Abstract
CD40 ligand is a costimulatory molecule which acts a potent immunomodulator. We found the mice inoculated with human CD40 ligand expression plasmid (pMEhCD40L) combined with human immunodeficiency virus type‐1 (HIV‐1) DNA vaccine exhibited both humoral and cellular antigen‐specific immunological enhancement. The expression of hCD40L induced predominantly antigen‐specific immunoglobulin G (IgG) antibody response while it failed to induce mucosal IgA response. Delayed‐type hypersensitivity (DTH) and cytotoxic T lymphocyte (CTL) activity were induced in a dose‐dependent manner. Examination of the relative levels of the two IgG subclasses showed that co‐injection of pMEhCD40L enhanced IgG2a response without suppressing IgG1 response. Similarly, the expression of pMEhCD40L enhanced not only T helper 1 (Th1)‐ but also Th2‐type cytokine production. In conclusion, co‐inoculation of pMEhCD40L with DNA vaccine was shown to be a useful way to enhance CTL responses without suppressing the humoral immune response in acquired immune deficiency syndrome (AIDS) patients.
Introduction
To control the acquired immune deficiency syndrome (AIDS) pandemic, development of an effective vaccine against human immunodeficiency virus type‐1 (HIV‐1) has a first priority in current immunology and vaccinology. Previous reports have demonstrated that HIV‐1‐specific cytotoxic T lymphocytes (CTLs) play an important role in preventing progression of AIDS or HIV disease.1–3 One of most promising techniques to achieve this goal is DNA vaccination.4–7 Although DNA vaccination is characterized by its preferential induction of CTL response, it might be more useful if it could elicit a high level of humoral response as well. For this purpose, we studied a CD40 ligand (CD40L) as a potent immunomodulator that activates both T and B lymphocytes. CD40L (CD154), a costimulatory molecule on the surface of activated helper T lymphocytes, binds to CD40 of the tumour necrosis factor‐α (TNF‐α) receptor family. In recent studies, CD40L was shown to play an important role in the interaction between antigen‐specific T lymphocyte and antigen‐presenting cells.8–13
The expression of CD40L is reported to enhance both T helper 1 (Th2)‐ and (Th1)‐type immune response.11,13–15 The expression of CD40L could therefore be expected to induce the enhancement of both humoral and cellular immune responses when used with DNA vaccine.
In the present study, we aimed to clarify (1) whether CD40L expression could enhance cellular and humoral immunity generated by DNA vaccine and (2) how Th1–Th2 dichotomy estimated from the pattern of cytokine production was changed in DNA immunization combined with CD40L.
Materials and methods
Animals and plasmids
BALB/c (H‐2d) mice (6–10 weeks old) were purchased from Japan SLC, Inc., Hamamatsu, Japan.
Plasmid pCMV160IIIB (IIIB) encoding gp160 of HIV‐1IIIB and plasmid pcREV (REV) encoding HIV‐1 rev were described previously.16
Plasmids, pMEhCD40L expressing human CD40L (hCD40L) and pMEhCD40 expressing human CD40 (hCD40) were kindly donated by Dr J. Inoue (The Institute of Medical Science, University of Tokyo, Japan). pME18S (pSR‐empty), a mock plasmid against hCD40L, was kindly donated by Dr K. Nakajima (Nagoya City University, Nagoya, Japan). As control plasmid for IIIB/REV, we used pCMVempty vector (pCMVemp).
Immunization protocol
Mice were immunized with 5 mg each of pCMV160IIIB and pcREV associated with 1–50 mg of pMEhCD40L, pMEhCD40, or mock plasmid twice at a three‐week interval. HIV‐DNA vaccine has been described previously.16–19 Direct inoculation of the DNA vaccine into the biceps femoris muscle was done as previously described.16–19 In some cases, pMEhCD40L and DNA vaccine were separately injected into contralateral legs (contralateral injection).
