Abstract
The interaction of immunoglobulin G (IgG) antibodies with FcγR constitutes a critical mechanism through which IgG antibody effector functions are mediated. In the current work we have examined whether human neutrophil FcγR exhibit pH dependence in their association with IgG. Binding assays were performed in culture medium adjusted to different pH values. It was found that the binding of either heat‐aggregated human IgG (AIgG), soluble immune complexes (sIC) or IgG‐coated erythrocytes (IgG‐E) was markedly higher at pH 6·5 than at pH 7·3. This effect was not observed when saturation of FcγR was achieved, suggesting that acidic pH increases the avidity of FcγR for IC without modifying the total binding capacity. Similar results were observed for the binding of AIgG to either monocytes, natural killer (NK) or K562 cells, suggesting that acidic pH increases the avidity of both, FcγRII and FcγRIII. Additional experiments were performed to analyse whether the binding of IgG to FcγRI also showed pH dependence. To this aim, we employed interferon‐γ‐treated human neutrophils and mouse inflammatory macrophages, previously incubated with blocking antibodies directed to FcγRII and FcγRIII. Acidic pH did not enhance the binding of AIgG nor monomeric IgG under these experimental conditions. Further studies are required to determine whether the enhancement of FcγR avidity for IC could be attributed to titration of histidine(s) residues on the Fc fragment of IgG.
Introduction
Three major classes of human receptors for the Fc portion of immunoglobulin G (IgG) molecules (FcγR) have been defined, FcγRIII (CD16), FcγRII (CD32), and FcγRI (CD64). They are present on most haematopoietic cells, including B, T, and natural killer (NK) cells, monocytes, macrophages and polymorphonuclear leucocytes. Most cell types co‐express different forms of FcγR which bind IgG with different affinities. Thus, FcγRI binds monomeric IgG with high affinity, while FcγRII and FcγRIII bind primarily immune complexes (IC), having low affinity for the monomeric ligand.1–3
FcγR act as signal‐transducing molecules and thus provide an important link between cellular and humoral branches of the immune system. In fact, the interaction of FcγR with IC triggers a variety of cell responses such as phagocytosis, antibody‐dependent cellular cytotoxicity (ADCC), and secretion of several inflammatory mediators and cytokines.1–5
The FcγR expressed by haematopoietic cells are structurally related, hetero‐oligomeric molecules in which the ligand‐binding subunit is represented by tandemly repeated immunoglobulin‐like domains.6,7 In contrast, neonatal Fc receptor (FcRn), which transports maternal IgG to the bloodstream of the fetus and the newborn and also regulates IgG homeostasis, is structurally similar to class I major histocompatibility complex (MHC) molecules.7,8 FcRn and FcγR appear also to differ in the stoichiometry of their interaction with IgG. An important feature of the interaction among the IgG Fc fragment and FcRn is that the Fc is functionally divalent, thus each Fc fragment may bind to two FcRn molecules simultaneously. Dimerization of FcRn molecules by bridging IgG Fc might lead to the appropriate trafficking of complexes within and across cells. By contrast, the IgG Fc fragment appears to interact with FcγR in a 1 : 1 stoichiometry. IgG monovalency for FcγR account for the inability of monomeric IgG to trigger cell responses, because it was unable to induce FcγR aggregation.9,10
Previous work has shown that FcRn exhibits pH dependence in its association with monomeric IgG.11,12 To our knowledge, no previous report has examined the effect of extracellular pH on the binding of IC to FcγR. Considering that acidic pH is a frequent feature in inflammatory areas,13–15 in the present study we analyse whether the binding of IC to leucocyte FcγR is increased at acidic pH.
