phase for 5 min. Subsequently, the cuvette was washed with PBS‐T and the dissociation phase was monitored for 5 min. Bound RF was removed (cuvette regeneration) by washing with either 10 mm HCl or the gentle Ag/Ab elution buffer (Pierce, Rockford, IL). The baseline was then re‐established after washing the cuvette with PBS‐T. In the HRG inhibition experiments, HRG (15 µg/ml) was added to the cuvette, allowed to reach equilibrium for 5 min and then unbound HRG washed away before addition of RF. As previously,23 we found no evidence that the 10 mm HCl cuvette regeneration wash significantly affected the ability of the proteins to subsequently interact with immobilized proteins on the cuvette surface.
Results
Formation of IIC containing human IgG and anti‐human IgG antibodies
Previous studies have shown that the formation of IIC between ovalbumin and anti‐ovalbumin IgG can be studied in vitro by monitoring changes in IIC particle size by absorbance measurements at 350 nm.23 In the present work IIC containing human IgG and anti‐human IgG antibodies were formed by: (1) the incubation of polyclonal rabbit anti‐human IgG (specific for the Fc region of human IgG) with monomeric human IgG; (2) the incubation of human RF with heat‐aggregated human IgG; and (3) the incubation of human RF with monomeric human IgG.
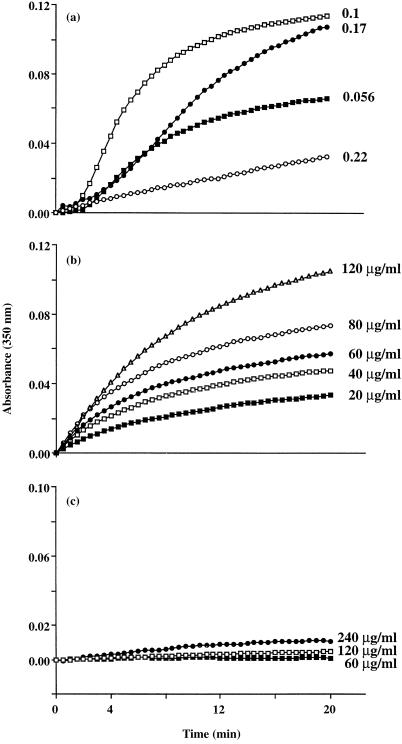
To study the formation of IIC between rabbit anti‐human IgG and monomeric human IgG, rabbit anti‐human IgG (69 µg/ml) was incubated in PBS‐BSA buffer and the formation of IIC was initiated by the addition of human IgG at different concentrations in the range 3·9–15·5 µg/ml (Ag : Ab ratio of ˜0·056–0·22). The increase in the average size of the IIC formed in the suspension was monitored by measuring the absorbance of the turbid suspension at 350 nm (as described in Materials and Methods) for 20 min (Fig. 1a). These data show that incubation of rabbit anti‐human IgG with monomeric human IgG (from pooled human serum) resulted in the formation of IIC, with maximum formation occurring at an Ag : Ab ratio of 0·1. To determine whether the nature of the light chain of human IgG (κ or λ), as antigen, affects the formation of IIC, IIC formation also was carried out by mixing human IgG1κ or IgG1λ with rabbit anti‐human IgG. These experiments indicated that the light chain of human IgG (as antigen) has no effect on the formation and precipitation of IIC (not shown).
Figure 1.
Formation of IIC between human IgG and either rabbit anti‐human IgG antibodies or RF as measured by light scattering at 350 nm. The results in (a) show the increase in absorbance as a function of time caused by formation of IIC containing rabbit anti‐human IgG (69 µg/ml) and human IgG at the antigen : antibody ratios of 0·056 (▪), 0·1 (□). 0·17 (•) and 0·22 (○). The results in (b) show the increase in absorbance as a function of time caused by formation of IIC containing human RF (8 IU/ml) and aggregated human IgG at concentrations of 20 (▪), 40 (□), 60 (•), 80 (○) and 120 (▵) µg/ml. The results in (c) show the increase in absorbance as a function of time caused by formation of IIC containing human RF (8 IU/ml) and monomeric human IgG at concentrations of 60 (▪), 120 (□) and 240 (•) µg/ml. Data are representative of three separate experiments.