Antigen‐specific antibody enzyme‐linked immunosorbent assay (ELISA)
ELISA was performed as described previously.18 Blood and fecal samples were collected before immunizations and 1 week after the second immunization and stored at –40°. Fecal samples were prepared as described elsewhere.18 Recombinant gp160 protein (5 mg/ml) donated by the National Institutes of Health AIDS Research and Reference Reagent Program was coated onto 96‐microwell plates (Nunc, Roskilde, Denmark) and the wells were treated with blocking solution (1% bovine serum albumin (BSA) in phosphate‐buffered saline (PBS)). Serial dilutions of sample sera were added and allowed to react for 2 hr at 37°. After washing with 0·05% Tween‐20/PBS, the wells were treated with second antibodies, anti‐mouse immunoglobulin G (IgG), A and M antibodies. Specific antibody titres were expressed as the reciprocal value of the final detectable dilution, which gave an optical density (OD415) of ≥ 0·2 OD units compared with each preimmunized sample. IgG1 and G2a antibody titres were determined by comparison with a standard curve generated using known dilutions of high‐titred antisera.
Footpad swelling test
Footpad swelling was measured as previously described.20 Ten days after the first immunization, mice were injected with 40 mg of R10I (RGPGRAFVTI, corresponding to amino acids 318–327 from the V3 loop of HIV IIIB gp120; H‐2d restricted epitope) into each footpad. After 24 hr, the extent of footpad swelling was measured as the difference between the pre‐ and postinjected footpad thickness using a Peacock dial thickness gauge (Ozaki Co., Tokyo, Japan). Apart from these series, some immunized mice were injected with other 10‐mer peptide as control peptide for R10I.
CTL assay
Spleen cells isolated from immunized or non‐treated mice were cultured for 5 days in the presence of irradiated syngeneic spleen cells pulsed with R10I. CTL activity was determined by effector‐cell‐mediated lysis of P815 mastocytoma cells (H‐2d) pulsed with R10I in a 6‐hr chromium release assay. The effector:target (E:T) ratio ranged from 5 : 1 to 80 : 1. The percentage of specific 51Cr release was calculated as:
ELISA and enzyme linked immunosorbent spot assay (ELIspot) for cytokine measurement
Two million spleen cells from inoculated mice were restimulated in vitro with 10 mg/ml of R10I or 5 mg/ml of concanavalin A (Con A) in a 24‐well plate. After 48 hr, interleukin‐2 (IL‐2), IL‐4, and interferon‐γ (IFN‐γ) in the culture media were quantitated by Cytoscreen immunoassay kit (BioSource International, Camarillo, CA). The concentration of cytokines was determined using a standard curve prepared with recombinant murine IL‐2, IFN‐γ or IL‐4.
Cytokine ELIspot assay was performed as previously described.21 Cells were isolated from spleen and inguinal or para‐aortic lymph nodes 7 days after the second immunization. Serial threefold dilutions of a single‐cell suspension, starting with 5 × 106 cells/well, were incubated at 5% CO2, 37° for 12 hr with or without 10 mg/ml of R10I. Spots were counted in each well and the dilution was used to calculate the total number of cytokine secreting cells/sample.
Data analysis
All values were expressed as mean ± SEM. Statistical analysis of the experimental data and controls were performed using the two‐tailed Student’s t‐test or one‐way anova.
Results
The effect of co‐inoculation with hCD40L expression plasmid on immunoglobulin production
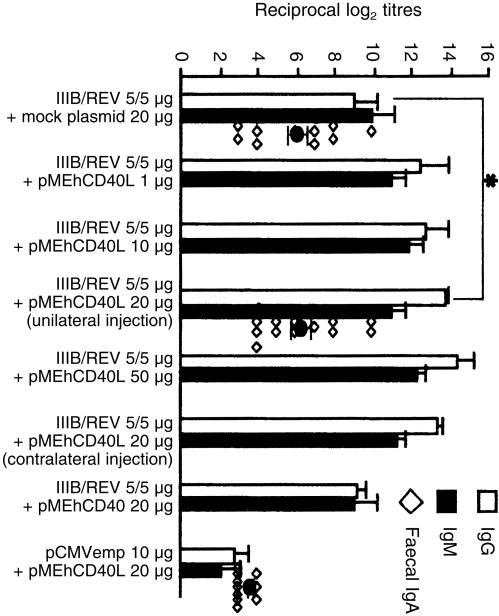
Figure 1 shows HIV‐1IIIB‐specific serum IgG and IgM titres of groups with or without pMEhCD40L. Compared with a mock plasmid group, the groups co‐inoculated with pMEhCD40L showed higher titres of antigen‐specific antibody in both IgG and IgM isotypes. Especially in IgG response, there was a significant difference between the two groups (13·8 ± 0·2 versus 9·0 ± 1·23, P = 0·023). There was no difference in fecal IgA response between these two groups (6·05 ± 0·60 versus 6·29 ± 0·61, P = 0·78). Co‐injection with pMEhCD40 did not enhance either IgG or IgM response when compared with a mock plasmid (IgG: 9·2 ± 0·49, IgM: 9·0 ± 1·225). It was suggested that the co‐inoculation with pMEhCD40L predominantly induced antigen‐specific IgG production.
