Abstract
As the burden of infectious diseases becomes reduced in many countries, a remarkable increase in the incidence of allergies has occurred. The basis for the rise in atopic disorders as a correlate of the decline in infectious diseases has not been defined. In the present study, we tested experimentally whether prior systemic infection with Mycobacterium bovis bacillus Calmette Guérin (BCG) had any effect on ovalbumin (OVA) Al(OH)3 (alum)-induced immunoglobulin E (IgE) production, airway mucus production and eosinophilic inflammation. The data showed that allergen-specific IgE production and OVA-induced eosinophilia and goblet cell development were significantly inhibited by prior infection with BCG. Correspondingly, following immunization with OVA alum, BCG-infected mice exhibited significantly higher levels of allergen-driven interferon-γ (IFN-γ) production than the mice without infection. The ratio of IFN-γ: interleukin (IL)-4 production was higher in OVA-sensitized mice with prior BCG infection than in those without infection. The abrogation of OVA-induced mucus production and pulmonary eosinophilia in BCG-infected mice correlated with significantly decreased IL-5 production and increased IFN-γ and IL-12 production. These data provide direct evidence that intracellular bacterial infection (i.e. BCG) can inhibit antigen-specific IgE and airway reactivity induced by environmental allergen. Furthermore, the results suggest that changes in cytokine-producing patterns of T lymphocytes and other cells may be the mechanism by which infections influence allergies.
INTRODUCTION
Allergy is a state of immediate hypersensitivity that is mediated by immunoglobulin E (IgE) in response to normally harmless environmental antigens, termed allergens. In most developed countries, ≈20–30% of the population currently suffer from various forms of allergies.1,2 A well documented, but presently unexplained, epidemiological finding is that allergic diseases appear to increase with advancing socioeconomic development and occur more often in industrialized countries than in developing areas.3–5 As a correlate, a significant increase in the prevalence of allergy over the recent decades in developed countries has been associated with a striking reduction in childhood infectious diseases and reduction in vaccinations.6,7 Epidemiological studies suggest an innate connection between the increased incidence of allergy and reduced incidence of infectious diseases. In particular, epidemiological surveys carried out recently in Japan and Africa clearly showed an inverse correlation between delayed-type hypersensitivity (DTH) responses to tuberculin (and therefore current or previous mycobacterial infection) or history of measles infection, and immediate atopic reactions.8,9 Although epidemiological studies suggest a causal relationship between the reduction of infectious diseases and the increase of allergic disorders, few model-based, hypothesis-driven studies have been performed thus far to address the following questions: can certain existing or previous infections inhibit IgE and allergic responses induced by allergens and, if so, what is the underlying mechanism by which infections manipulate atopic allergies?
Cytokines produced by CD4 T lymphocytes and other cells play a critical role in the regulation of immune responses, both to allergens and to infectious agents.10–14 It has been widely demonstrated that in the two types of diseases, namely intracellular bacterial infection and allergy, differential cytokine patterns are often induced.12,13 Allergen-specific T cells in atopic individuals belong disproportionately to the T helper 2 (Th2) subset, which produces relatively high levels of interleukin (IL)-4 and IL-5 and low, or undetectable, levels of interferon-γ (IFN-γ).15,16,17 In contrast, most intracellular bacterial infections induce strong Th1-like responses characterized by strong cell-mediated immunity and IFN-γ production.15,18–20 We have shown in previous studies that the type of adjuvants used in immunization can significantly influence the IgE responses and cytokine patterns induced by an allergen, ovalbumin (OVA).21 Specifically, OVA induces Th2-like responses when Al(OH)3 (alum) is used as adjuvant, while it induces Th1-like responses when complete Freund’s adjuvant (CFA) is used as adjuvant. Although the mechanism of the adjuvant effect is unclear, the presence of dead mycobacteria in CFA may be one of the reasons for the inhibitory effect of CFA on OVA-induced Th2-like responses because a recent study showed that treatment of mice with dead Brucella abortus, immediately before allergen immunization, inhibited IgE responses and Th2 cytokine production to the allergen.22
Antigen-specific IgE production, excessive bronchial mucus production and goblet cell development, and pulmonary eosinophilic inflammation are among the hallmarks of atopic asthmatic reaction. To directly address the question regarding the causal relationship between intracellular bacterial infection and the reduction of allergies, we examined in the present study whether systemic mycobacterial infection has any influence on OVA-induced IgE production and airway mucus production and eosinophilic inflammation. The data show that prior systemic infection with bacillus Calmette Guérin (BCG) is inhibitory for allergen-specific IgE production, goblet cell development and pulmonary eosinophilic inflammation induced by OVA. As a correlate, allergen-driven cytokine-producing patterns following OVA immunization in BCG-infected mice were significantly different from those in mice without BCG infection. Specifically, following OVA immunization, BCG-infected mice exhibited significantly elevated IFN-γ production and decreased IL-4 production. The ratio of IFN-γ: IL-4 production induced by OVA alum immunization was significantly higher in mice infected previously with BCG than in mice without prior BCG infection. The abrogation of bronchial mucus production and eosinophilia induced by intranasal challenge with OVA correlated with significantly decreased IL-5 and increased IFN-γ and IL-12 production by splenocytes and correlated with elevation of IL-12 production in local (lung) tissues. The data provide evidence that intracellular bacterial infection (BCG) can inhibit antigen-specific IgE responses and airway reactivity induced by environmental allergen, and that changes in cytokine patterns may be the mechanism by which infections influence allergies.