Materials and methods
Reagents
Monoclonal antibodies (mAb) 3G8 F(ab′)2 and IV3 (IgG2b), which recognize human FcγRIII and FcγRII, respectively, were obtained from Medarex, Inc. (West Lebanon, NH). Human IgG was purchased from Sigma Lab. (St Louis, MO). IgG aggregates were prepared by heating human IgG at a concentration of 5 mg/ml for 12 min at 63°. Then, AIgG was centrifuged at 10 000 g for 5 min and the precipitate was discarded. Soluble IC, prepared at fivefold antigen excess using human IgG as antigen and specific rabbit IgG antibodies to human IgG and monomeric human IgG, were obtained as we previously described.16
Culture medium
The standard medium used in this study was RPMI‐1640 (Gibco, Detroit, MI) supplemented with 1% heat‐inactivated fetal calf serum (Gibco) (tissue culture medium (TCM), pH 7·3). It was adjusted to different pH values by the addition of different amounts of isotonic HCl or NaOH.
Cell isolation
Blood samples were obtained from healthy donors who had taken no medication for at least 10 days before the day of sampling. Blood was obtained by venepuncture of the forearm vein, and it was drawn directly into plastic tubes containing 3·8% sodium citrate (1 : 9 v/v). Human neutrophils were isolated by dextran sedimentation and Ficoll–Hypaque gradient centrifugation (Ficoll, Pharmacia, Uppsaala, Sweden; Hypake, Winthrop Products Inc., Buenos Aires, Argentina), as described.17 Contaminating erythrocytes were removed by hypotonic lysis. After washing, the cells (more than 96% of neutrophils on May–Grunwald/Giemsa stained cytopreps) were resuspended at 5 × 106/ml in TCM. Peripheral blood mononuclear cells were harvested from the interphase of Ficoll–Hypaque gradient. After washing, the cells were resuspended in TCM. The purified cells contained 95–98% mononuclear cells and 2–5% polymorphonuclear cells (PMN). Murine peritoneal macrophages were obtained from BALB/c mice, injected intraperitoneally 3 days before macrophage harvest with 1·5 ml of aged 3% sterile thioglycollate broth. The peritonea of the animals were surgically exposed by use a midline incision. Cells (70–80% macrophages) were harvested by injecting 5 ml of saline into the peritoneal cavity followed by aspiration.
Flow cytometry analysis
Human neutrophils (5 × 106/ml) were incubated for different times at 4° with AIgG or sIC (10–3000 µg/ml) in culture medium adjusted to different pH values. Then, cells were fixed with 1% paraformaldehyde (1 hr at room temperature), washed six times with phosphate‐buffered saline (PBS) and stained for 30 min at 4° with fluoroscein isothiocyanate (FITC)‐conjugated F(ab′)2 rabbit antihuman IgG. To determine the binding of AIgG to human monocytes and NK cells, peripheral blood mononuclear cells (PBMC) (5 × 106/ml) were incubated, at different pH, for 30 min at 4° with 50 µg/ml AIgG. Then, cells were fixed with 1% paraformaldehyde and the binding of AIgG to each population was determined by two‐colour immunofluorescence using phycoerythrin (PE)‐conjugated mAb anti‐CD14 and anti‐CD56, respectively, and FITC‐conjugated F(ab′)2 rabbit anti‐human IgG. Flow cytometry was performed on a fluorescence‐activated cell sorter (FACS) analyser (Becton‐Dickinson Immunocytometry System, San Jose, CA). Fluorescence intensity was determined on 10 000 cells from each sample. As fluorescence distribution was unimodal, results were expressed as specific mean fluorescence intensities (MFI) calculated after subtracting non‐specific fluorescence.
EA rosetting
Rosetting was performed as we previously described.18 Briefly, human neutrophils (15 µl, 5 × 106/ml) and chicken erythrocytes (E) (100 µl, 2% v/v) sensitized with subagglutinating amounts of rabbit IgG anti‐E, were incubated for 45 min at 4° in culture medium adjusted to different pH values. Rosetting was evaluated by microscopic examination. At least 100 cells were scored in each sample.
Statistical analyses
Student’s paired t‐test was used to determine the significance of differences between means and P < 0·05 was taken as statistically significant.