Figure 1.
Antigen‐specific serum and fecal antibody titre with or without pMEhCD40L. Mice were immunized twice in 3‐week intervals with 5/5 mg of IIIB/REV associated with indicated dose of pMEhCD40L. ‘unilateral injection’ represent that IIIB/REV and pMEhCD40L were injected into the same leg and ‘contralateral injection’ represent that each of IIIB/REV and pMEhCD40L was separately injected into different legs. The fecal IgA titres were plotted as the symbol ◊. Blood and fecal samples were collected at 1 week after the second immunization. The symbol * indicates a significant difference between them (P < 0·05). Data represented the means ± SEM of five to five mice in each group. Similar experiments were performed three times.
IL‐4 promotes the switch from IgM to IgG1 while IFN‐γ promotes the switch from IgM to IgG2a, and we previously demonstrated that the balance between Th1 and Th2 response could be reflected by the ratio of IgG2a and IgG1 antibodies.22 To examine Th1/2 balance, IgG1 and IgG2a subclasses of HIV‐1IIIB‐specific serum IgG were assayed. The group co‐injected with pMEhCD40L showed slightly increased titre of HIV‐1‐specific IgG1 antibody compared with the group co‐injected with mock plasmid (161·4 ± 17·3 versus 131·5 ± 17·5, P = 0·24). The titre of antigen‐specific IgG2a antibody was significantly elevated by pMEhCD40L co‐injection compared with mock plasmid co‐injection (214·7 ± 19·9 versus 134·5 ± 19·5, P = 0·014). IgG subclasses were switched to predominant IgG2a response by addition of pMEhCD40L expression plasmid. These results suggest that expression of CD49L preferentially augments the Th1‐dependent response reflected by the IgG2a response.
Enhancement of delayed‐type hypersensitivity (DTH) response by co‐inoculation with HIV‐1 DNA vaccine and hCD40L expression plasmid
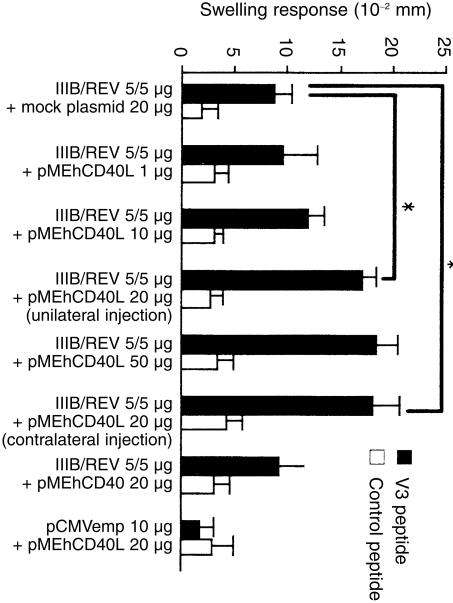
The HIV‐1‐specific DTH reaction was analysed by assessing the footpad swelling response 10 days after the first immunization. As shown in Fig. 2, mice given a co‐injection of IIIB/REV and pMEhCD40L exhibited dose‐dependent enhancement of footpad swelling response, and there was a significant difference between the pMEhCD40L 20 mg group and the mock plasmid group (17·0 ± 1·02 versus 8·67 ± 1·67 × 10–2 mm, P = 0·013). An injection of pMEhCD40L to the contralateral leg enhanced DTH response equally well (17·9 ± 2·52 versus 17·0 ± 1·02 × 10–2 mm, P = 0·75). There is no difference in DTH response (8·67 ± 1·67 versus 9·20 ± 2·6 × 10–2 mm) between the mock group and the group co‐injected with pMEhCD40. No enhancement was observed in DTH response against control peptide. The group co‐injected with control plasmid (pCMVemp) and pMEhCD40L did not show any response against IIIB peptide (1·67 ± 1·45 × 10–2 mm). These results clearly demonstrated that co‐injection with pMEhCD40L contributed to the enhancement of antigen‐specific DTH response.