MATERIALS AND METHODS
Animals
Female C57BL/6 mice (6–10 weeks old) were obtained from Charles River Canada (Montreal, Quebec, Canada). Female Sprague-Dawley rats were bred at the University of Manitoba (Winnipeg, Canada) breeding facility. Animals were used in accordance with the guidelines issued by the Canadian Council on Animal Care.
Organism and infection
M. bovis BCG (BCG Vaccine; Connaught Laboratories Ltd, Willowdale, ON, Canada) was grown as dispersed cultures in Middlebrook’s 7H9 broth (Difco Laboratories Inc., Detroit, MI) containing 0·2% (v/v) glycerol and 0·05% (v/v) Tween-80 and supplemented with 10% (v/v) Middlebrook ADC enrichment (Difco). The stock culture was stored at −80° until used. The number of BCG bacilli, expressed as colony-forming units (CFUs), was determined by plating diluted culture on plates of Middlebrook 7H11 agar (Difco) containing 0·5% (v/v) glycerol and supplemented with 10% (v/v) Middlebrook OADC enrichment (Difco). For infection, different numbers of BCG bacilli (1–10×105 CFUs), diluted in 200 μl of sterile saline, were injected into the lateral tail vein of the mouse. For monitoring infection, the numbers of viable bacteria in the lungs, liver, spleen, heart and kidney were determined by plating serial dilutions of individual whole-organ homogenates onto Middlebrook 7H11 agar containing glycerol and Middlebrook enrichment. The number of CFUs were counted after incubation for 3 weeks at 37° in an atmosphere of 9% CO2.
Sensitization and challenge with allergen
Mice infected with BCG or treated with phosphate-buffered saline (PBS) were immunized intraperitoneally (i.p.) with 2 μg of OVA (ICN Biomedicals, Montreal, Canada) in 2 mg of Al(OH)3 adjuvant (alum) 2 weeks following the infection or treatment, as described previously.21 Mice were bled for analysis of serum antibody production on days 10 and 14, respectively, post-OVA immunization. On day 15 post-OVA immunization, mice were challenged intranasally with 100 μg of OVA (40 μl) and were killed 7 days later. Bronchial and pulmonary cellular infiltration and local cytokine production were examined by differential cell counts and determination of cytokine proteins, respectively, in the bronchoalveolar lavages (BALs).
BAL and differential cell counts
The mouse trachea was cannulated and the lungs were washed twice with 1 ml of PBS. The BAL fluid was centrifuged immediately and cells were resuspended in 0·5 ml of PBS. The cells were counted under a microscope and a drop of the cell suspension was applied onto the surface of a glass slide and spread to form a BAL smear. The slide was air-dried, fixed with ethanol and stained with Fisher Leukostat Stain Kit (Fisher Scientific, Ontario, Canada) to stain leucocytes. The number of monocytes, neutrophils, lymphocytes and eosinophils per 200 cells were counted, based on morphology and staining characteristics.
Histopathological analysis
Seven days following OVA challenge, lungs were collected and fixed in 10% buffered formalin. The fixed lungs were embedded in paraffin, sectioned, stained by haematoxylin and eosin, and examined for pathological changes under light microscopy. Bronchial mucus and mucus-containing goblet cells were stained by thionin using the method of Mallory.23
Cell culture
For examination of cytokine patterns in splenocytes, mice were killed either at day 5 postinitial OVA immunization or at day 7 following intranasal challenge with OVA. The spleens were isolated aseptically and single cell suspensions were cultured as described previously.24 Briefly, cells were cultured at a concentration of 7·5×106 cells/ml (2 ml/well), alone or with OVA (1 mg/ml) or immobilized anti-CD3 monoclonal antibody (mAb; 145-2C11; PharMingen, San Diego, CA), in 24-well plates at 37° in complete culture medium: RPMI-1640 containing 10% heat-inactivated fetal calf serum (FCS), 1% l-glutamine and 5×10−5 2-mercaptoethanol (2-ME) (Kodak, Rochester, NY). Duplicate cultures were established from the spleen cells of individual mice in each group. Culture supernatants were harvested at various time-points for measurement of cytokines.24
Determination of cytokines
Cytokines in the supernatants of spleen cell cultures, BALs and lung homogenates were analysed by enzyme-linked immunosorbent assays (ELISAs) using purified (for capture) and biotinylated (for detection) antibodies (PharMingen), as described previously.25 IFN-γ levels in 72-hr culture supernatants were measured by a two-mAb sandwich ELISA (XMG1·2 for capture and R4-6A2 for detection). IL-5 levels in 72-hr culture supernatants were measured using mAb TRFK as capture antibody and mAb TRFK4 as detection antibody. IL-4 levels were measured using 11B11 as capture antibody and BVD6-24G2 as detection antibody. IL-12 p40 levels were measured using mAb C15·6 and mAb C17·8 as capture and detection antibodies, respectively.
Determination of serum antibodies
The level of allergen-specific IgE was determined by passive cutaneous anaphylaxis (PCA) of Sprague-Dawley rats, as described previously.21 Allergen-specific immunoglobulin G (IgG)1 and IgG2a were measured using goat anti-mouse IgG1 or goat anti-mouse IgG2a antibodies (Southern Biotechnology Associates, Inc., Birmingham, AL), as described previously.21
Statistical analysis
Antibody titres (ELISA or PCA) were log transformed and analysed using the unpaired Student’s t-test. Cytokine levels in different groups were analysed using the unpaired Student’s t-test.