Results
Acidic pH increases the avidity of neutrophil FcγR for AIgG
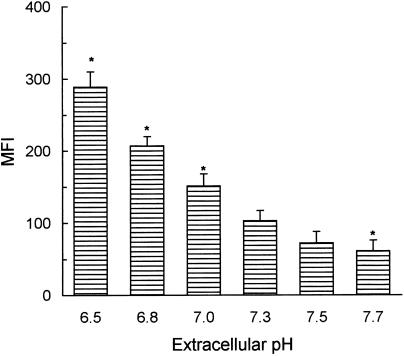
Neutrophils constitutively express two low‐affinity FcγR: FcγRII (10 000 sites/cell) and FcγRIIIb (100 000 sites/cell).1–3 In a first set of experiments we examined whether neutrophil FcγR exhibit pH dependence in their association with IC. Binding assays were performed in culture medium adjusted to different pH values using AIgG, as an IC analogous. As shown in Fig. 1, the binding of AIgG was significantly higher at acidic pH (pH ≤ 7·0) than at neutral (7·3) or basic (≥ 7·5) pH.
Figure 1.
Effect of extracellular pH on the binding of AIgG to human neutrophils. Neutrophils (5 × 106/ml) were incubated for 30 min at 4° with 50 µg/ml AIgG, in culture medium adjusted to different pH values. Cells were then fixed and stained with FITC‐conjugated F(ab′)2 rabbit anti‐human IgG. Results are expressed as MFI and represent the arithmetic mean ± SEM from 11 to 13 experiments. *Statistical significance: P < 0·01 compared to pH 7·3.
To exclude the possibility that the increased binding of AIgG at acidic pH involves non‐specific interactions between AIgG and neutrophil surface, we evaluated the ability of mAb directed to neutrophil FcγR to prevent AIgG binding. To this aim, neutrophils were treated for 30 min at 4° with the mAb 3G8 F(ab′)2 and IV.3, directed to FcγRIII and FcγRII, respectively. Concentrations of mAb three‐ to fivefold higher than those required to saturate all binding sites as determined by FACS analysis were used in these studies. After blockage, binding assays were performed by incubating neutrophils with AIgG (50 µg/ml) for an additional period of 30 min at 4° either at pH 7·3 or 6·5. It was found that, in all cases, binding of AIgG was almost completely prevented by simultaneous incubation of neutrophils with both anti‐FcγR mAb (% binding inhibition > 87, n = 5).
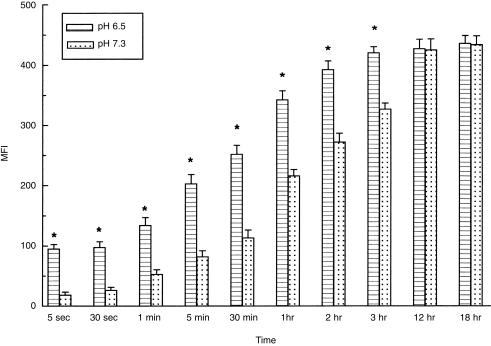
Kinetic studies were then performed by incubating neutrophils with 50 µg/ml of AIgG for different times at 4°. Results in Fig. 2 show that AIgG binding increased progressively to reach a maximum at 3 hr and 12 hr, for pH 6·5 and 7·3, respectively. Differences in the binding profiles at acidic and neutral pH were higher at early incubation times. Thus, at 5 and 30 s, binding of AIgG was almost four‐ to fivefold higher at acidic pH. At 30 min there was a two‐ to threefold higher binding at acidic pH. In contrast, no significant differences were observed at the equilibrium.
Figure 2.
Kinetics of the binding of AIgG to human neutrophils at acidic or neutral pH. Neutrophils (5 × 106/ml) were incubated for different times at 4° with 50 µg/ml AIgG, in culture medium adjusted to pH 6·5 or 7·3. Cells were then fixed and stained with FITC‐conjugated F(ab′)2 rabbit anti‐human IgG. Results are expressed as MFI and represent the arithmetic mean ± SEM from 6 to 10 experiments. *Statistical significance: P < 0·01 compared to pH 7·3.