Figure 2.
Footpad swelling response induced by DNA vaccine plus pMEhCD40L. Ten days after first immunization, mice were injected with 40 mg of R10I or sperm whale myoglobin peptide. The DTH responses were evaluated 24 hr after peptide injection. The symbol * indicates a significant difference between them (P < 0·05). Data represent the means ± SEM of five to five mice in each group. Similar experiments were performed three times.
Enhancement of CTL activity by co‐inoculation with HIV‐1 DNA vaccine and hCD40L expression plasmid
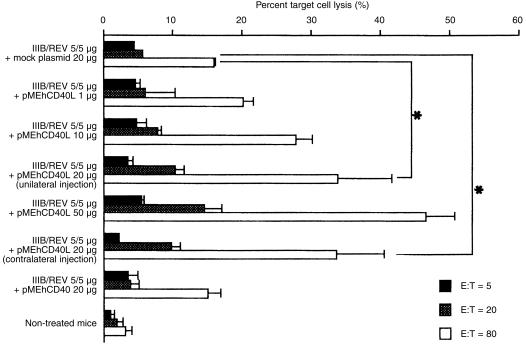
To confirm the enhancement of antigen‐specific CTL activity, we assayed V3 peptide from gp160 specific CTL activity. As shown in Fig. 3, there was a dose‐dependent enhancement of CTL activity in the group co‐injected with pMEhCD40L (F = 3·76 > Fα = 3·20). Compared with a mock plasmid group, the group co‐injected with same amount of pMEhCD40L plasmid showed a significant enhancement of CTL activity at E/T ratio of 20 and 80 (E/T = 20; 10·30 ± 0·1 versus 5·56 ± 1·37%, P = 0·050, E/T = 80; 33·89 ± 7·06 versus 16·01 ± 0·16%, P = 0·048). The group injected with pMEhCD40L and the DNA vaccine in the contralateral legs exhibited the same enhancement of CTL activity as the co‐injected group. There was no enhancement of CTL activity in the group co‐injected with pMEhCD40 compared with the mock group (15·18 ± 1·748 versus 16·01 ± 0·16%)
Figure 3.
Antigen‐specific CTL activity of pMEhCD40L‐injected mice. Two weeks after second immunization, spleen cells were isolated and restimulated in vitro with R10I peptide. Syngeneic spleen cells pulsed with R10I peptide were used as targets. Data represent the means (SEM of five mice from three separate experiments. * indicates CTL activity were significantly enhanced compared with mock group at E/T ratio of 40 and 80 (P < 0·05).
The cytokine production profile using hCD40L expression plasmid
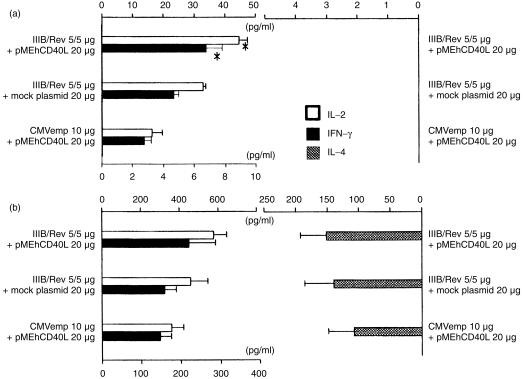
As shown in Fig. 4, the group co‐injected with pMEhCD40L exhibited higher levels of IL‐2 and IFN‐γ in the culture supernatant stimulated with R10I than the group co‐injected with mock plasmid (IL‐2: 44·47 ± 3·086 versus 32·85 ± 0·973 pg/ml, P = 0·045, IFN‐γ: 6·77 ± 1·037 versus 4·664 ± 0·332 pg/ml, P = 0·038). This suggested that pMEhCD40L plasmid enhanced antigen‐specific Th1 cytokine production. At an optimal concentration of Con A, there was no significant difference in IL‐2, IL‐4 and IFN‐γ production among these three groups.
Figure 4.