RESULTS
BCG-infected mice show decreased serum allergen-specific IgE production and elevated antigen-specific IgG2a production
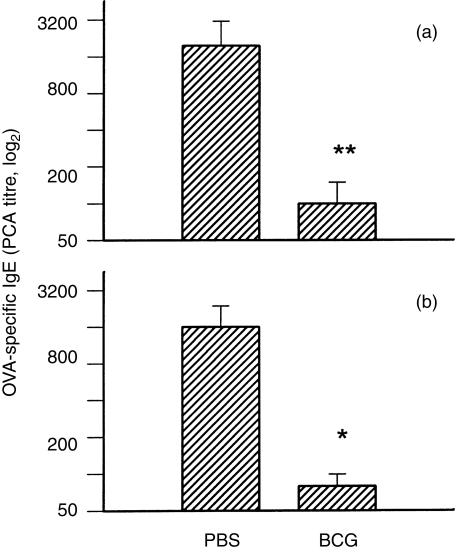
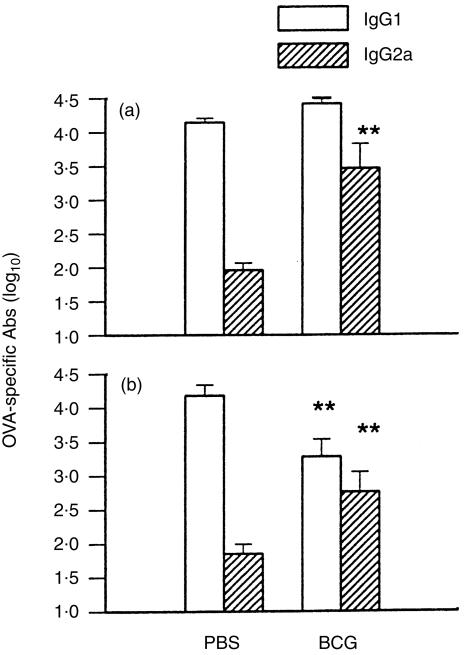
Serum antibodies, specific for OVA, in mice with or without prior BCG infection, were measured at day 10 (IgE) or day 14 (IgG1 and IgG2a) following OVA alum immunization. As shown in Fig. 1, allergen-specific IgE production in the groups of mice with previous BCG infection (1×105 or 1×106 CFUs) was >10-fold lower than that in the uninfected control mice (P<0·01) (Fig. 1). Some BCG-infected mice showed undetectable (titre <20) antigen-specific IgE production following OVA alum immunization. In contrast, following OVA alum immunization, OVA-specific IgG2a production was 20–300-fold higher in mice with previous BCG infection than in control mice (P<0·01) (Fig. 2). OVA-specific IgG1 levels in BCG-infected mice, subsequently immunized with OVA, were lower than those in control mice (with no BCG infection) which were immunized with OVA (alum) (Fig. 2). Unlike antigen-specific IgE responses, however, total serum IgE levels after OVA immunization were comparable among the groups of mice with or without prior BCG infection (data not shown). This data suggests that prior systemic BCG infection can modulate the isotypes of antibody response, including IgE, to subsequent unrelated antigen (allergen) immunization.
Figure 1.
Prior bacillus Calmette Guérin (BCG) infection inhibited ovalbumin (OVA)-specific immunoglobulin E (IgE) production. Data from two independent experiments are shown. C57BL/6 mice were infected intravenously with 1×105 (a) or 1×106 (b) colony-forming units (CFUs) of Mycobacterium bovis BCG or treated with phosphate-buffered saline (PBS) alone. Two weeks after BCG inoculation, mice were immunized intraperitoneally with 2 μg of OVA in 2 mg of Al(OH)3 (alum) adjuvant. Mice were bled at day 10 postimmunization for analysis of serum IgE production. Allergen (OVA)-specific IgE levels in individual mice were determined by passive cutaneous anaphylaxis (PCA) using female Sprague-Dawley rats. Geometric mean titres (±SEM) of each group, plotted on a log2 scale, are presented. *represents P<0·05.
Figure 2.
Bacillus Calmette Guérin (BCG) infection increased allergen-specific immunoglobulin (Ig)G2a and decreased allergen-specific IgG1 production. Mice were infected with BCG (or treated with phosphate-buffered saline, PBS) and subsequently immunized with ovalbumin (OVA) in alum, as described in the legend to Fig. 1. Mice were bled at day 14 post-OVA immunization, and allergen (OVA)-specific IgG1 and IgG2a antibodies in serum samples from individual mice were determined by enzyme-linked immunosorbent assay (ELISA). Titres were transformed to log10 and presented as mean±SEM. Data from two independent experiments are shown. Panel (a), mice were infected with 1×105 colony-forming units (CFUs) of BCG. Panel (b), mice were infected with 1×106 CFUs of BCG. **represents P<0·01.
