Abstract
The α‐galactosylceramide KRN7000 was reported to be presented by CD1d to natural killer (NK) T cells, cells that are thought to play an important role in the rejection of malignant tumours and in the regulation of several autoimmune diseases. Here we analysed human peripheral blood (PB) NK T cells (Vα24+ Vβ11+ T cells) before and after a short‐term culture in the presence of KRN7000. KRN7000 strongly activated PB Vα24+ Vβ11+ T cells and, when stimulated, the vast majority of these cells expressed interferon‐γ (IFN‐γ). Exposure of these KRN7000‐cultured Vα24+ Vβ11+ T cells to interleukin‐12 (IL‐12), but not to IL‐7, resulted in a relative increase in IFN‐γ‐expressing Vα24+ Vβ11+ T cells, compared with IL‐4‐expressing Vα24+ Vβ11+ T cells, indicating a shift towards a T‐helper type 1 (Th1) phenotype. KRN7000 strongly up‐regulated the expression of the cytotoxic molecule granzyme B (GrB) in Vα24+ Vβ11+ T cells. Although IL‐7 resulted in a decrease in GrB levels in KRN7000‐cultured Vα24+ Vβ11+ T cells, IL‐12 increased GrB levels in both Vα24+ Vβ11+ T cells and in Vα24+ Vβ11+ T‐cell clones and increased cytotoxicity against hCD1d‐transfected HeLa cells. Our data provide further insight into the characteristics of human Vα24+ Vβ11+ T cells and indicate that KRN7000 is a potent activator of Vα24+ Vβ11+ T cells. Combined with the established anti‐tumour effects of KRN7000 in mouse models, these results may support the use of KRN7000 as an anti‐tumour agent in man.
Introduction
Natural killer (NK) T cells, which share characteristics of both T cells and NK cells, have an extremely restricted T‐cell receptor (TCR) repertoire, in man consisting of a Vα24‐chain preferentially paired with a Vβ11‐chain.1–5 In contrast to classical peptide antigen‐presenting major histocompatibility complex (MHC) class I and II molecules, CD1 molecules present lipid antigens or hydrophobic peptide antigens.6–8 Both mouse and human CD1d molecules can present the glycolipid α‐galactosylceramide (KRN7000) to NK T cells from either species,9,10 illustrating a strong conservation of recognition. Because of the capacity of both human and mouse NK T cells to rapidly produce large amounts of interleukin‐4 (IL‐4) and interferon‐γ (IFN‐γ) upon activation, a possible role of NK T cells in the regulation of immune responses has been hypothesized.11,12 Important roles have been suggested in the rejection of malignant tumours and in the regulation of autoimmune diseases.13–16 The importance of NK T cells in anti‐tumour immunity has been demonstrated in Vα14 NK T‐cell‐deficient mice that could no longer mediate IL‐12‐induced rejection of tumours.13 It has been reported that both IL‐12 and IL‐7 can influence the cytokine profiles of mouse NK T cells. IL‐12 can induce production of perforin in mouse NK1+ T cells and can bias IL‐4‐producing NK T cells towards IFN‐γ production.17,18 Furthermore, IL‐7 was shown to reverse NK1+ T‐cell‐defective IL‐4 production in thenon‐obese diabetic mouse.19
KRN7000 was reported to have strong anti‐tumour activity in mice with colon26 hepatic metastases. Its administration resulted in a high percentage of cured mice that acquired tumour‐specific immunity.20 Data on the characteristics of human peripheral blood (PB) NK T cells (Vα24+ Vβ11+ T cells) are limited. Considering the potential of KRN7000 to act as an anti‐tumour agent, it is essential to elucidate further the effects of KRN7000 on human Vα24+ Vβ11+ T cells.
Here, we analysed healthy donor‐derived PB Vα24+ Vβ11+ T cells before and after a short‐term culture in the presence of KRN7000. Furthermore, the effects of IL‐12 and IL‐7 on the cytokine profile and granzyme B (GrB) content of KRN7000‐cultured Vα24+ Vβ11+ T cells were analysed. KRN7000 caused strong activation of PB Vα24+ Vβ11+ T cells, the vast majority of which was capable of producing IFN‐γ. KRN7000 up‐regulated the expression of GrB, a molecule which cleaves peptide bonds after aspartic acid residues and induces apoptosis in target cells,21,22 in Vα24+ Vβ11+ T cells. IL‐12 and IL‐7 resulted in a further increase and decrease in GrB levels, respectively. Finally, we showed that the IL‐12‐induced increase in GrB expression in Vα24+ Vβ11+ T cells was related to an increase in cytotoxicity. These data, combined with the reported anti‐tumour effects of KRN7000 in mice, further support the rationale for the evaluation of KRN7000 as an anti‐tumour agent in man.
Materials and methods
Cell preparation
Peripheral blood mononuclear cells (PBMC) were obtained from the blood of healthy volunteers (four males; two females; median age 32 years, range 25–37 years) by Lymphoprep® (Nycomed Pharma AS, Oslo, Norway) density gradient centrifugation and were washed twice with phosphate‐buffered saline (PBS).