The formation of IIC between human RF and aggregated human IgG was carried out by the addition of RF (8 IU/ml) to different concentrations of heat‐aggregated human IgG (20–120 µg/ml) in PBS‐BSA buffer. Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) analysis showed that the majority of RF used in these studies is the IgG form (not shown). As can be seen from the data in Fig. 1(b) the formation of IIC under these conditions was dependent on the concentration of heat‐aggregated IgG used in the incubations. Interestingly, our experiments also indicate that little if any insolubilization and formation of IIC between RF and monomeric human IgG occurs in this system (Fig. 1c).
HRG blocks the formation of IIC between human IgG and anti‐human IgG antibodies
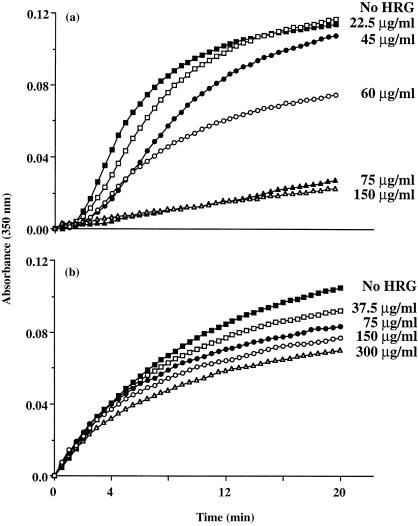
The effect of HRG on the formation of IIC between rabbit anti‐human IgG (69 µg/ml) and monomeric human IgG (6·9 µg/ml) was examined. As shown in Fig. 2(a) preincubation of rabbit anti‐human IgG and human IgG with HRG at 22·5, 45 and 60 µg/ml inhibited the formation of IIC in a dose‐dependent manner. Although little effect was seen at 22·5 µg/ml HRG, higher concentrations (75 and 150 µg/ml) of HRG resulted in an almost complete blockage of the formation of IIC in this system.
Figure 2.
Effect of HRG on the formation of IIC between human IgG and either rabbit anti‐human IgG antibodies or human RF as measured by light scattering at 350 nm. The results in (a) show the increase in absorbance as a function of time due to formation of IIC containing human IgG and rabbit anti‐human IgG (69 µg/ml) at equivalence antigen : antibody ratio for a control experiment (▪ no additions), and for experiments carried out in the presence of 22·5 (□), 45 (•), 60 (○), 75 (▴) and 150 (▵) µg/ml of human HRG. The results in (b) show the increase in absorbance as a function of time due to formation of IIC containing RF (8 IU/ml) and aggregated human IgG (120 µg/ml) for a control experiment (▪ no additions), and for experiments carried out in the presence of 37·5 (□), 75 (•), 150 (○) and 300 (▵) µg/ml of human HRG. Data are representative of three separate experiments.
Interestingly, the preincubation of RF (8 IU/ml) with different concentrations of HRG in the range 37·5–300 µg/ml resulted in a HRG concentration‐dependent inhibition of the formation of IIC between RF and heat‐aggregated human IgG (˜120 µg/ml) (Fig. 2b). However, HRG was much less effective at inhibiting IIC formation in this system (compare Fig. 2a, b)
HRG blocks the formation of IIC between RF and STP‐aggregated biotinylated human IgG
Because HRG was relatively ineffective at blocking the formation of IIC between human RF and heat‐aggregated human IgG another form of aggregated human IgG was investigated as antigen. In particular, there was the concern that heat treatment may destroy HRG binding sites on human IgG. Thus, it was considered essential to establish whether HRG could block the formation of IIC between RF and biotinylated human IgG (b‐IgG) aggregated with STP.
Experiments were conducted to determine the optimum ratio of STP: b‐IgG to produce aggregated b‐IgG which subsequently formed IIC upon addition of RF (8 IU/ml). The b‐IgG (30 µg/ml) was incubated with different concentrations of STP (6–60 µg/ml) in PBS‐BSA‐Zn in a quartz reaction cuvette for 20 min at 37°. It should be noted that cross‐linking b‐IgG with STP resulted in no significant detectable light scattering (Fig. 3a). However, addition of RF to some solutions of STP‐b‐IgG resulted in an increase in absorbance due to light scattering by IIC. These experiments indicated that the formation of IIC with RF (8 IU/ml) and STP‐b‐IgG is optimal when 18 µg/ml of STP is used for b‐IgG cross‐linking (Fig. 3a).