Cytokine production in the culture media of the splenocytes with R10I or Con A. Mice were inoculated with the vaccine formulated as shown. Spleen cells were harvested at two weeks after second immunization. These cells were cultured in the presence of the 10 mg/ml of R10I (a) or 5 mg/ml of Con A (b). The results were determined by a duplicate assay and presented as means ± SEM for three mice in each group. The symbol * means significant enhancement in the cytokine production compared with mock group (P < 0·05).
For further analysis of cytokine secretion profile, we used the cytokine ELIspot technique, which has been shown to have higher sensitivity than ELISA in detecting cytokine production. As shown in Table 1, we could detect the spots of antigen‐specific IL‐4‐producing cells with R10I peptide stimulation. The group co‐injected with pMEhCD40L showed twice the number of spots of IL‐4 producing cells and approximately threefold increase in the number of spots of IFN‐γ‐producing cells in spleen cells, compared with the group co‐injected with mock plasmid. In cells from lymph nodes, the group with pMEhCD40L had a ninefold increase in the number of spots of IL‐4 producing cells and an eightfold increase in the number of spots of IFN‐γ‐producing cells. Without stimulation, there was a similar number of spots among all groups for IL‐4 and IFN‐γ production.
Table 1.
ELIspot assay of mice co‐inoculated with HIV‐1 DNA vaccine and hCD40L expression plasmid
| 10 mg/ml openface> R10I (CTL epitope) cocultured | ||||||
|---|---|---|---|---|---|---|
| Spleen (spots/106 cells) | Lymphnode (spots/106 cells) | |||||
| +hCD40L* | emp† | +mock§ | +hCD40L* | emp† | +mock§ | |
| IL‐4 | 11·4 ± 3·21 | 5·02 ± 1·59 | 6·39 ± 1·29 | 80 ± 24·4§ | 7·54 ± 2·98 | 8·82 ± 2·49 |
| IFN‐γ | 19·1 ± 5·45 § | 6·32 ± 1·29 | 5·71 ± 1·26 | 213 ± 64·4 § | 8·33 ± 1·83 | 24 ± 6·75 |
| +hCD40L* | emp† | +mock‡ | +hCD40L* | emp† | +mock‡ | |
| IL‐4 | 5·43 ± 4·31 | 5·6 ± 3·23 | 2·83 ± 1·27 | 5 ± 2·5 | 4·76 ± 2·38 | 2·94 ± 1·47 |
| IFN‐γ | 5·36 ± 2·86 | 4·41 ± 2·21 | 2·6 ± 1·31 | 11·3 ± 8·75 | 8·33 ± 5·95 | 4·78 ± 2·57 |
Mice were injected with 5/5 mg of IIIB/REV and 10 mg of pMEhCD40L twice in 3‐week intervals.
Instead of IIIB/REV, CMV‐emp vector (10 mg) was inoculated associated with pMEhCD40L (10 mg).
Mock plasmid (10 mg) was inoculated instead of pMEhCD40L.
This value is significantly different (P < 0·05) from that obtained with mock group.
Discussion
We performed our experiments using pMEhCD40L, a vector encoding hCD40L, which is identical to mCD40L. The cross‐reactivity between human and murine CD40L was reported in previous reports.8,9,14,23 The two sequences exhibit 78% amino acid identity. There is 81% amino acid identity between the cytoplasmic domains, and 75% between the extracellular domains.8 In several papers, it was reported that hCD40L could affect murine cells.8,14,23–26 Previously, Mendoza et al.31 reported the immunostimulatory effect of a plasmid expressing CD40L on gene immunization. We therefore examined in detail whether inoculation with a DNA vaccine combined with hCD40L expression vector would be effective against HIV.
Co‐inoculation with pMEhCD40L enhanced the production of antigen‐specific serum antibody, especially in IgG titre, while there was no difference in whole IgG and IgM production among the groups (data not shown). Therefore, it was suggested that the expression of hCD40L would enhance not polyclonal but antigen‐specific antibody production. There are two possible explanations for this humoral enhancement. First, antigen‐specific B lymphocytes activated by DNA vaccination are thought to express more CD40 on their surface, and hCD40L could act selectively on these antigen‐specific B lymphocytes. Second, antigen‐specific CD4+ T‐lymphocyte response could be enhanced by co‐injection with pMEhCD40L, which thereafter help to activate antigen‐specific B lymphcytes.