Flow cytometric analysis
To reduce non‐specific binding, cells were incubated with human immunoglobulin G (IgG) (Calbiochem‐Novabiochem Corporation, La Jolla, CA) and washed twice. For detection of intracellular antigens, cells were fixed with either 50 µl of buffered formaldehyde acetone composed of 0·2 mg Na2HPO4 ·2 H2O/ml, 1 mg KH2PO4/ml, 45% (v/v) acetone, 9·75% (v/v) formaldehyde and 45·25% (v/v) distilled water (pH 6·8) for 90 seconds (for detection of GrB) or with 4% paraformaldehyde for 10 min (for detection of IL‐4 and IFN‐γ) and, thereafter, washed in PBS supplemented with 0·5% bovine serum albumin (BSA) and 0·1% (w/v) sodium azide containing 0·1% (w/v) saponin and 50 mm d‐glucose to permeabilize cells. For intracellular blocking of non‐specific binding, cells were again incubated with human IgG. Fluorescein isothiocyanate (FITC)‐labelled IgG1, phycoerythrin (PE)‐labelled IgG2a, PE‐labelled CD4, PE‐labelled CD8α, PE‐labelled CD56 and PE‐labelled CD161 were purchased from Becton Dickinson (San Jose, CA). FITC‐labelled anti‐human Vα24 and biotin‐labelled anti‐human Vβ11 were purchased from Immunotech (Marseille, France). Streptavidin‐RPE‐Cy5 was obtained from Dako (Glostrup, Denmark). PE‐labelled CD25, PE‐labelled anti‐IL‐4 and PE‐labelled anti‐IFN‐γ were obtained from Pharmingen (San Diego, CA). PE‐labelled anti‐granzyme B11 was a gift from the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service (CLB, Amsterdam, the Netherlands).23 Flow cytometric analysis was performed on a fluorescence‐activated cell sorter (FACStar plus), equipped with an argon‐ion laser (Becton Dickinson).
KRN7000
KRN7000 ((2S,3S,4R)‐1‐O (α‐d‐galactopyranosyl)‐2‐(N‐hexacosanoylamino)‐1,3‐4‐octadecanetriol) was synthesized by the Pharmaceutical Research Laboratory, Kirin Brewery. KRN7000 was dissolved in 100% dimethyl sulphoxide (DMSO). Final concentration of DMSO in cultures was 0·1%.
Expansion and culture of Vα24+ Vβ11+ T cells
For expansion of Vα24+ Vβ11+ T cells, total human PBMC were cultured for 7 days in Iscove's modified Dulbecco's medium (IMDM, BioWhitaker, Verviers, Belgium) supplemented with 50 U/ml penicillin–streptomycin, 0·01 mm 2‐mercaptoethanol (2‐ME), 1·6 mm l‐glutamine, 25 mm HEPES, 20% human pooled serum (HPS, CLB) and 100 ng/ml of KRN7000. Part of the cultures received in addition 10 ng/ml (final concentration) of recombinant human (rh) IL‐12 (Hoffmann La Roche Inc., Nutley, NJ) or 10 ng/ml of rhIL‐7 (R & D systems, Minneapolis, MN) during the last 24 hr. Cells were then used for FACS analysis. In order to analyse cellular IL‐4 and IFN‐γ content after stimulation, cells were stimulated with calcium‐ionophore (1 µm, Sigma, St. Louis, MO) and phorbol 12‐myristate 13‐acetate (PMA; 20 ng/ml, Sigma) in the presence of monensin (3 µm, Sigma) for 4 hr. The human Vα24+ Vβ11+ T‐cell clones B11 and C14, previously described,24,25 were cultured in RPMI‐1640 medium supplemented with 50 U/ml penicillin–streptomycin, 0·01 mm 2‐ME, 1·6 mm l‐glutamine, 25 mm HEPES, 8% HPS and 150 U/ml rhIL‐2 (Hoffmann La Roche Inc.). For analysis of the effects on GrB expression in Vα24+ Vβ11+ T‐cell clones, 10 ng/ml of either rhIL‐12 or rhIL‐7 was added to the cultures.
Culture of monocyte‐derived dendritic cells (moDC)
PBMC were allowed to adhere to culture flasks for 2 hr at 37°. Adherent cells were cultured for 7 days in the presence of rhIL‐4 (1000 U/ml, CLB) and recombinant human granulocyte–macrophage colony‐stimulating factor (rh GM‐CSF; 100 ng/ml, Kirin Brewery) in IMDM supplemented with 50 U/ml penicillin–streptomycin, 0·01 mm 2‐ME and 10% FCS (Integro b.v., Zaandam, the Netherlands). Immature moDC were then cultured for 3 days with tumour necrosis factor‐α (TNF‐α; 50 ng/ml, Cetus, Amsterdam, the Netherlands) in the presence of either KRN7000 (100 ng/ml) or an equal amount of vehicle (DMSO). Mature moDC were washed and co‐cultured with clone C14 for 48 hr. Cells were then used for FACS analysis.