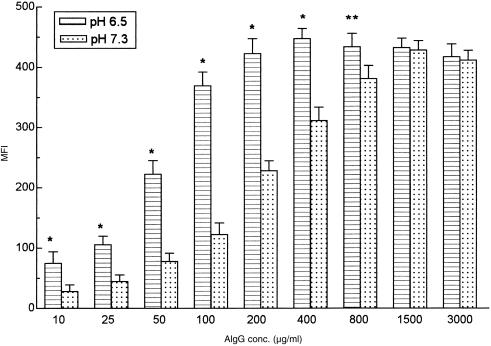
Binding studies at variable concentrations of AIgG were then performed. Data in Fig. 3 show that acidic pH markedly increased AIgG binding, when concentrations lower than those required to saturate all binding sites were employed. By contrast, there were no differences when saturation was achieved. It is important to note that this dose curve was carried out with a 30‐min incubation period, time at which binding equilibrium was only achieved at extremely high concentrations of AIgG (800–3000 µg/ml). Taking this into account, we next examined the kinetics of AIgG binding using lower concentrations of AIgG (1·0 µg/ml). Our results showed that while at early time points (30 min, 90 min and 270 min) binding of AIgG was significantly higher at acidic than at neutral pH (P < 0·001, pH 6·5 versus 7·3), there were no differences at the equilibrium (18 hr at 4°): MFI = 82 ± 19 versus 77 ± 14, pH 6·5 versus 7·3, n = 4.
Figure 3.
Effect of extracellular pH on the binding of different concentrations of AIgG to human neutrophils. Neutrophils (5 × 106/ml) were incubated for 30 min at 4° with AIgG (10–3000 µg/ml) in culture medium adjusted to pH 6·5 or 7·3. Cells were then fixed and stained with FITC‐conjugated F(ab′)2 rabbit anti‐human IgG. Results are expressed as MFI and represent the arithmetic mean ± SEM from 6 to 9 experiments. Statistical significance: *P < 0·01 and **P < 0·05, compared to pH 7·3.
Acidic pH increases the avidity of neutrophil FcγR for sIC and IgG‐E
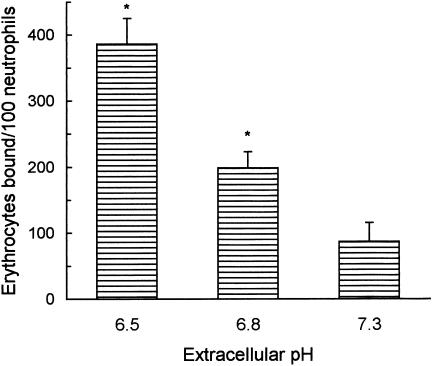
Next, we examined the effect of acidic pH on the binding of two different types of IC; particulate IC, prepared with chicken erythrocytes (E) and subagglutinating amounts of rabbit anti‐E IgG antibodies, and soluble immune complexes (sIC), prepared with human IgG and rabbit‐IgG antibodies to human IgG. Acidic pH greatly enhanced the percentage of neutrophils which bound at least one erythrocyte (% EA rosettes = 56 ± 12 versus 27 ± 7, pH 6·5 versus pH 7·3, mean ± SEM, n = 6, P < 0·01) as well as the number of erythrocytes bound to each neutrophil (Fig. 4). This effect cannot be attributable to an increased avidity of anti‐E IgG antibodies to erythrocyte surface antigens because of the acidification of culture medium. In fact, when IgG‐E were incubated for 30 min at neutral (7·3) or acidic pH (6·5), and the amount of IgG associated to erythrocyte surface was then analysed by flow cytometry, there were no differences in MFI values (245 ± 18 versus 239 ± 15, neutral versus acidic pH, X ± SEM, n = 4). In no case rosette formation was observed using unsensitized E.