Systemic BCG infection modulates cytokine patterns of immunocompetent cells elicited by allergen
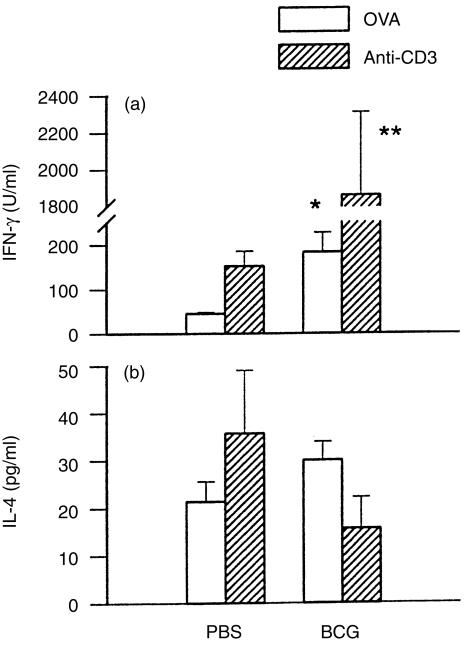
The decrease of IgE and increase of IgG2a responses to OVA in mice with prior BCG infection suggest that T-cell responses to allergen were modulated by the infection. To examine directly the effect of BCG infection on allergen-specific T-cell activation, we compared Th1-related (IFN-γ) and Th2-related (IL-4) cytokine production in OVA-immunized mice with or without prior BCG infection. Cytokine production by ex vivo spleen cells from OVA-immunized mice was analysed using allergen (OVA)-specific and polyclonal T-cell (anti-CD3) stimulation. As shown in Fig. 3, upon either allergen-specific or polyclonal T-cell stimulation, IFN-γ production in BCG-infected mice was significantly higher than in mice without prior BCG infection. The absolute value of IL-4 levels was not significantly different between OVA-immunized mice with or without prior BCG infection. Notably, however, largely as a result of the increased IFN-γ production, the ratio of IFN-γ:IL-4 upon allergen-specific or polyclonal T-cell (anti-CD3) stimulation was markedly increased in OVA-immunized mice with prior BCG infection (P<0·05). IL-4 and IFN-γ were produced mainly by CD4 T cells because the presence of anti-CD4 mAb (YTS 191·1) inhibited most (>80%) of the production of these cytokines (data not shown). These results indicate that prior BCG infection can modulate cytokine-producing patterns of CD4 T cells to allergen (OVA) exposure, predominantly enhancing IFN-γ production and thus increasing the ratio of IFN-γ:IL-4.
Figure 3.
Effect of prior bacillus Calmette Guérin (BCG) infection on allergen (ovalbumin, OVA)-driven cytokine production by splenocytes. Mice were infected with 1×106 colony-forming units (CFUs) of BCG, or treated with PBS, and were immunized with OVA (2 μg) in alum at 2 weeks postinfection. Mice were killed at day 5 following OVA immunization and splenocytes from individual mice were cultured at 7·5×106 cells/ml and stimulated with OVA (1 mg/ml) or immobilized anti-CD3 monoclonal antibody (mAb; 145-2C11). Culture supernatants were harvested at 72 hr and tested for interferon-γ (IFN-γ) and interleukin-4 (IL-4) using enzyme-linked immunosorbent assays (ELISAs). *represents P<0·05; **represents P<0·01.
Systemic BCG infection inhibits bronchial mucus production and pulmonary eosinophilia induced by local challenge with allergen
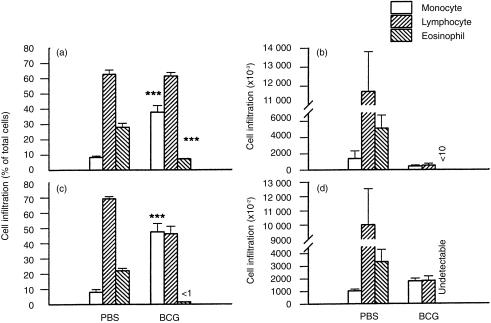
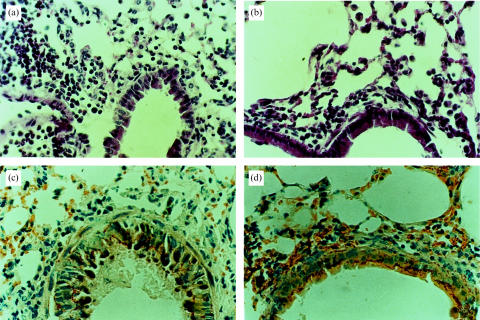
Cellular infiltration in the lungs of the mice challenged intranasally with OVA was examined by differential counting of BAL cells. In general, mice with prior BCG infection showed less cellular infiltration following challenge with OVA than those without the infection (Fig. 4). Mice without prior BCG infection showed massive pulmonary eosinophilia with 0·3–6×106 eosinophils recovered from the BAL of each mouse. In contrast, mice with prior BCG infection showed marginal or undetectable eosinophil infiltration following the same intranasal challenge with OVA. Intravenous injection of BCG at a dose of 1×105 CFUs inhibited eosinophilia, as shown by comparing the number of eosinophils among control and infected mice: 5×106 eosinophils in control mice (without BCG infection) versus 1×104 eosinophils in BCG-infected mice, representing a 500-fold decrease in eosinophilia (Fig. 4b). Eosinophils comprised 25–30% of the total BAL cells in the mice without BCG infection but <5% of the total BAL cells following OVA challenge in the BCG-infected mice (Fig. 4a). Intravenous injection with 1×106 CFUs of BCG almost completely inhibited pulmonary eosinophilia induced by OVA (Fig. 4c, 4d). In parallel with the large proportion of eosinophils in the BALs of the OVA-challenged mice without prior BCG infection, a significant increase in the proportion of macrophage/monocytes in the BALs was observed in mice with prior BCG infection (Fig. 4a, 4d). Macrophage/monocytes comprised <10% of total BAL cells in the OVA-challenged mice without BCG infection but >35% of the total BAL cells in OVA-challenged mice with prior BCG infection (Fig. 4a, 4c). The most common cell population in the BAL was lymphocytes, the proportion of which was not significantly different among OVA-challenged mice either with or without BCG infection. Histological analysis also showed remarkably reduced eosinophil infiltration in the bronchial and pulmonary tissues in mice with BCG infection that were OVA sensitized and -challenged (Fig. 5). Mice without prior BCG infection showed massive eosinophilia in bronchial submucosa, alveolar and perivascular sheaths, with infiltration of lymphocytes and a few monocytes following OVA challenge. In contrast, BCG-infected mice developed infiltrations of mainly macrophage/monocytes and lymphocytes around the small bronchi, bronchioles and blood vessels in the lungs with very few eosinophils. Moreover, the mucus-containing goblet cells, mucus secretion and bronchial epithelial hyperplasia induced by OVA sensitization/challenge were also markedly decreased in the BCG-infected mice (Fig. 5b, 5d). These results demonstrate that systemic mycobacterial infection can suppress not only allergen-specific IgE responses but also local allergic inflammatory responses (eosinophilia) and mucus production induced by allergen (OVA).