Figure 3.
Effect of HRG on the formation of IIC between RF and STP aggregated biotinylated human IgG (b‐IgG). In (a) b‐IgG (30 µg/ml) was incubated with STP at 6 (▪), 12 (□), 18 (•), 24 (○) and 60 (▴) µg/ml for 20 min, then RF (8 IU/ml) was added to the STP–b‐IgG complexes and IIC formation was monitored by light scattering. In (b) b‐IgG was preincubated without (▪) or with human HRG at 15 (□), 30 (•), 75 (○), and 150 (▴) µg/ml for 20 min before aggregation by streptavidin (STP) (18 µg/ml) for 20 min and the addition of RF (8 IU) to form IIC. Data are representative of three separate experiments.
The effect of HRG on the formation of IIC between RF and STP‐b‐IgG was examined by preincubating b‐IgG (30 µg/ml) with different concentrations of HRG (15–150 µg/ml) for 20 min before the addition of STP (18 µg/ml). After incubating this mixture for 20 min RF was added to initiate the formation of IIC. Gel filtration studies showed that the presence of HRG had no effect on the cross‐linking of b‐IgG by STP (data not shown). The data show that preincubation of IgG with HRG inhibits formation of IIC; inhibition was significant at 15 and 30 µg/ml HRG, with over 60% to 80% blockage of IIC formation occurring at 75 and 150 µg/ml HRG, respectively (Fig. 3b). This is similar to the inhibitory effect of HRG on the formation of IIC between human IgG and rabbit anti‐human IgG (compare Figs 2a and 3b).
Use of optical biosensor to examine the effect of HRG on RF–IgG interaction
Because HRG is an IgG binding protein23 it may mask epitopes on IgG recognized by RF. Thus, an IAsys optical biosensor was used to study the binding of human RF to IgG and the effect of human HRG on this interaction. Human IgG1 possessing the κ light chain (IgG1κ) was biotinylated and bound to STP immobilized onto the sensing surface of an IAsys dextran cuvette. The binding of RF to immobilized IgG1κ, which may resemble immobilized IC, was carried out by the addition of RF to the cuvette at different concentrations in the range 2–16 IU/ml in PBS‐T‐BSA. In this study, the concentration of RF is expressed as IU/ml (instead of molarity) as most published work and clinical evaluations of RF levels in patients use this unit. As shown in Fig. 4(a) the binding of RF to IgG1κ over a 10‐min period exhibited saturation kinetics with near maximal binding occurring at ˜16 IU/ml RF. The biosensor signal obtained was specific for the binding of RF to the immobilized IgG1κ because preincubation of the RF (8 IU/ml) with 120 µg/ml of soluble IgG1κ inhibited the binding of RF to immobilized IgG1κ (not shown). Analysis of the data using the ‘Fast Fit’ program showed that the association curves could be fitted to a single exponential. For each binding curve the observed rate constant (kobs) was determined and plotted against the titre of RF. As shown in Fig. 4(b) the plot of kobs against the RF concentration (IU/ml) approximated a straight line with the slope or on‐rate being 14·92 ± 2·43 × 10–5 IU/ml/s and the y‐intercept (off‐rate) for the interaction being 1·69 ± 0·41 × 10–3/s.
Figure 4.
Interaction of RF with immobilized human IgG1κ. (a) Human IgG1κ immobilized onto the sensing surface of a biosensor cuvette was reacted with different concentrations (2–16 IU/ml) of RF, the overlay plots representing the binding of different RF concentrations to the immobilized IgG1κ. (b) The value of kobs for the binding curve for each RF titre was determined using the linearization method (Fast Fit program) and each value plotted against the concentration of RF. The plot of kobs against RF concentration (IU/ml) approximates a straight line (•); the slope represents kon and the y‐intercept represents koff for this interaction. (c) In some experiments before monitoring the binding of RF (8 IU/ml) to IgG1κ the cuvette was either untreated (no HRG) or pretreated (+HRG) with HRG (15 µg/ml) for 5 min. Each data point in (b) represents the mean ± SEM obtained from three separate experiments. The data in (a) and (c) are representative of three separate experiments.