Cell‐mediated cytotoxicity
Cytotoxicity was assessed using a standard 4‐hr 51Cr‐release assay at the indicated effector : target ratios. Cells from clone B11, cultured in the presence or absence of rhIL‐12 (10 ng/ml) for 48 hr, were used as effector cells. HeLa/CD1d cells were used as target cells.
Statistical analysis
Statistical analysis was performed using the paired Student'st‐test. P < 0·05 was considered significant.
Results
Phenotype of peripheral blood Vα24+ Vβ11+ T cells
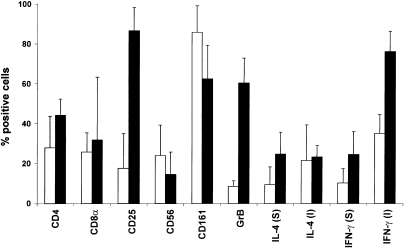
Flow cytometry was used to analyse the phenotype of Vα24+ Vβ11+ T cells in freshly isolated PBMC from six healthy volunteers (Fig. 1, open bars). CD161 (NKR‐P1A), an NK locus‐encoded C‐type lectin, was expressed on the vast majority of Vα24+ Vβ11+ T cells in all donors tested. CD4 was expressed by 27·9% (mean) and the CD8α chain was expressed by 25·7% of Vα24+ Vβ11+ T cells. CD56 was expressed by 24·0% of PB Vα24+ Vβ11+ T cells. Expression of GrB was confined to 8·4% of Vα24+ Vβ11+ T cells, and 17·6% expressed CD25. IL‐4 was expressed by 9·3% of Vα24+ Vβ11+ T cells, while 10·2% expressed IFN‐γ. When Vα24+ Vβ11+ T cells were stimulated with PMA and calcium‐ionophore, 21·4% expressed IL‐4 and 34·8% expressed IFN‐γ.
Figure 1.
Phenotype of PB Vα24+ Vβ11+ T cells before and after a 7‐day culture in the presence of KRN7000. PB Vα24+ Vβ11+ T cells (open bars, n = 6) and KRN7000‐cultured Vα24+ Vβ11+ T cells (closed bars, n = 3) were phenotypically analysed using three‐colour flow cytometry. Mean + SD of Vα24+ Vβ11+ T cells positive for various markers is shown. IL‐4 and IFN‐γ expression were assessed before [spontaneous (S)] and after [induced (I)] a 4‐hr stimulation with PMA and calcium‐ionophore.
Phenotype of Vα24+ Vβ11+ T cells after a short‐term culture in the presence of KRN7000
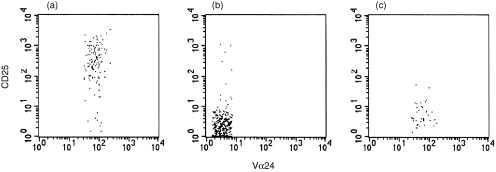
The effect of KRN7000 on the phenotype of Vα24+ Vβ11+ T cells was analysed in three healthy donors (Fig. 1, closed bars and Table 1). Vα24+ Vβ11+ T cells constituted 0·09% ± 0·02% (mean ± standard error of mean, n = 5) of the PB lymphocyte population. During a 7‐day culture in the presence of KRN7000, Vα24+ Vβ11+ T cells expanded approximately 2·7‐fold, while no expansion was observed using the vehicle control (N. Nishi et al. submitted for publication). Before culture, 10·7% ± 11·0% [mean ± standard deviation (SD)] of Vα24+ Vβ11+ T cells expressed the activation marker CD25, while this increased to 86·4% ± 11·6% after culture (P = 0·0085). Activation of Vα24+ Vβ11+ T cells was not accompanied by similar activation in either Vα24– Vβ11+ T cells or Vα24+ Vβ11– T cells, indicating that the KRN7000 induced activation was not a general phenomenon (Fig. 2). The expression of GrB was strongly up‐regulated in Vα24+ Vβ11+ T cells (P = 0·0135), since 6·5% ± 2·8% of Vα24+ Vβ11+ T cells expressed GrB before and 60·3% ± 12·5% after culture. We observed a small, but not significant, decrease in the percentage of cells expressing CD161 after culture in the presence of KRN7000. When cultured in the presence of KRN7000, 24·5% ± 11·0% and 24·3% ± 11·5% of Vα24+ Vβ11+ T cells spontaneously expressed IL‐4 and IFN‐γ, respectively. After stimulation with PMA and calcium‐ionophore, 23·0% ± 5·9% and 76·0% ± 10·1% of cultured Vα24+ Vβ11+ T cells expressed IL‐4 and IFN‐γ, respectively.
Table 1.