Figure 4.
Prior bacillus Calmette Guérin (BCG) infection decreased pulmonary eosinophilic infiltration induced by ovalbumin (OVA). Mice were injected intravenously with phosphate-buffered saline (PBS) or BCG and were subsequently (2 weeks later) immunized intraperitoneally with OVA (2 μg) in alum. The mice were challenged with OVA (100 μg) at day 15 following OVA immunization and were killed at day 7 post challenge. Pulmonary cellular infiltration were examined by differential cell counts of the bronchoalveolar lavage (BAL). Data from two independent experiments using different doses of BCG for infection are presented as mean±SEM. Experiment 1 (a, b) used BCG at 1×105 colony-forming units (CFUs) and Exp. 2 (c, d) used BCG at 1×106 CFUs for infections. The figure shows the absolute number of each infiltrating cell population (b, d) and the proportion (a, c) of each cellular component comprising the total BAL cells. Experiment 1 (panel B) showed very few (10 000±3000) eosinophils in the lung and Exp. 2 (d) showed undetectable eosinophil infiltration. **represents P<0·01; ***represents P<0·001.
Figure 5.
Histological analysis of bronchial inflammatory reaction and mucus production induced by allergen challenge in mice with or without prior bacillus Calmette Guérin (BCG) infection. Mice were injected intravenously with phosphate-buffered saline (PBS) or BCG and subsequently (2 weeks later) immunized intraperitoneally with ovalbumin (OVA) (2 μg) in alum. The mice were challenged with OVA (100 μg) at day 15 following OVA immunization and were killed at day 7 postchallenge. Lung tissues were fixed routinely and sections were stained either with haematoxylin and eosin (a, c) for infiltrating cells and lung structure, or with thionin (b, d) for mucus and mucus-containing goblet cells. (a) and (b), OVA-sensitized and -challenged mice without prior BCG infection. (c) and (d), BCG-infected mice that were subsequently sensitized/challenged with OVA.
Systemic BCG infection inhibits allergen-driven IL-5/IL-4 production and increases IFN-γ/IL-12 (p40) production by splenocytes following intranasal challenge with OVA
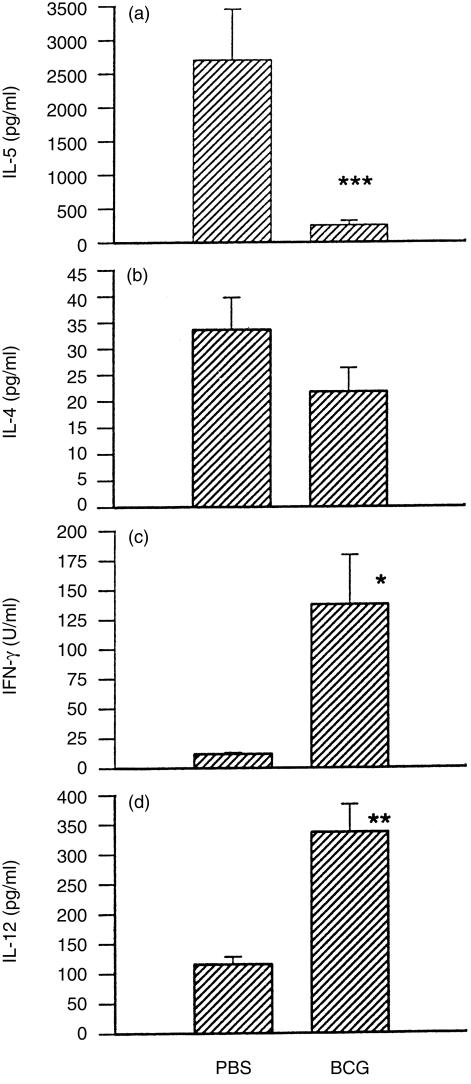
To elucidate the mechanism by which BCG inhibits bronchial and pulmonary eosinophilic inflammation induced by allergen (OVA), we examined antigen-driven Th1-related (IFN-γ/ IL-12) and Th2-related (IL-4/IL-5) cytokine production by spleen cells collected from OVA-sensitized and -challenged mice with or without prior BCG infection. Allergen-driven IL-5 and IL-4 production in mice with prior BCG infection was significantly lower than in those without BCG infection (Fig. 6a, 6b). In contrast, OVA-driven IFN-γ and IL-12 (p40) production in mice with prior BCG infection was significantly higher than in those without infection (Fig. 6c, 6d). These results indicate that the inhibition of eosinophilia and the enhancement of monocyte/macrophage infiltration caused by prior BCG infection correlates with a switch of cytokine patterns from dominant IL-5 and IL-4 production to dominant IFN-γ and IL-12 production.
Figure 6.