The ability of HRG to bind with high affinity to immobilized IgG23 raised the question of whether HRG can mask epitopes on IgG1κ recognized by RF. Consistent with the data shown in Fig. 4(a), the addition of 8 IU/ml of RF in PBS‐T‐BSA to the cuvette increased the binding signal (association pattern, no HRG) (Fig. 4c). However, the addition of RF (8 IU/ml) to a cuvette containing immobilized IgG1κ that had been pretreated with 15 µg/ml HRG did not result in an increased binding signal, suggesting that HRG blocks the binding of RF to immobilized IgG1κ. Interestingly, under these conditions the addition of RF to the HRG pretreated cuvette actually showed a dissociation response, rather than the expected association (see Fig. 4c, +HRG). This result may be caused by RF displacing some of the HRG bound to the immobilized IgG1κ. As the immobilization of b‐IgG on the sensing surface may have created cross‐linked IgG molecules resembling IC, the biosensor data further supports the view that HRG has the ability to block the binding of RF to IgG containing IC.
HRG promotes solubilization of already formed IIC
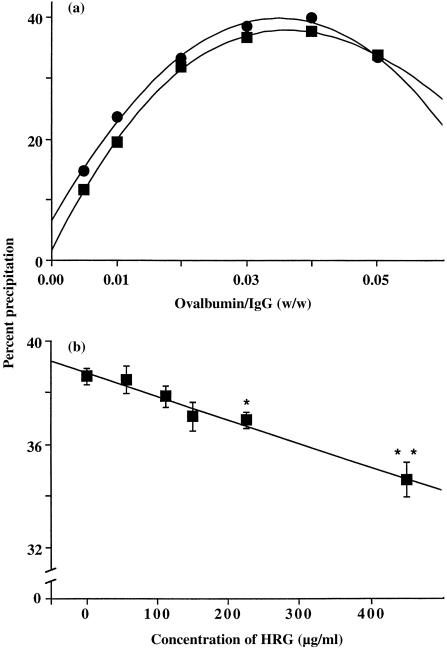
Activation of the classic and alternative complement pathways has been shown to inhibit the formation of IIC and to promote solubilization of already formed IIC.30 Therefore, it was important to examine whether HRG, apart from inhibiting the formation of IIC and regulating the binding of RF to IgG containing IC, could promote the solubilization of already formed IIC. Different concentrations of ovalbumin were added to rabbit anti‐ovalbumin IgG (900 µg/ml) to form IIC and the IIC either left untreated, or treated with HRG (150 µg/ml), and then incubated overnight at 37°. After incubation, the IIC that remained in each sample were collected by centrifugation, dissolved in NaOH, and the absorbance of each solution was measured to determine the percent IgG precipitation as described in the Materials and Methods. A comparison of the precipitin curves shown in Fig. 5(a) indicates that there was slightly less precipitate in the incubations in which HRG was included with the preformed IIC at almost all Ag : Ab ratios.
Figure 5.
Effect of HRG on solubilization of already formed IIC. In (a) IIC were formed between ovalbumin and anti‐ovalbumin IgG (900 µg/ml) at different antigen : antibody ratios (0·005–0·05) and then HRG (150 µg/ml) was examined for its ability to solubilize the IIC overnight at 37° (▪) and compared with IIC incubated in the absence of HRG (•). Data expressed as percent precipitation of anti‐ovalbumin IgG (for details see Materials and methods). (b) Shows the ability of different concentrations of HRG, following incubation overnight at 37°, to solubilize ovalbumin antiovalbumin IgG (900 µg/ml) IIC formed at an equivalence (0·03) antigen: antibody ratio. Each data point in (b) represents mean ± SEM of three experiments. Asterisks indicate the ability of different concentrations of HRG to significantly solubilize the preformed IIC when compared to IIC formation in the absence of HRG, i.e. *P = 0·02, **P = 0·006.
To explore whether the effect of HRG on the solubilization of already formed IIC is dependent on the concentration of HRG, IIC were formed at the equivalence Ag : Ab ratio (i.e. 0·03), and then incubated with different concentrations (56–450 µg/ml) of HRG. The results indicate that HRG promotes solubilization of already formed IIC, and that the solubilization is dependent on the concentration of HRG (Fig. 5b), with very significant solubilization (P < 0·006) occurring when the molar ratio of HRG : IgG is ˜1, i.e. 450 µg/ml HRG. There was also significant solubilization of IIC at 225 µg/ml (P = 0·02) of HRG, with 150 µg/ml of HRG producing almost significant solubilization (P = 0·07).