Figure 4.
Effect of extracellular pH on EA rosette formation. Neutrophils (15 µl, 5 × 106/ml) and chicken erythrocytes (E) (100 µl, 2% v/v) sensitized with subagglutinating amounts of rabbit IgG anti‐E were incubated for 45 min at 4° in culture medium adjusted to different pH values. Rosetting was then evaluated by microscopic examination. At least 100 cells were scored in each sample. Results are expressed as the arithmetic mean ± SEM of seven experiments. *Statistical significance: P < 0·01 compared to pH 7·3.
By employing sIC (50 µg/ml), on the other hand, we also found that binding was markedly higher at acidic pH than at neutral pH (MFI = 135 ± 23 versus 72 ± 17, pH 6·5 versus 7·3, n = 6, P < 0·05). Gel filtration analysis of sIC carried out under neutral (pH 7·3) and acidic conditions (pH 6·5) shows no differences in the size distribution of IC (not shown), suggesting that the enhancing effect of acidic pH on sIC binding cannot be attributable to changes in the IC composition.
Acidic pH increases the avidity of FcγR expressed by monocytes, NK, and K562 cells for AIgG
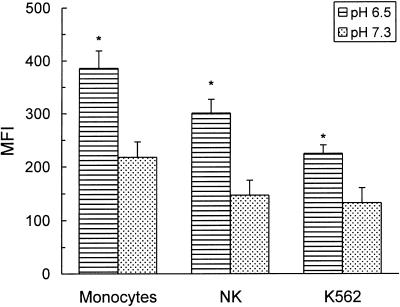
To determine whether acidic pH enhanced IgG binding to leucocyte populations other than neutrophils, PBMC were incubated with AIgG at either pH 6·5 or 7·3 for 30 min at 4°. Then, cells were fixed and the binding of AIgG to monocytes and NK cells was analysed by two‐colour immunofluorescence employing PE‐conjugated mAb anti‐CD14 and anti‐CD56, respectively. Results in Fig. 5 show that in both populations AIgG binding was significantly higher at pH 6·5 than at pH 7·3. Similar findings were observed in the erythroleukaemia cell line K562 (Fig. 5).
Figure 5.
Effect of extracellular pH on the binding of AIgG to monocytes, NK and K562 cells. PBMC (5 × 106/ml) were incubated for 30 min at 4° with 50 µg/ml AIgG in culture medium adjusted to pH 6·5 or 7·3. Then, cells were fixed and the binding of AIgG to monocytes and NK cells was determined by two‐colour immunofluorescence using PE‐conjugated mAb anti‐CD14 and anti‐CD56, respectively, and FITC‐conjugated F(ab′)2 rabbit IgG antihuman IgG. Binding of AIgG to K562 cells was measured after incubation of K562 cells (2 × 106/ml) and AIgG (50 µg/ml) for 30 min at 4°. After this time, cells were fixed and the binding of AIgG was determined as described above. Results are expressed as the arithmetic mean ± SEM of five to nine experiments. *Statistical significance: P < 0·05 compared to pH 7·3.
Acidic pH does not increase the affinity/avidity of FcγRI
The above mentioned data strongly suggest that acidic pH increases the avidity of low affinity FcγR (i.e. FcγRII and FcγRII) for immune complexes. To determine whether FcγRI also showed pH‐dependence in their association with IgG, we performed additional experiments using neutrophils treated with interferon‐γ (IFN‐γ; 100 U/ml, 18 hr at 37°). As expected, IFN‐γ‐induced FcγRI expression as revealed by flow cytometry using the mAb anti‐FcγRI 32.2 (Medarex) (not shown). Binding of AIgG (30 min at 4°) was assessed in IFN‐γ‐treated neutrophils, after blocking FcγRIII and FcγRII with the mAb 3G8 F(ab′)2 and IV.3. Our results showed that acidic pH did not enhance the binding of AIgG or monomeric IgG (50 µg/ml) to FcγRII/III‐blocked neutrophils: MFI for AIgG binding = 41 ± 9 versus 45 ± 5; MFI for monomeric IgG = 29 ± 4 versus 33 ± 5 (pH 6·5 versus 7·3, n = 4). In all cases, binding was inhibited to background levels by the mAb 197.1, a blocking antibody directed to FcγRI.