Concerning protection against HIV, mucosal immunity is an important factor. Hence, the increase in antigen‐specific mucosal IgA production was one of requirements for an effective HIV vaccine. In our experiments, however, there was no enhancement in fecal antibody in the group co‐inoculated with pMEhCD40L. It was supposed that either the expression of hCD40L could not work on the production of mucosal IgA antibody or that intramuscular injection was not a proper route for the induction of mucosal antibody.27
More importantly, the expression of hCD40L enhanced HIV‐1‐specific cellular immune response assessed by DTH swelling in a dose‐dependent manner. DTH is supposed to reflect mainly the response of CD4+ T lymphocytes. The expression of hCD40L is suggested to induce antigen‐specific CD4+ T‐lymphocyte response effectively. The expression of hCD40L also enhanced CTL response.
These results together demonstrated that the expression of hCD40L could enhance cellular immunity as well as humoral immunity. Armitage et al. showed that both soluble and membrane‐bound CD40L act as a T‐cell growth factor.14 Sallusto et al. reported that surface major histocompatibility complex (MHC) class I and class II molecules were increased when dendritic cells (DCs) were incubated with CD40L.28 Interestingly, Bennett and Schoenberger showed that signalling through CD40 on the antigen‐presenting cell (APC) can replace CD4+ helper T lymphocytes in priming helper‐dependent CD8+ CTL responses.29,30 In our present study, there are three possible explanations for this enhancement. First, both pMEhCD40L and IIIB/REV are taken up by APCs and hCD40L was then expressed as membrane‐bound form on APCs to stimulate themselves with each other, which could result in the augmentation of immune response. Second, the inoculated pMEhCD40L is expressed on helper T cells separately from IIIB/REV and expressed on T lymphocytes, which then serve to activate APCs. Finally, pMEhCD40L is taken up by certain cells and express in soluble form to stimulate APCs involved in presenting the antigens.
To examine the actual mechanism, each of pMEhCD40L and IIIB/REV were separately injected into opposite legs. The results demonstrated that hCD40L could show its immunostimulatory properties even through contralateral injection. Our results suggested that a soluble form of hCD40L or hCD40L expressing cells might be carried and bound to CD40 on antigen‐presenting APCs. Mendoza et al. reported that animals injected with DNA vaccine and pCD40L at different sites did not show enhanced CTL response.31 We have no way of explaining the difference between their results and ours. It might depend on the difference between mCD40L and hCD40L or the difference between the vectors studied.
Next we analysed cytokine production using ELISpot assay. This method enables us to count directly the number of cytokine‐producing cells and to evaluate the balance between Th1 and Th2 activation more precisely; this is because the ELISpot data was shown to reflect only the rate of cytokine production while ELISA data reflect not only the rate of production but also the rate of decay and consumption of cytokine. Using this ELISpot technique, we found that the number of IFN‐γ‐producing cells and IL‐4‐producing cells were both increased prominently by the expression of hCD40L. This result indicates that the expression of hCD40L caused the stimulation of both Th1 and Th2 cells which was not indicated by the analysis using ELISA. The increased production of IgG2a without suppression of IgG1 production is consistent with bilateral stimulation of Th1 and Th2 cells.
Generally, an adjuvant is supposed to enhance either Th1‐ or Th2‐type immune response. In contrast, the addition of pMEhCD40L could enhance both humoral and cellular immunity without changing the balance between Th1 and Th2 under DNA vaccination and can enhance the production of both IL‐4 and IFN‐γ, so it is thought to be a unique adjuvant.
Our results showed that the cells from inguinal or para‐aortic lymph nodes had more antigen‐specific cytokine‐producing cells than the cells from spleen. This is an important observation to show the exact site where antigen presentation occurs in the DNA vaccine system. Robinson et al. reported that immune response was obtained even they ablated the immunized mouse muscle immediately after immunization. They hypothesized that antigen presentation occurred not at the injected focus but at a close lymph node or in the spleen via the blood stream where DNA vaccination was given intramuscularly.32,33 Our results support their hypothesis.
The present study clearly demonstrated adjuvant activity of hCD40L in DNA vaccination against HIV and the vaccination combined with pMEhCD40L is expected to contribute to the development of more effective vaccination than one combined with conventional adjuvant. Especially, in AIDS patients with decreased CD4+ helper T lymphocytes, hCD40L in DNA vaccination may provide an opportunity for enhancement of CTL activity. In fact, we planned the experiment using this plasmid in severe combined immunodeficiency (SCID)/Hu mice because this model can simulate immunologically the human being infected with HIV.