Discussion
Our previous studies23 demonstrated that HRG can inhibit the formation of IIC. Thus, it was important to examine whether HRG could also inhibit formation of IIC resulting from the interaction of RF with human IgG. In order to answer this question three RF models were used. The first involved a xenogeneic system, namely the interaction of rabbit IgG specific for human IgG (Fc region‐specific) with monomeric human IgG. The other two systems entailed the formation of IIC between human RF and human IgG aggregated either by heat or by cross‐linking with STP following biotinylation. It was found that, in order for RF to form IIC, the human IgG needed to be aggregated (Fig. 1b, c), indicating that RF may bind weakly to monomeric IgG, and that multimeric interactions may be required for the formation of IIC. These results are consistent with the reported binding affinity of RF for IC being 100‐fold higher than for monomeric IgG.4,11
A major finding from the present work is that in all three models of the RF–human IgG interaction, HRG blocks the formation of IIC. It is interesting to note that with the rabbit anti‐human IgG–human IgG interaction and the RF‐STP‐aggregated b‐IgG interaction, HRG could completely inhibit IIC formation (Figs 2a and 3b). This effect was not observed in the rabbit anti‐ovalbumin/ovalbumin interaction, where HRG only partially inhibited IIC formation.23 This difference is probably caused by HRG both directly blocking the epitopes on human IgG recognized by RF and inhibiting insolubilization of RF‐human IgG‐containing IC. In the case of the ovalbumin/anti‐ovalbumin system HRG would only inhibit IC insolubilization. The failure of high concentrations (300 µg/ml) of HRG to completely block the formation of IIC between human RF and heat‐aggregated human IgG (Fig. 2b) may be an indication of inefficient binding of the HRG to aggregated human IgG and/or possible alterations in the HRG binding site upon exposure to heat.
To clarify whether HRG inhibits IIC formation by masking epitopes on IgG recognized by RF, additional studies were performed using the optical biosensor. These studies indicated that human RF binds to immobilized human IgG1κ (presumably mimicking immobilized IC) in a concentration‐dependent and saturable manner (Fig. 4a). In contrast, preincubation of IgG1κ immobilized on the biosensor surface with HRG resulted in a total inhibition of RF binding and, in fact, significant dissociation of bound HRG was observed. Dissociation of HRG presumably occurs through the competitive binding of the HRG to RF in solution. These biosensor data are consistent with HRG masking the site on IgG1κ recognized by the RF used in this study.
Another important finding from the present work is that the presence of HRG can promote solubilization of already formed IIC. HRG was much more effective at inhibiting the formation of IIC (50% inhibition when the HRG : IgG molar ratio was ˜1),23 than at promoting solubilization (15% solubilization occurred when the HRG was incubated with IIC at a HRG : IgG molar ratio of ˜1) (Fig. 5b), although such a result is not that surprising when one considers the poor accessibility of HRG to IIC. Nevertheless, the ability of HRG to promote solubilization of already formed IIC in vitro, suggests that HRG may play an important role in the solubilization and subsequent clearance of IIC in vivo. Furthermore, we have recently shown that the incorporation of HRG into IC leads to enhanced uptake of the IC by monocytes.24
All of the experiments described in this paper were carried out in a plasma‐free system. However, our previous studies have shown that HRG can inhibit IIC formation in whole human plasma.23 In fact, HRG appears to be the major endogenous inhibitor of IIC formation in normal human plasma, plasma depleted of HRG enhancing rather than inhibiting IIC formation and readdition of HRG restoring the inhibitory activity of plasma.23 Preliminary experiments were also performed in which it was shown that HRG could still inhibit IIC formation between RF and aggregated human IgG when 10% human plasma, depleted of HRG, was added to the reaction mixture (not shown).