Phenotype of PB Vα24+Vβ11+‐T cells before (day 0) and after (day 7) a 7‐day culture in the presence of KRN7000
| Donor 1 | Donor 2 | Donor 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Change | Day 0 | Day 7 | Change | Day 0 | Day 7 | Change | |
| CD4 | 22·7 | 51·0 | + 28·3 | 26·3 | 35·1 | + 8·8 | 58·8 | 46·3 | − 12·5 |
| CD8α | 24·3 | 9·2 | − 15·1 | 23·1 | 71·6 | + 48·5 | 20·7 | 14·0 | − 6·7 |
| CD25 | 23·1 | 88·6 | + 65·5 | 7·1 | 96·2 | + 89·1 | 2·0 | 74·3 | + 72·3 |
| CD56 | 14·8 | 3·0 | − 11·8 | 19·4 | 25·3 | + 5·9 | 9·7 | 15·1 | + 5·4 |
| CD161 | 79·6 | 50·3 | − 29·3 | 96·0 | 81·4 | − 14·6 | 64·8 | 55·4 | − 9·4 |
| GrB | 4·0 | 60·6 | + 56·6 | 9·6 | 72·6 | + 63·0 | 5·9 | 47·6 | + 41·7 |
| IL‐4 (s) | 2·6 | 25·0 | + 22·4 | 3·5 | 13·3 | + 9·8 | 4·3 | 35·2 | + 30·9 |
| IL‐4 (i) | 20·0 | 26·3 | + 6·3 | 4·5 | 16·1 | + 11·6 | 3·8 | 26·5 | + 22·7 |
| IFN‐γ (s) | 6·4 | 17·0 | + 11·6 | 3·8 | 18·3 | + 14·5 | 2·8 | 37·5 | + 34·7 |
| IFN‐γ (i) | 25·4 | 80·8 | + 55·4 | 23·6 | 82·7 | + 59·1 | 36·0 | 64·4 | + 28·4 |
Percentages of Vα24+ Vβ11+ T cells expressing the various markers are shown. Expression of IL‐4 and IFN‐γ was assessed both before (s) and after (i) a 4‐hr stimulation with PMA and calcium‐ionophore. The increase in GrB expression, the increase in CD25 expression and the increase in IFN‐γ (i) expression were significant (P = 0·0135, P = 0·0085 and P = 0·0389, respectively, paired Student's t‐test). Data of three donors are shown.
Figure 2.
KRN7000 induces activation of PB Vα24+ Vβ11+ T cells but not of PB Vα24– Vβ11+ T cells or of PB Vα24+ Vβ11– T cells. PBMC were cultured in the presence of KRN7000 for 7 days. Using three‐colour flow‐cytometry, CD25 expression was analysed in Vα24+ Vβ11+ T cells (a), Vα24– Vβ11+ T cells (b) and Vα24+ Vβ11– T cells (c). Representative dot‐plots of donor 1 are shown.
Effects of IL‐12 and IL‐7 on granzyme B expression and cytokine profile of Vα24+ Vβ11+ T cells
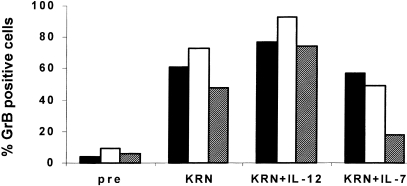
As mentioned, KRN7000 induced the expression of GrB in Vα24+ Vβ11+ T cells. Although IL‐12, added during the last 24 hr of a culture of PBMC in the presence of KRN7000, resulted in an even higher expression of GrB, IL‐7 exerted the opposite effect, resulting in a decrease in GrB content in KRN7000‐cultured Vα24+ Vβ11+ T cells (Fig. 3). Representative data from one donor are shown in Fig. 4. IL‐12 and IL‐7 not only modified the percentage of Vα24+ Vβ11+ T cells that expressed GrB, but also influenced the amount of GrB per cell (as indicated by the mean fluorescence). When IL‐12 was added during the last 24 hr of the culture, we observed a shift in the spontaneously expressed cytokine balance of Vα24+ Vβ11+ T cells towards a T‐helper type 1 (Th1) phenotype in all three donors tested (Table 2). IL‐7, on the other hand, failed to show a consistent effect on the balance of cytokines (Table 2). In all donors, we observed expression of IFN‐γ in the vast majority of KRN7000‐cultured Vα24+ Vβ11+ T cells, when cells were stimulated with PMA and calcium‐ionophore. In donor 2, IL‐12 induced a further increase in the percentage of Vα24+ Vβ11+ T cells that expressed IFN‐γ after stimulation with PMA and calcium‐ionophore, and also resulted in an increase in the mean amount of IFN‐γ in these cells. In contrast, the addition of IL‐7 resulted in a decrease in both the percentage of Vα24+ Vβ11+ T cells that expressed IFN‐γ and in the amount of IFN‐γ in these cells (Table 2 and data not shown). No clear effects ofIL‐12 and IL‐7 on the percentage of Vα24+ Vβ11+ T cells expressing IL‐4 or IFN‐γ could be observed in the other donors when cells were stimulated with PMA and calcium‐ionophore.