Abrogation of eosinophilic inflammation correlated with a reduction of interleukin (IL)-5 and increases of interferon-γ (IFN-γ) and IL-12 production. Ovalbumin (OVA)-driven cytokine production by splenocytes of the mice described in Expt. 2 in Fig. 4 were examined at day 7 postintranasal challenge with OVA. Data are presented as mean±SEM. *represents P<0·05; **represents P<0·01; and ***represents P<0·001.
Systemic BCG infection increases IL-12 (p40) production in the lung
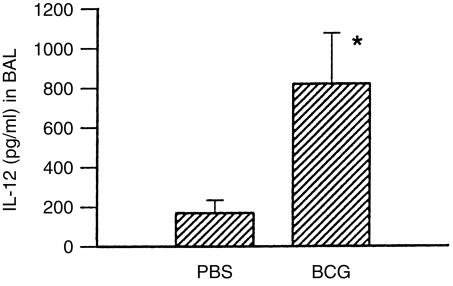
To elucidate the relationship between bronchial mucus production and pulmonary eosinophilic inflammation and local cytokine synthesis induced by allergen exposure, we examined the level of Th1-related and Th2-related cytokines in BALs collected from OVA-sensitized/challenged mice with or without BCG infection. Most local cytokines that were analysed in the study (IL-4, IL-5 and IFN-γ) were present at the lower limit of detection at the time of analysis, with a trend of slightly lower IL-4 levels in the mice with prior BCG infection, compared with those without prior BCG infection (data not shown). IL-12 (p40) was the only local cytokine that was readily detectable at the time of determination and its production significantly differed between OVA-sensitized/challenged mice with or without BCG infection (Fig. 7). The data suggest that the increase in local IL-12 production, and thus inhibition of Th2-like cytokine production, may contribute to the abrogation of eosinophilia induced by allergen.
Figure 7.
Abrogation of pulmonary eosinophilic inflammation correlated with an increase in local bronchoalveolar lavage (BAL) interleukin (IL)-12 production. The BALs of the mice described in Fig. 4 were analysed for IL-12 production using a sandwich enzyme-linked immunosorbent assay (ELISA). Data are presented as mean±SEM. *represents P<0·05.
DISCUSSION
Recently, Ern et al.26 reported that intranasal, but not intradermal or subcutaneous, inoculation of M. bovis BCG abrogated murine eosinophilia, in the lung, induced by OVA sensitization/challenge. Our data confirmed their finding and extended it by showing that systemic BCG infection can dramatically inhibit allergen-specific IgE responses and bronchial epithelial hyperplasia and mucus production. To our knowledge, this is the first experimental study that shows inhibition of allergen-specific IgE and mucus production following allergen exposure caused by intracellular bacterial infection. As allergen-specific IgE is the key element in most allergic diseases, our data suggest that the epidemiologically demonstrated inverse association between infection and allergies is a causal relationship. Moreover, as prior BCG infection can inhibit de novo IgE responses, it is possible that early vaccination with attenuated intracellular bacteria is useful for preventing atopic allergies (not limited to asthma) induced by environmental allergens. It should be noted, however, that this study was carried out using only a single strain of mouse, C57BL/6. Further studies using multiple mouse strains with different genetic backgrounds is necessary to test whether the inhibitory effects of BCG infection on allergy, as observed in this study, are related to the host’s susceptibility to infection and/or allergy.
The demonstration of reduced bronchial mucus production and epithelial hyperplasia is particularly important in evaluating the effect of intracellular bacterial infection on the bronchial asthmatic reaction. Although the work of Ern et al. has demonstrated a reduction of pulmonary eosinophilia (induced by allergen) caused by intranasal BCG infection, the relevance of this change in infiltrating cells to a clinical asthmatic reaction is not clear because the real role of eosinophilia in asthma is still debatable. There are numerous reports which show an asthmatic reaction induced by various allergens in the absence of bronchial eosinophilic inflammation.27–30 Therefore, abrogation of pulmonary eosinophilia may not necessarily mean reduction of the asthmatic reaction by BCG infection. Our data, however, showed not only the abrogation of pulmonary eosinophilia, but also decreases of mucus production and epithelial hyperplasia, which are important components of the asthmatic reaction, thus unambiguously indicating the inhibitory effect of intracellular bacterial infection (i.e. BCG) on the atopic asthmatic reaction.