To confirm that acidic pH was unable to increase FcγRI affinity/avidity, we performed another set of experiments using inflammatory murine macrophages, which express FcγRI, II and III. As expected acidic pH enhanced the binding of AIgG (50 µg/ml) to untreated macrophages (MFI = 310 ± 25 versus 596 ± 41, pH 7·3 versus 6·5, P < 0·01, n = 4). By contrast, it did not modify the binding of AIgG to macrophages blocked with the rat mAb 2·4G2, which recognizes both, FcγRII and FcγRIII (MFI = 133 ± 15 versus 119 ± 11, pH 7·3 versus 6·5, n = 4). It was also observed that the binding of monomeric IgG to either untreated or FcγRII/III‐blocked macrophages did not differ when carried out under neutral or acidic conditions (not shown).
Discussion
In the present work, we show that the binding of AIgG, sIC or IgG‐E to human neutrophils is markedly higher at pH 6·5 than at pH 7·3. This effect appears to be due to specific interactions established between the Fc portion of IgG molecules and FcγR as blocking FcγR by specific mAb almost completely abrogates IC binding. On the other hand, the possibility that cell exposure to acidic pH could induce an over‐expression of FcγR should be ruled out considering that incubation procedures were always performed at 4°. Furthermore, when neutrophil expression of FcγRII and FcγRIII was examined using specific mAb and flow cytometry analysis, there were no differences between cells incubated at different pH values (unpublished data). A similar mechanism seems to be operative in other cell populations. Thus, we have also found that binding of AIgG to either monocytes, NK or K562 cells was significantly higher at pH 6·5 than at pH 7·3, suggesting that acidic pH increases the avidity of both low affinity FcγR, FcγRII and FcγRIII. By contrast, experiments performed with human neutrophils treated with IFN‐γ as well as with mouse inflammatory macrophages showed that acidic pH does not increase FcγRI affinity/avidity.
Neutrophil binding assays performed at variable concentrations of AIgG as well as kinetic studies showed that acidic pH enhances FcγR binding when concentrations and/or incubation periods shorter than those required to saturate all binding sites and/or achieved the equilibrium were assessed. By contrast, no enhancing effect was observed when saturation or equilibrium was achieved, suggesting that acidic pH increases the avidity of FcγR for IC without modifying total binding capacity.
Our results are consistent with the hypothesis that titration of surface accessible histidyl side chains, which show pKa values in the range of 6–7,8–10,19 could explain, at least in part, the enhancing effect of acidic pH on FcγR avidity. To our knowledge, no previous report has examined the impact of extracellular pH on the binding of IC to leucocyte FcγR. It is important to note, however, that other proteins also show pH dependence in their association with the Fc fragment of IgG. The heterodimeric FcRn, structurally similar to MHC class I molecules, binds monomeric IgG at pH 6·5 but not at pH 7·3.7–12 Although the mechanism(s) involved remains undefined, it appears to require the ionization of three to four histidines on the Fc fragment, that enables them to establish electrostatic interactions with amino acid residues sited on the FcRn.7,8 The involvement of Fc histidines suggests a mechanism through which pH‐dependent binding is brought about, as the pKa of the imidazole side‐chain is in the pH range of 6–7, resulting in an affinity of FcRn/Fc interaction in the nanomolar range.20 Previous works have also stated that another protein, the modified form of the acute phase reactant C‐reactive protein (mCRP), which is formed by a non‐proteolytic conformational change of CRP, also shows pH dependence in its association with AIgG. Binding is markedly higher at pH 5·0–6·5 than at pH 7·0–7·5.21 As in the case of FcRn, the enhancing effect of acidic pH seems to be a result of the titration of selected histidine(s) residues on the Fc fragment of IgG.21