Figure 3.
Effects of KRN7000, IL‐12 and IL‐7 on GrB expression in Vα24+ Vβ11+ T cells. GrB expression was assessed in Vα24+ Vβ11+ T cells that were either freshly isolated (pre), cultured for 7 days in the presence of KRN7000 (KRN) or cultured for 7 days in the presence of KRN7000 plus IL‐12 or IL‐7 during the last 24 hr. Data of three donors are shown (donor 1, closed bars; donor 2, open bars; donor 3,hatched bars).
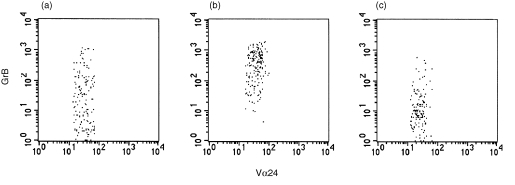
Figure 4.
Effects of KRN7000, IL‐12 and IL‐7 on GrB expression by Vα24+ Vβ11+ T cells. GrB expression was analysed using three‐colour flow cytometry. Expression of GrB in PB Vα24+ Vβ11+ T cells that were cultured for 7 days in the presence of KRN7000 is shown (a). Addition of IL‐12 for 24 hr increased GrB levels (b), while addition of IL‐7 decreased GrB levels (c). Representative dot‐plots of donor 2 are shown.
Table 2.
Effects of IL‐12 and IL‐7 on the percentages of Vα24+ Vβ11+ T cells that express IL‐4 and IFN‐γ
| Donor 1 | Donor 2 | Donor 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| KRN | KRN+IL‐12 | KRN+IL‐7 | KRN | KRN+IL‐12 | KRN+IL‐7 | KRN | KRN+IL+12 | KRN+IL‐7 | |
| IL‐4 (s) | 25·0 | 14·8 | 23·7 | 13·3 | 4·8 | 6·8 | 35·2 | 15·2 | 21·8 |
| IFN‐γ (s) | 17·0 | 58·1 | 37·7 | 18·3 | 17·3 | 4·4 | 37·5 | 40·0 | 19·1 |
| IFN‐γ : IL‐4 (s) | 0·7 | 3·9 | 1·6 | 1·4 | 3·6 | 0·6 | 1·1 | 2·6 | 0·9 |
| IL‐4 (i) | 26·3 | 26·8 | 34·4 | 16·1 | 9·7 | 15·2 | 26·5 | 22·7 | 23·3 |
| IFN‐γ (i) | 80·8 | 82·4 | 77·1 | 82·7 | 96·8 | 72·6 | 64·4 | 62·3 | 61·5 |
PBMC were cultured for 7 days in the presence of KRN7000 (KRN) or cultured for 7 days in the presence of KRN7000 plus IL‐12 or IL‐7 during the last 24 hr. The percentages of IL‐4‐ and IFN‐γ‐expressing cells were assessed both before (s) and after (i) a 4‐hr stimulation with PMA and calcium‐ionophore. IFN‐γ : IL‐4 ratios were calculated by dividing the percentages of Vα24+ Vβ11+ T cells spontaneously expressing IFN‐γ and IL‐4, respectively. Data of three donors are shown.
Effects of KRN7000, IL‐12 and IL‐7 on human Vα24+ Vβ11+ T‐cell clones
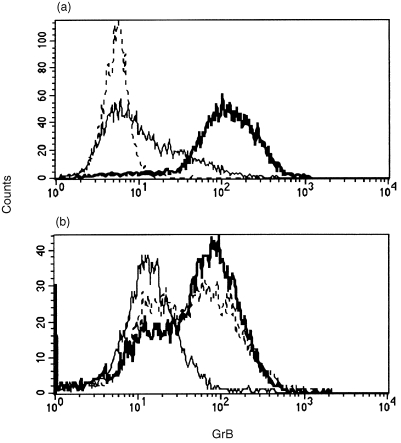
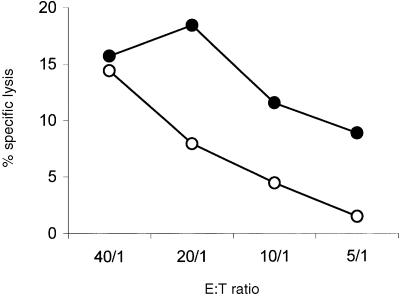
To determine whether KRN7000 could also result in up‐regulation of GrB expression in a Vα24+ Vβ11+ T‐cell clone, moDC and clone C14 were co‐cultured for 48 hr. Clone C14 was selected for co‐culture experiments since clone B11 was previously shown to be CD1d autoreactive.24 The addition of KRN7000 during maturation of the moDC clearlyup‐regulated the expression of GrB in C14 cells as compared to the vehicle control (Fig. 5a). The effects of IL‐12 and IL‐7 on the expression of GrB were studied in clones B11 and C14. IL‐12 induced a time‐dependent up‐regulation of the expression of GrB in both clones (Fig. 5b) while IL‐7 did not affect the expression of GrB in these clones (not shown). Clone B11, cultured in the presence of IL‐12 for 48 hr, showed an increase in cytotoxicity towards HeLa/CD1d at various effector to target ratios (Fig. 6). Both clones spontaneously expressed IL‐4 and, to a lesser extent, IFN‐γ, and could be induced to express high levels of both IL‐4 and IFN‐γ after a 4‐hr stimulation with PMA and calcium‐ionophore. However, no consistent effects on the expression of IL‐4 and IFN‐γ were observed when either IL‐12 or IL‐7 was added to the cultures (not shown).