The present work represents the first report that a plasma protein (i.e. HRG) is able to block the binding of RF to IC. The results also suggest that when it is incorporated in IC, HRG may prevent IC from being recognized by the surface immunoglobulins on RF‐producing B cells. From these results it is proposed therefore that HRG may directly interfere with the production of RF in vivo. These findings have major implications for the role of HRG in regulating the production of RF, and hence the regulation of the humoral immune response. Similarly, HRG‐mediated solubilization of already formed IIC has important implications for the prevention of the deposition of pathogenic IIC in tissues (e.g. synovia, kidney, blood vessel wall) in some IC‐associated diseases. The presence of HRG, by promoting the solubilization and enhanced clearance of the IIC trapped in tissues, may reduce the pathogenic effects and immunological consequences associated with the deposition of IIC.
References
- 1.Panush RS, Bianco NE, Schur DH. Serum and synovial fluid IgG, IgA, and IgM anti‐immunoglobulins in rheumatoid arthritis. Arth Rheum. 1971;14:737. doi: 10.1002/art.1780140609. [DOI] [PubMed] [Google Scholar]
- 2.Stewart JJ, Agosto H, Litwin S, et al. A solution to the rheumatoid paradox – pathologic rheumatoid factors can be tolerized by competition with natural rheumatoid factors. J Immunol. 1997;159:1728. [PubMed] [Google Scholar]
- 3.Nasu H, Chia DS, Knutson DW, Barnett EV. Naturally occuring human antibodies to the F (ab′)2 portion of IgG. Clin Exp Immunol. 1980;42:378. [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson PM, Page Faulk W. Rheumatoid factor: its nature, specificity, and production in rheumatoid arthritis. Clin Immunol Immunopathol. 1976;6:414. doi: 10.1016/0090-1229(76)90094-5. [DOI] [PubMed] [Google Scholar]
- 5.Capra JD, Winchester RJ, Kunkel HG. Cold‐reactive rheumatoid factors in infectious mononucleosis and other diseases. Arthr Rheum. 1969;12:67. doi: 10.1002/art.1780120202. [DOI] [PubMed] [Google Scholar]
- 6.Carson DA, Pasquali J‐l, Tsoukas CD, et al. Physiology and pathology of rheumatoid factors. Springer Semin Immunopathol. 1981;4:161. doi: 10.1007/BF01857093. [DOI] [PubMed] [Google Scholar]
- 7.Schifferli JA. Complement and immune complexes. Res Immunol. 1996;147:109. doi: 10.1016/0923-2494(96)87183-5. [DOI] [PubMed] [Google Scholar]
- 8.Walport MJ, Davies KA. Complement and immune complexes. Res Immunol. 1996;147:103. doi: 10.1016/0923-2494(96)87182-3. [DOI] [PubMed] [Google Scholar]
- 9.Mellow GH, Clarkson AB. Trypanosoma lewisi: enhanced resistance in naive lactating rats and their suckling pups. Exp Parasitol. 1982;53:217. doi: 10.1016/0014-4894(82)90063-7. [DOI] [PubMed] [Google Scholar]
- 10.Heyman B. The immune complex: possible ways of regulating the antibody response. Immunol Today. 1990;11:310. doi: 10.1016/0167-5699(90)90126-t. [DOI] [PubMed] [Google Scholar]
- 11.Normansell DE. Anti‐globulins in rheumatoid arthritis sera. II. The reactivity of anti‐globulin rheumatoid factors with altered G‐globulin. Immunochemistry. 1971;8:593. doi: 10.1016/0019-2791(71)90200-x. [DOI] [PubMed] [Google Scholar]
- 12.Pisko EJ, Turner RA, Foster SL. Induction of human rheumatoid factor producing cells by aggregated IgG. Arthr Rheum. 1982;25:1108. doi: 10.1002/art.1780250912. [DOI] [PubMed] [Google Scholar]
- 13.Coulie P, Van Snick J. Rheumatoid factors and secondary immune responses in the mouse. II. Incidence, kinetics and induction mechanism. Eur J Immunol. 1983;13:895. doi: 10.1002/eji.1830131107. [DOI] [PubMed] [Google Scholar]