Our data also suggest that alteration in cytokine patterns is crucial for bacterial infection-mediated suppression of the allergic reaction. The decrease in allergen-specific IgE in BCG-infected mice correlated with an elevation in IL-12 and IFN-γ production and an increase in the ratio of IFN-γ:IL-4 production. We and others have demonstrated that the ratio of Th1:Th2 cytokine synthesis elicited by allergen exposure is critical in determining the type of immune responses and class/subclass of antibodies, including IgE production, which is probably more relevant to antibody class switch and clinical status than the absolute value of particular cytokines.24,31–35 Thus, chemically modified OVA (OA-POL) treatment can inhibit IgE production induced by native OVA (alum) immunization, mainly via a change (increase) in the ratio of IFN-γ:IL-4 synthesis.24,34 Local cytokine analyses in the present study demonstrated significantly increased IL-12 production following OVA challenge in BCG-infected mice, also suggesting increased Th1 responses in these mice. The failure to detect IL-5 in BALs of allergen-sensitized and -challenged mice, with or without BCG infection, is rather surprising. It may be because of the time-point used in this study. Nevertheless, cytokine analysis of splenocytes following OVA exposure showed significantly less IL-5 production in BCG-infected mice than in mice without BCG infection. The latter point may be important because of evidence that shows circulating, but local lung, IL-5 is required for the development of airway eosinophilia.36
How can prior intracellular bacterial infection modulate the host’s cytokine-producing patterns and airway reactivity induced by allergen? Recent studies show that Th1/Th2 cells are derived from a common precursor lineage37,38 whose selective differentiation is affected by microenvironmental factors that regulate transcriptional activation of cassettes of specific cytokine genes.39–42 The early presence of IFN-γ and IL-12 favours Th1 polarization whereas IL-4 is the potent stimulus for Th2 polarization. IL-12 is the most important cytokine, which has the ability to enhance the differentiation of Th-cell precursors into Th1-like cells. IL-12 can activate transcription factors related to the Th1 phenotype, including Stat4,43 which is activated solely by IL-12.44,45 In addition, human T cells fail to differentiate into Th2-like cells if IL-4 is absent at the site of antigen-specific cell antigen–peptide T-cell interaction.41 Similarly, mice with germline disruption of the IL-4 gene fail to generate Th2-like cells and fail to produce IgE antibodies.46–48 IL-4 induces the development of Th2-like cells, via signalling, through the activation of Stat6, a transcriptional factor for the Th2 phenotype.49–51 Intracellular bacteria often induce Th1-like responses because they can innately activate macrophages, which produce IFN-γ and IL-12, and natural killer (NK) cells, which produce IFN-γ, thereby establishing a setting for Th1-like T-cell priming. In contrast, allergens are poor stimulators of NK cells and macrophages. They may fail to establish a setting for Th1 priming when being exposed in vivo, resulting in the development of Th2-like cells. It is possible therefore that the Th1-like setting in the microenvironment of antigen-presenting cell–allergen peptide–T-cell interaction, established by previous intracellular bacterial infection, can direct the allergen-specific T cells to Th1-like cells when allergen is subsequently exposed.
This study is informative for understanding the influence of environmental factors on antigen-specific T-cell responses. The modifying effect of microenvironmental factors on immune responses to antigen in natural conditions in vivo is largely unknown. Although the studies using cytokine-blocking antibodies or gene knockout animals have demonstrated a role of environmental cytokines in determining T-cell differentiation, they all examine the influence of environmental cytokines in a condition of general or complete deficiency of these cytokines.10–12 Studies are very limited regarding the effect of alteration in cytokine microenvironment induced by natural physiological or pathological processes on T-cell responses to unrelated antigens.52 From this point of view, the present study provides convincing evidence that non-specific microenvironmental factors induced by a natural infection may play an important role in regulating antigen-driven cytokine patterns to unrelated antigen.
Acknowledgments
This work was supported by a grant from the Medical Research Council of Canada (MRC) to X.Y. (MT-14680). X.Y. is the recipient of a salary (Scholar) award from the MRC.
References
- 1.Wuthrich B. Epidemiology of the allergic diseases: are they really on the increase? Int Arch Allergy Appl Immunol. 1989;90:3. doi: 10.1159/000235067. [DOI] [PubMed] [Google Scholar]
- 2.Ishizaka K, Ishizaka T, Hornbrook MJ. Physicochemical properties of human reaginic antibody. V. Correlation of reaginic activity with γE globulin antibody. Immunology. 1966;97:840. [PubMed] [Google Scholar]
- 3.Cookson WO.C.M, Moffatt MF. Asthma: an epidemic in the absence of infection? Science. 1997;275:41. doi: 10.1126/science.275.5296.41. [DOI] [PubMed] [Google Scholar]
- 4.Springett VH, Darbyshire JH, Nunn AJ, Sutherland I. Changes in tuberculosis notification rates in the white ethnic group in England and Wales between 1953 and 1983. J Epidemiol Commun Health. 1988;42:370. doi: 10.1136/jech.42.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce IN, Harland RW, McBride NA, MacMahon J. Trends in the prevalence of asthma and dyspnoea in first year university students. Quart J Med. 1993;86:1993. [PubMed] [Google Scholar]
- 6.Schultz-Larsen F. The epidemiology of atopic dermatitis. Monogr Allergy. 1993;31:9. [PubMed] [Google Scholar]
- 7.Doll R. Health and the environment in the 1990s. Am J Public Health. 1992;82:933. doi: 10.2105/ajph.82.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;775:77. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 9.Shaheen SO, Aaby P, Hall AJ, et al. Measles and atopy in Guinea-Bissau. Lancet. 1996;347:1997. doi: 10.1016/s0140-6736(96)91617-7. [DOI] [PubMed] [Google Scholar]