By employing the specific histidine‐modifying reagent diethylpyrocarbonate (DEPC),22 previous reports have established that histidine residues on the Fc portion of IgG play an important role in both, the binding to staphylococcal protein A and the homologous interactions among Fc regions required to the formation of insoluble IC.23,24 To our knowledge no previous works have analysed the role of histidine residues on the Fc fragment of IgG in the binding of IC to FcγR. In an attempt to determine whether titration of these residues could account for the enhancing effect of acidic pH on IC binding to FcγR, we performed additional experiments using AIgG treated with DEPC. Assays were carried out in conditions under which other amino acids such as tyrosine and lysine were not modified. Unexpectedly, we found that DEPC‐treatment almost completely abrogated AIgG binding to neutrophils assessed either at acidic or neutral pH (% inhibition > 87% in both conditions, n = 7). This effect was reversed by hydroxilamine (D. H. López, unpublished observations), a reagent able to cleave the carbethoxyhistidine bond formed during DEPC‐treatment. While these results support a critical role for histidine residues in the binding of IgG to neutrophil FcγR, they do not allow us to define whether titration of histidine residues on the Fc fragment of IgG mediates the enhancement of FcγR avidity observed at acidic pH. Further studies using protein engineering to replace specific histidines on the Fc fragment of IgG are needed to define this point.
Immune complexes play a critical role in the pathogenesis of several infectious and autoimmune diseases.25 They interact with FcγR expressed by phagocytic cells and trigger the release of inflammatory cytokines, proteolytic enzymes, oxidative agents and other toxic molecules.1–5 Considering that acidic pH is a common feature in inflammatory areas,13–15 our results support the idea that acidosis may exacerbate the course of certain inflammatory processes by increasing the avidity of the interactions between IC and phagocytic cells. Further studies will be required to define the mechanisms through which extracellular pH affect the binding of IC to FcγR, as well as the overall impact of extracellular pH on leucocyte physiology.
Acknowledgments
This work was supported by grants from the ‘Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Buenos Aires University School of Medicine, and “Antorchas’, and “Alberto J. Roemmers” Foundations, Buenos Aires, Argentina.
Glossary
Abbreviations
- AIgG
heat‐aggregated human IgG
- IC
immune complexes
- sIC
soluble immune complexes
- IgG‐E
IgG‐coated erythrocytes
- PBMC
peripheral blood mononuclear cells
References
- 1.Unkeless JC. Function and heterogeneity of human Fc receptors for immunoglobulin G. J Clin Invest. 1989;83:355. doi: 10.1172/JCI113891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 3.Van De Winkel JGL, Capel PJA. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993;14:215. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 4.Van De Winkel JGL, Anderson CL. Biology of human immunoglobulin G Fc receptors. J Leukocyte Biol. 1991;49:511. doi: 10.1002/jlb.49.5.511. [DOI] [PubMed] [Google Scholar]
- 5.Fridman WH, Bonnerot C, Daeron M, Amigorena S, Teillaud JL, Sautes C. Structural bases of FcγR functions. Immunol Rev. 1992;125:49. doi: 10.1111/j.1600-065x.1992.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 6.Williams AF, Barclay AN. The immunoglobulin superfamily domains for cell surface recognition. Annu Rev Immunol. 1988;6:381. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- 7.Ravetch JV, Margulies DH. New tricks for old molecules. Nature. 1994;372:323. doi: 10.1038/372323a0. [DOI] [PubMed] [Google Scholar]