- 14.Nemazee DA, Sato VL. Induction of rheumatoid antibodies in the mouse: regulated production of autoantibody in the secondary humoral response. J Exp Med. 1983;158:529. doi: 10.1084/jem.158.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lijnen HR, De Cock F, Collen D. Turn over of histidine‐rich glycoprotein in healthy subjects and during thrombolytic therapy. Thromb Res. 1981;23:121. doi: 10.1016/0049-3848(81)90245-0. [DOI] [PubMed] [Google Scholar]
- 16.Morgan WT. The histidine‐rich glycoprotein of serum has a domain rich in histidine, proline, and glycine that binds heme and metals. Biochemistry. 1985;24:1496. doi: 10.1021/bi00327a031. [DOI] [PubMed] [Google Scholar]
- 17.Heimburger N, Haupt H, Kranz T, Baudner S. Human serum proteine mit hoher affinitat zu carboxymethylcellulose. II. Physikalisch‐chemische und immunologische: charakterisierung eines histidinreichen 3,8s‐α2‐glykoproteins (Cm‐Protein I) Hoppe‐Seyler’s Z Physiol Chem. 1972;353:1133. [PubMed] [Google Scholar]
- 18.Brown KJ, Parish CR. Histidine‐rich glycoprotein and platelet factor 4 mask heparan sulfate proteoglycans recognized by acidic and basic fibroblast growth factor. Biochemistry. 1994;33:13918. doi: 10.1021/bi00250a047. [DOI] [PubMed] [Google Scholar]
- 19.Leung LLK, Nachman RL, Harpel PC. Complex formation of platelet thrombospondin with histidine‐rich glycoprotein. J Clin Invest. 1984;73:5. doi: 10.1172/JCI111206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung LLK. Interaction of histidine‐rich glycoprotein with fibrinogen and fibrin. J Clin Invest. 1986;77:1305. doi: 10.1172/JCI112435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang N‐s, Leu RW, Rummage JA, Anderson JK, Mole JE. Regulation of complement functional efficiency by histidine rich glycoprotein. Blood. 1992;79:2973. [PubMed] [Google Scholar]
- 22.Borza D‐b, Morgan WT. Histidine‐proline‐rich glycoprotein as plasma pH sensor. J Biol Chem. 1998;273:5493. doi: 10.1074/jbc.273.10.5493. [DOI] [PubMed] [Google Scholar]
- 23.Gorgani NN, Parish CR, Easterbrook‐Smith SB, Altin JG. Histidine‐rich glycoprotein binds to human IgG and C1q and inhibits the formation of insoluble immune complexes. Biochemistry. 1997;36:6653. doi: 10.1021/bi962573n. [DOI] [PubMed] [Google Scholar]
- 24.Gorgani NN, Altin JG, Parish CR. Histidine‐rich glycoprotein regulates the binding of monomeric IgG and immune complexes to monocytes. Int Immunol. 1999;II:1275. doi: 10.1093/intimm/11.8.1275. [DOI] [PubMed] [Google Scholar]
- 25.Rylatt DB, Sia DY, Mundy JP, Parish CR. Autorosette inhibition factor: Isolation and properties of the human plasma protein. Eur J Biochem. 1981;119:641. doi: 10.1111/j.1432-1033.1981.tb05655.x. [DOI] [PubMed] [Google Scholar]
- 26.Easterbrook‐Smith SB, Vandenberg RJ, Alden JA. The role of Fc : Fc interactions in insoluble immune complex formation. Molec Immunol. 1988;25:1331. doi: 10.1016/0161-5890(88)90048-x. [DOI] [PubMed] [Google Scholar]
- 27.Cush R, Cronin JM, Stewart WJ, Maule CH, Molloy J, Goddard NJ. The resonant mirror: a novel optical biosensor for direct sensing of biomolecular interactions. Part I: Principles of operation and associated instrumentation. Biosensors Bioelectron. 1993;8:347. [Google Scholar]
- 28.Buckle PE, Davies RJ, Kinning T, et al. The resonant mirror: a novel optical biosensor for direct sensing of biomolecular interactions. Part II: applications. Biosensor Bioelectron. 1993;8:355. [Google Scholar]
- 29.George AJT, French RR, Glennie MJ. Measurement of kinetic binding constants of a panel of anti‐saporin antibodies using a resonant mirror biosensor. J Immunol Methods. 1995;183:51. doi: 10.1016/0022-1759(95)00031-5. [DOI] [PubMed] [Google Scholar]
- 30.Schifferli JA, Steiger G, Paccaud JP. Complement mediated inhibition of immune precipitation and solubilization generate different concentrations of complement anaphylatoxins. Clin Exp Immunol. 1986;64:407. [PMC free article] [PubMed] [Google Scholar]