Figure 5.
Effects of KRN7000 and IL‐12 on GrB expression by Vα24+ Vβ11+ T‐cell clones. Clone C14 and allogeneic moDC, matured in the presence of either KRN7000 (bold line) or vehicle (line), were co‐cultured for 48 hr and GrB expression was assessed by flow cytometry, the dotted line represents isotype control (a). IL‐12 induces a time‐dependent up‐regulation of GrB expression in clone B11. Zero hours (solid line), 24 hr (dotted line) and 48 hr (bold line) (b).
Figure 6.
Analysis of the effect of IL‐12 on the cytotoxicity of Vα24+ Vβ11+ T‐cell clone B11 against HeLa/CD1d. Clone B11 was cultured for 48 hr in the presence (•) or absence (○) of IL‐12. Cytolytic potential against CD1d‐transfected HeLa cells was assessed.
Discussion
The combination of a highly conserved recognition system, involving CD1d,9,10 and the potential of both mouse and human NK T cells to produce large amounts of IL‐4 and IFN‐γ rapidly upon activation,11,12 have drawn attention to the special nature of NK T cells. Synthetic glycolipids with an α‐anomeric structure, including KRN7000, can be recognized by NK T‐cell clones and can trigger proliferation, cytokine release (IL‐4 and IFN‐γ) and cytotoxic activity.10 KRN7000 could therefore be a useful agent in the modulation of immune responses and, more specifically, has the potential to act as an anti‐tumour agent.20
In the present study, we analysed the phenotype of PB Vα24+ Vβ11+ T cells both before and after culture in the presence of KRN7000. Several reports described the phenotype and function of Vα24+ Vβ11+ T‐cell clones and cell lines.11,25,26 Little, however, is currently known on the phenotype of uncultured PB Vα24+ Vβ11+ T cells. It was reported that these cells are CD8αdim/β– and show significantly enhanced expression of both IL‐2Rβ and the memory marker CD45RO compared to mainstream αβ T cells, while PB Vα24+ Vβ11+ T cells did not appear to be constitutively activated.27 In contrast to the reported absence of CD56 on Vα24+ Vβ11+ T‐cell clones,11 we show that approximately 24·0% of PB Vα24+ Vβ11+ T cells expressed CD56. Expression of the NK locus‐encoded C‐type lectin CD161 (NKR‐P1A) has been reported to be quite heterogeneous between different donors,27 but our data show expression of CD161 on a high proportion of Vα24+ Vβ11+ T cells in all donors tested. However, upon culture in the presence of KRN7000 a decrease in the percentage of Vα24+ Vβ11+ T cells expressing CD161 was observed. Since CD161 was reported to be involved in triggering cytotoxicity,28,29 this decrease might indicate that CD161 does not play a major role in the cytotoxicity mediated by KRN7000‐activated Vα24+ Vβ11+ T cells. This is supported by the demonstration that CD1d‐restricted target cell lysis by activated invariant Vα24 T cells was CD161 independent.30 KRN7000 induced high levels of CD25 in Vα24+ Vβ11+ T cells, but not in Vα24– Vβ11+ T cells and Vα24+ Vβ11– T cells. Since KRN7000‐cultured Vα24+Vβ11+ T cells strongly expressed CD25 (IL‐2Rα), it can be expected that cytokines targeting the IL‐2R will have potent effects on the expansion of Vα24+ Vβ11+ T cells.
Previous reports showed that mouse NK T cells induced cytotoxicity through an NK‐like effector mechanism, involving the release of cytotoxic granules.13,31 Since KRN7000 has been shown to be capable of inducing a strong anti‐tumour immune response in mouse models,20 we investigated the effects of KRN7000 on the expression of GrB. It is known that GrB, which cleaves peptide bonds after aspartic acid residues, causes apoptosis in the presence of perforin, through the activation of caspases.21,22 Using both PB Vα24+ Vβ11+ T cells and human Vα24+ Vβ11+ T‐cell clones, we showed that KRN7000 strongly up‐regulated GrB expression and that IL‐12, previously shown to induce GrB expression in NK cells and T cells,32,33 resulted in a further increase in GrB expression in KRN7000‐cultured PB Vα24+ Vβ11+ T cells and Vα24+ Vβ11+ T‐cell clones. Furthermore, this IL‐12‐induced increase in GrB expression in the Vα24+ Vβ11+ T‐cell clone B11 was related to an increase in cytotoxicity, illustrating the relation between an increased expression of GrB in Vα24+ Vβ11+ T cells and an increase in their cytotoxic potential. KRN7000 might therefore, at least in part, exert its anti‐tumour effect through an increase in GrB expression in Vα24+ Vβ11+ T cells and IL‐12 might potentiate this effect.