- 8.Burmeister WP, Gastinel LN, Simister NE, Blum ML, Bjorkman PJ. Crystal structure at 2.2 Å resolution of the MHC‐related neonatal Fc receptor. Nature. 1994;372:336. doi: 10.1038/372336a0. [DOI] [PubMed] [Google Scholar]
- 9.Burton DR, Woof JM. Human antibody effector function. Adv Immunol. 1992;51:1. doi: 10.1016/s0065-2776(08)60486-1. [DOI] [PubMed] [Google Scholar]
- 10.Jefferis R, Lund J, Pound JD. IgG‐Fc‐mediated effector functions: molecular definition of interaction sites for effector ligands and the role of glycosylation. Immunol Rev. 1998;163:59. doi: 10.1111/j.1600-065x.1998.tb01188.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodewald R. pH‐dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J Cell Biol. 1976;71:666. doi: 10.1083/jcb.71.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Story CM, Mikulska JE, Simister NE. A major histocompatibility complex class I‐like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med. 1994;180:2377. doi: 10.1084/jem.180.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edlow DW, Sheldon WH. The pH of inflammatory exudates. Proc Soc Exp Biol Med. 1971;137:1328. doi: 10.3181/00379727-137-35782. [DOI] [PubMed] [Google Scholar]
- 14.Ward TT, Steibigel RT. Acidosis of synovial fluid correlates with synovial fluid leukocytosis. Am J Med. 1978;64:933. doi: 10.1016/0002-9343(78)90446-1. [DOI] [PubMed] [Google Scholar]
- 15.Tannok IF, Rotin D. Acidic pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373. [PubMed] [Google Scholar]
- 16.Schattner M, Lazzari M, Trevani AS, et al. Activation of human platelets by immune complexes prepared with cationized human IgG. Blood. 1993;82:3045. [PubMed] [Google Scholar]
- 17.Boyum A. Separation of leukocytes from blood and bone marrow. Scand J Lab Invest. 1968;22(Suppl. 97):77. [PubMed] [Google Scholar]
- 18.Trevani AS, Andonegui GA, Isturiz MA, Schatner M, Serebrinsky G, Geffner JR. Effect of proteolytic enzymes on neutrophil FcγRII activity. Immunology. 1994;82:632. [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JK, Tsen MF, Ghetie V, Ward ES. Localization of the site of the murine IgG1 molecule that is involved in binding to the murine intestinal Fc receptor. Eur J Immunol. 1994;24:2429. doi: 10.1002/eji.1830241025. [DOI] [PubMed] [Google Scholar]
- 20.Ghete V, Ward ES. FcRn: the MHC class I‐related receptor that is more than an IgG transporter. Immunol Today. 1997;18:592. doi: 10.1016/s0167-5699(97)01172-9. [DOI] [PubMed] [Google Scholar]
- 21.Motie M, Brockmeier S, Potempa LA. Binding of model soluble immune complexes to modified C‐reactive protein. J Immunol. 1996;156:4435. [PubMed] [Google Scholar]
- 22.Shriver Z, Hu Y, Sasisekharan R. Heparinase II from Flavobacterium heparinum. Role of histidine residues in enzymatic activity as probed by chemical modification and site‐directed mutagenesis. J Biol Chem. 1998;273:10160. doi: 10.1074/jbc.273.17.10160. [DOI] [PubMed] [Google Scholar]
- 23.Haake DA, Franklin EC, Frangione B. The modification of human immunoglobulin binding to staphylococcal protein A using diethylpyrocarbonate. J Immunol. 1982;129:190. [PubMed] [Google Scholar]
- 24.O’Brien RO, Roeth PJ, Thomson SA, Bartell G, Easterbrook‐Smith SB. The effects of histidine residue modification on the immune precipitating ability of rabbit IgG. Arch Biochem Biophys. 1994;310:25. doi: 10.1006/abbi.1994.1135. [DOI] [PubMed] [Google Scholar]
- 25.Nydegger VE, Kazatchkine MD, Lamber PH. Involvement of immune complexes in diseases. In: Fougereau M, Dausset J, editors. Progress in Immunology. Vol. 4. New York: Academic Press; 1980. p. 1025. [Google Scholar]