References
- 1.Carmichael A, Jin X, Sissons P, Borysiewics L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV‐1)‐specific cytotoxic T lymphocyte (CTL) response at different stages of HIV‐1 infection. Differential CTL response to HIV‐1 and Epstein–Barr virus in late disease. J Exp Med. 1993;177:249. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langlade‐Demoyen P, Ngo‐Giang‐Huong N, Ferchal F, Oksenhendler E. Human immunodeficiency virus (HIV) nef‐specific cytotoxic T lymphocytes in noninfected heterosexual contact of HIV‐infected patients. J Clin Invest. 1994;93:1293. doi: 10.1172/JCI117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowland‐Jones S, Sutton J, Ariyoshi K, et al. HIV‐specific cytotoxic T‐cells in HIV‐exposed but uninfected Gambian women. Nature Med. 1995;1:59. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 4.Liu MA. Overview of DNA vaccines. Ann N Y Acad Sci. 1995:15–20. doi: 10.1111/j.1749-6632.1995.tb44727.x. [DOI] [PubMed] [Google Scholar]
- 5.Manickan E, Karem KL, Rouse BT. DNA vaccines – a modern gimmick or a boon to vaccinology. Crit Rev Immunol. 1997;17:139. doi: 10.1615/critrevimmunol.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- 6.McDonnell WM, Askari FK. DNA vaccines. N Engl J Med. 1996;334:42. doi: 10.1056/NEJM199601043340110. [DOI] [PubMed] [Google Scholar]
- 7.Ulmer JB, Deck RR, Dewitt CM, Friedman A, Donnelly JJ, Liu MA. Protective immunity by intramuscular injection of low doses of influenza virus DNA vaccines. Vaccine. 1994;12:1541. doi: 10.1016/0264-410x(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 8.Spriggs MK, Armitage RJ, Stockbine L, et al. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J Exp Med. 1992;176:1543. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laman JD, Claassen E, Noelle RJ. Functions of CD40 and its ligand, gp39 (CD40L) Crit Rev Immunol. 1996;16:59. doi: 10.1615/critrevimmunol.v16.i1.40. [DOI] [PubMed] [Google Scholar]
- 10.Han S, Hathcock K, Zheng B, Kepler TB, Hodes R, Kelsoe G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7 in established germinal centers. J Immunol. 1995;155:556. [PubMed] [Google Scholar]
- 11.Grewal IS, Flavell RA. The CD40 ligand. At. the center of the immune universe? Immunol Res. 1997;16:59. doi: 10.1007/BF02786323. [DOI] [PubMed] [Google Scholar]
- 12.Foy TM, Durie FH, Noelle RJ. The expansive role of CD40 and its ligand, gp39, in immunity. Semin Immunol. 1994;6:259. doi: 10.1006/smim.1994.1034. [DOI] [PubMed] [Google Scholar]
- 13.Campbell KA, Ovendale PJ, Kennedy MK, Fanslow WC, Reed SG, Maliszewski CR. CD40 ligand is required for protective cell‐mediated immunity to Leishmania major. Immunity. 1996;4:283. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 14.Armitage RJ, Tough TW, Macduff BM, et al. CD40 ligand is a T cell growth factor. Eur J Immunol. 1993;23:2326. doi: 10.1002/eji.1830230941. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Wilson JM. CD40 ligand‐dependent T cell activation. requirement of B7–CD28 signaling through CD40. Science. 1996;273:1862. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 16.Okuda K, Bukawa H, Hamajima K, et al. Induction of potent humoral and cell‐mediated immune responses following direct injection of DNA encoding the HIV type 1 env and rev gene products. AIDS Res Hum Retroviruses. 1995;11:933. doi: 10.1089/aid.1995.11.933. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji T, Hamajima K, Ishii N, et al. Immunomodulatory effects of a plasmid expressing B7 on human immunodeficiency virus‐1‐specific cell‐mediated immunity induced by a plasmid encoding the viral antigen. Eur J Immunol. 1997;27:782. doi: 10.1002/eji.1830270329. [DOI] [PubMed] [Google Scholar]