It has been found that both IL‐12 and IL‐7 can influence the cytokine profiles of mouse NK T cells.17,19 Culture with KRN7000 resulted in an increase in Vα24+ Vβ11+ T cells that spontaneously expressed IL‐4 and IFN‐γ. However, when cultured Vα24+ Vβ11+ T cells were stimulated with PMA and calcium‐ionophore, a large proportion of these cells expressed IFN‐γ, while the percentage of Vα24+ Vβ11+ T cells expressing IL‐4 could not be increased in a similar fashion. This suggests a Th1 phenotype for this KRN7000‐cultured PB Vα24+ Vβ11+ T‐cell population. The spontaneously expressed cytokine balance of KRN7000‐cultured PB Vα24+ Vβ11+ T cells could be modified, IL‐12 resulting in a relative increase in IFN‐γ‐expressing Vα24+ Vβ11+ T cells, compared to IL‐4‐expressing Vα24+ Vβ11+ T cells, indicating a shift towards a Th1 phenotype. It is therefore tempting to assume that, as has been hypothesized in mice,17 cytokines present in the microenvironment contribute to directing the immune response of Vα24+ Vβ11+ T cells.
In conclusion, our data demonstrate that KRN7000 can modify the phenotype and cytokine profile of PB Vα24+ Vβ11+ T cells. KRN7000 showed strong activation of PB Vα24+ Vβ11+ T cells that, when stimulated, expressed high levels of the Th1‐type cytokine IFN‐γ. These activated cells could, however, still produce both IL‐4 and IFN‐γ and the cytokine profile of these activated Vα24+ Vβ11+ T cells could be altered by cytokines in the environment. The observed activation of Vα24+ Vβ11+ T cells combined with the KRN7000‐induced up‐regulation of the cytotoxic molecule GrB further strengthen the rationale for the evaluation of the use of KRN7000 in anti‐tumour immune therapy. A phase I clinical trial of KRN7000 is currently being conducted in our hospital in patients with solid tumours.
Acknowledgments
We thank Dr Tanja D. de Gruijl (Department of Medical Oncology, University Hospital Vrije Universiteit, Amsterdam, the Netherlands) and Dr Marc Bonneville (INSERM, Nantes, France) for critical reading of the manuscript and Dr Laurent Brossay (La Jolla Institute of Allergy and Immunology, San Diego, CA) for providing human CD1d‐transfected HeLa cells. This work was supported by a grant from the Netherlands Organization for Scientific Research (NWO)
Glossary
Abbreviations
- GrB
granzyme B
- HeLa/CD1d
hCD1d‐transfected HeLa cells
- IL
interleukin
- IFN
interferon
- moDC
monocyte‐derived dendritic cells
- PB
peripheral blood
- PBMC
peripheral blood mononuclear cells
References
- 1.Lantz O, Bendelac A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I‐specific CD4+ and CD4–CD8– T cells in mice and humans. J Exp Med. 1994;180:1097. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koseki H, Asano H, Inabe T, et al. Dominant expression of a distinctive V14+ T‐cell antigen receptor α chain in mice. Proc Natl Acad Sci USA. 1991;88:7518. doi: 10.1073/pnas.88.17.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dellabona P, Casorati G, Friedli B, et al. In vivo persistence of expanded clones specific for bacterial antigens within the human T cell receptor α/β CD4–CD8– subset. J Exp Med. 1993;177:1763. doi: 10.1084/jem.177.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant Vα24‐JαQ/Vβ11 T cell receptor is expressed in all individuals by clonally expanded CD4–CD8– T cells. J Exp Med. 1994;180:1171. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell receptor (TCR) expression by human peripheral blood CD4–CD8– α/β T cells demonstrates preferential use of several Vβ genes and an invariant α chain. J Exp Med. 1993;178:1. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porcelli SA. The CD1 family: a third lineage of antigen presenting molecules. Adv Immunol. 1995;59:1. doi: 10.1016/s0065-2776(08)60629-x. [DOI] [PubMed] [Google Scholar]
- 7.Beckman EM, Brenner MB. MHC class I‐like, class II‐like and CD1 molecules: distinct roles in immunity. Immunol Today. 1995;16:349. doi: 10.1016/0167-5699(95)80154-5. [DOI] [PubMed] [Google Scholar]
- 8.Castano AR, Tangri S, Miller JE, et al. Peptide binding and presentation by mouse CD1. Science. 1995;269:223. doi: 10.1126/science.7542403. [DOI] [PubMed] [Google Scholar]