- 10.Mosmann TR, Subash S. The expanding universe of T cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 11.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 12.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 13.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 14.de Vries JE. Atopic allergy and other hypersensitivity. Curr Opin Immunol. 1994;6:835. doi: 10.1016/0952-7915(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann SH. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 16.Romagnani S. Induction of TH1 and TH2 responses: a key role for the ‘natural’ immune response? Immunol Today. 1992;13:379. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- 17.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:295. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 18.Liu MK, Brownsey RW, Reiner NE. Gamma interferon induces rapid and coordinate activation of mitogen-activated protein kinase (extracellular signal-regulated kinase) and calcium-independent protein kinase C in human monocytes. Infect Immun. 1994;62:2722. doi: 10.1128/iai.62.7.2722-2731.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller TE, Mackaness GB, Lagrange PH. Immunopotentiation with BCG. II. Modulation of the response to sheep red blood cells. J Natl Cancer Inst. 1973;51:1669. doi: 10.1093/jnci/51.5.1669. [DOI] [PubMed] [Google Scholar]
- 20.Mackaness GB, Lagrange PH, Ishibashi T. The modifying effect of BCG on the immunological induction of T cells. J Exp Med. 1974;139:1540. doi: 10.1084/jem.139.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Hayglass KT. Allergen-dependent induction of interleukin-4 synthesis in vivo. Immunology. 1993;78:74. [PMC free article] [PubMed] [Google Scholar]
- 22.Scott DE, Agranovich I, Inman J, Gober M, Golding B. Inhibition of primary and recall allergen-specific T helper cell type 2-mediated responses by a T helper cell type 1 stimulus. J Immunol. 1997;1997:107. [PubMed] [Google Scholar]
- 23.Mallory FB. Pathological Techniques. Philadelphia: W. B. Saunders; 1944. [Google Scholar]
- 24.Yang X, Gieni RS, Mosmann TR, Hayglass KT. Chemically modified antigen preferentially elicits induction of Th1-like cytokine synthesis patterns in vivo. J Exp Med. 1993;178:349. doi: 10.1084/jem.178.1.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Brunham RC. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol. 1998;161:1439. [PubMed] [Google Scholar]
- 26.Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-bacillus Calmette-Guérin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998;187:561. doi: 10.1084/jem.187.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsubara SK, Fushimi K, Ogawa H, et al. Inhibition of pulmonary eosinophilia does not necessarily prevent the airway hyperresponsiveness induced by Sephadex beads. Int Arch Allergy Immunol. 1998;116:67. doi: 10.1159/000023927. [DOI] [PubMed] [Google Scholar]
- 28.Hessel EM, Van Oosterhout AJ, Van Ark I, et al. Development of airway hyperresponsiveness is dependent on interferon-gamma and independent of eosinophil infiltration. Am J Respir Cell Mol Biol. 1997;16:325. doi: 10.1165/ajrcmb.16.3.9070618. [DOI] [PubMed] [Google Scholar]
- 29.Garssen J, van Loveren H, van der Vliet H, et al. T cell-mediated induction of airway hyperresponsiveness and altered lung functions in mice are independent of increased vascular permeability and mononuclear cell infiltration. Am Rev Respir Dis. 1993;147:307. doi: 10.1164/ajrccm/147.2.307. [DOI] [PubMed] [Google Scholar]
- 30.Garssen J, Nijkam FP, van der Vliet H, van Loveren H. T-cell-mediated induction of airway hyperreactivity in mice. Am Rev Respir Dis. 1991;144:931. doi: 10.1164/ajrccm/144.4.931. [DOI] [PubMed] [Google Scholar]
- 31.Swain SL, McKenzie DT, Weinberg AD, Hancock H. Characterization of T helper 1 and 2 cell subsets in normal mice: helper T cells responsible for IL-4 and IL-5 production are present as precursors which require priming before they develop into lymphokine-secreting cells. J Immunol. 1988;141:3445. [PubMed] [Google Scholar]
- 32.Bass H, Mosmann T, Strober S. Evidence for mouse Th1- and Th2-like cells after total lymphoid irradiation. J Exp Med. 1989;170:1495. doi: 10.1084/jem.170.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romagnani S. Human Th1 and Th2 subsets: doubt no more. Immunol Today. 1991;12:256. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- 34.Gieni RS, Yang X, Hayglass KT. Allergen-specific modulation of cytokine synthesis patterns and IgE responses in vivo with chemically modified allergen. J Immunol. 1993;150:302. [PubMed] [Google Scholar]
- 35.Finkelman FD, Holmes J, Katona IM, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe A, Mishima H, Renzi PM, Xu LJ, Hamid Q, Martin JG. Transfer of allergic airway responses with antigen-primed CD4+ but not CD8+ T cells in brown Norway rats. J Clin Invest. 1995;96:1303. doi: 10.1172/JCI118165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robert AS, Paul WE, Davis MM, de St. Groth BF. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4 T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steven LR, Wang ZE, Hatam F, Scott P, Locksley RM. Th1 and Th2 cell antigen receptor in experimental leishmaniasis. Science. 1993;259:1457. doi: 10.1126/science.8451641. [DOI] [PubMed] [Google Scholar]
- 39.Lohoff M, Ferrick D, Mittrucker HW, et al. Interferon regulatory factor-1 is required for a T helper 1 immune response in vivo. Immunity. 1997;6:681. doi: 10.1016/s1074-7613(00)80444-6. [DOI] [PubMed] [Google Scholar]
- 40.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587. doi: 10.1016/s0092-8674(00)80240-8. 1997. [DOI] [PubMed] [Google Scholar]
- 41.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 42.Constant SL, Bottomly K. Induction of Th1 and Th2, CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 43.Jacobson NG, Szabo SJ, Weber-Nordt RM, et al. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat) 3 and Stat4. J Exp Med. 1995;181:1755. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magram J, Connaughton SE, Warrier RR, et al. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 45.Thierfelder WE, van-Deursen JM, Yamamoto K, et al. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 46.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 47.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 48.Kopf M, Le GG, Bachmann M, Lamers MC, Blueth-mann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;245:1993. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 49.Lederer JA, Perez VL, Desroches L, Kim SM, Abbas AK, Lichtman AH. Cytokine transcriptional events during helper T cell subset differentiation. J Exp Med. 1996;184:397. doi: 10.1084/jem.184.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 51.Akimoto T, Numata F, Tamura M, et al. Abrogation of bronchial eosinophilic inflammation and airway hyperreactivity in signal transducers and activatiors of transcription (STAT) 6-deficient mice. J Exp Med. 1998;187:1537. doi: 10.1084/jem.187.9.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kline JN, Waldschmidt TJ, Businga TR, et al. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J Immunol. 1998;160:2555. [PubMed] [Google Scholar]