- 18.Okada E, Sasaki S, Ishii N, et al. Intranasal immunization of DNA vaccine with IL‐12‐ and granulocyte–macrophage colony‐stimulating factor (GM‐CSF)‐expressing plasmids in liposomes induces strong mucosal and cell‐mediated immune responses against HIV‐1 antigens. J Immunol. 1997;159:3638. [PubMed] [Google Scholar]
- 19.Tsuji T, Fukushima J, Hamajima K, et al. HIV‐1‐specific cell‐mediated immunity is enhanced by co‐inoculation of Tca3 expression plasmid with DNA vaccine. Immunology. 1997;90:1. doi: 10.1046/j.1365-2567.1997.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okuda K, Kaneko T, Yamakawa T, et al. Strong immunogenicity of a multicomponent peptide vaccine developed with the branched lysine oligopeptide method for human immunodeficiency virus infection. J Mol Recognit. 1993;6:101. doi: 10.1002/jmr.300060302. [DOI] [PubMed] [Google Scholar]
- 21.Shirai A, Holmes K, Klinman D. Detection and quantitation of cells secreting IL‐6 under physiologic conditions in BALB/c mice. J Immunol. 1993;150:793. [PubMed] [Google Scholar]
- 22.Shirai A, Conover J, Klinman DM. Increased activation and altered ration of interferon‐gamma. Interleukin‐4 secreting cells in MRL‐lpr/lpr mice. Autoimmunity. 1995;21:107. doi: 10.3109/08916939508993357. [DOI] [PubMed] [Google Scholar]
- 23.Fanslow WC, Clifford KN, Seaman M, et al. Recombinant CD40 ligand exerts potent biologic effects on T cells. J Immunol. 1994;152:4262. [PubMed] [Google Scholar]
- 24.Choe J, Kim HS, Zhang X, Armitage RJ, Choi YS. Cellular and molecular factors that regulate the differentiation and apoptosis of germinal center B cells. Anti‐Ig down‐regulates Fas expression of CD40 ligand‐stimulated germinal center B cells and inhibits Fas‐mediated apoptosis. J Immunol. 1996;157:1006. [PubMed] [Google Scholar]
- 25.Durie FH, Foy TM, Masters SR, Laman JD, Noelle RJ. The role of CD40 in the regulation of humoral and cell‐mediated immunity. Immunol Today. 1994;15:406. doi: 10.1016/0167-5699(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 26.Durie FH, Foy TM, Noelle RJ. The role of CD40 and its ligand (gp39) in peripheral and central tolerance and its contribution to autoimmune disease. Res Immunol. 1994;145:200. doi: 10.1016/s0923-2494(94)80184-3. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto M, Vancott JL, Okahashi N, et al. The role of Th1 and Th2 cells for mucosal IgA responses. Ann N Y Acad Sci. 1996;778:64. doi: 10.1111/j.1749-6632.1996.tb21115.x. [DOI] [PubMed] [Google Scholar]
- 28.Sallusto F, Lanzavacchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony‐stimulating factor plus interleukin 4 and downregulated by tumor necrosis Factor α. J Exp Med. 1994;179:1109. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenberger SP, Toes REM, van der Voort EIH, Offringa R, Melief CJM. T‐cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 30.Bennett SRM, Carbone FR, Karamalis F, Flavell RA, Miller JFAP, Heath WR. Help for cytotoxic‐T‐cell responses is mediated by CD40 signalling. Nature. 1998;393:478. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 31.Mendoza RB, Cantwell MJ, Kipps TJ. Immunostimulatory effects of a plasmid expressing CD40 ligand (CD154) on gene immunization. J Immunol. 1997;159:5777. [PubMed] [Google Scholar]
- 32.Robinson HL, Torres CAT. DNA vaccines. Semin Immunol. 1997;9(5):271–283. doi: 10.1006/smim.1997.0083. [DOI] [PubMed] [Google Scholar]
- 33.Winegar RA, Monforte JA, Suing KD, O’Loughlin KG, Rudd CJ, Macgregor JT. Determination of tissue distribution of an intramuscular plasmid vaccine using PCR and in situ DNA hybridization. Human Gene Therapy. 1966;7:2185. doi: 10.1089/hum.1996.7.17-2185. [DOI] [PubMed] [Google Scholar]