- 9.Brossay L, Chioda M, Burdin N, et al. CD1d‐mediated recognition of an α‐galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spada FM, Koezuka Y, Porcelli SA. CD1d‐restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1529. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Vα24+ CD4–CD8– T cells. J Exp Med. 1997;186:109. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Paul WE. Cultured NK1.1+ CD4+ T cells produce large amounts of IL‐4 and IFN‐γ upon activation by anti‐CD3 or CD1. J Immunol. 1997;159:2240. [PubMed] [Google Scholar]
- 13.Cui J, Shin T, Kawano T, et al. Requirement for Vα14 NKT cells in IL‐12‐mediated rejection of tumors. Science. 1997;278:1623. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 14.Wilson SB, Kent SC, Patton KT, et al. Extreme Th1 bias of invariant Vα24JαQ T cells in type I diabetes. Nature. 1998;391:177. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 15.Mieza MA, Itoh T, Cui JQ, et al. Selective reduction of Vα14+ NK T cells associated with disease development in autoimmune‐prone mice. J Immunol. 1996;156:4035. [PubMed] [Google Scholar]
- 16.Sumida T, Sakamoto A, Murata H, et al. Selective reduction of T cells bearing invariant Vα24JαQ antigen receptor in patients with systemic sclerosis. J Exp Med. 1995;182:1163. doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leite‐de‐moraes MC, Moreau G, Arnould A, et al. IL‐4‐producing NK T cells are biased towards IFN‐γ productionby IL‐12. Influence of the microenvironment on the functional capacities of NK T cells. Eur J Immunol. 1998;28:1507. doi: 10.1002/(SICI)1521-4141(199805)28:05<1507::AID-IMMU1507>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 18.Kawamura T, Takeda K, Mendiratta SK, et al. Critical role of NK1+ T cells in IL‐12‐induced immune responses in vivo. J Immunol. 1998;160:16. [PubMed] [Google Scholar]
- 19.Gombert JM, Tancrede‐bohin E, Hameg A, et al. IL‐7 reverses NK1+ T cell‐defective IL‐4 production in the non‐obese diabetic mouse. Int Immunol. 1996;8:1751. doi: 10.1093/intimm/8.11.1751. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa R, Motoki K, Ueno H, et al. Treatment of hepatic metastasis of the colon26 adenocarcinoma with an α‐galactosylceramide, KRN7000. Cancer Res. 1998;58:1202. [PubMed] [Google Scholar]
- 21.Liu CC, Young LHY, Young JDE. Mechanisms of disease: lymphocyte‐mediated cytolysis and disease. N Engl J Med. 1996;335:1651. doi: 10.1056/NEJM199611283352206. [DOI] [PubMed] [Google Scholar]
- 22.Talanian RV, Yang XH, Turbov J, et al. Granule‐mediated killing: pathways for granzyme B‐initiated apoptosis. J Exp Med. 1997;186:1323. doi: 10.1084/jem.186.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wever PC, van der Vliet HJJ, Spaeny LHA, et al. The CD8+ granzyme B+ T cell subset in peripheral blood from healthy individuals contains activated and apoptosis‐prone cells. Immunology. 1998;93:383. doi: 10.1046/j.1365-2567.1998.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couedel C, Peyrat MA, Brossay L, et al. Diverse CD1d‐restricted reactivity patterns of human T cells bearing ‘invariant’ AV24BV11 TCR. Eur J Immunol. 1998;28:4391. doi: 10.1002/(SICI)1521-4141(199812)28:12<4391::AID-IMMU4391>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Davodeau F, Peyrat MA, Necker A, et al. Close phenotypic and functional similarities between human and murine αβ T‐cell expressing invariant TCR α chains. J Immunol. 1997;158:5603. [PubMed] [Google Scholar]
- 26.Nieda M, Nicol A, Koezuka Y, et al. Activation of human Vα24NKT cells by α‐glycosylceramide in a CD1d‐restricted and Vα24TCR‐mediated manner. Hum Immunol. 1999;60:10. doi: 10.1016/s0198-8859(98)00100-1. [DOI] [PubMed] [Google Scholar]
- 27.Prussin C, Foster B. TCR Vα24 and Vβ11 coexpression defines a human NK1 T cell analog containing a unique Th0 subpopulation. J Immunol. 1997;159:5862. [PubMed] [Google Scholar]
- 28.Lanier LL, Chang C, Phillips JH. Human NKR‐P1A. A disulfide‐linked homodimer of the C‐type lectin superfamily expressed by a subset of NK and T lymphocytes. J Immunol. 1994;153:2417. [PubMed] [Google Scholar]
- 29.Poggi A, Rubartelli A, Moretta L, Zocchi MR. Expression and function of NKRP1A molecule on human monocytes and dendritic cells. Eur J Immunol. 1997;27:2965. doi: 10.1002/eji.1830271132. [DOI] [PubMed] [Google Scholar]
- 30.Exley M, Porcelli S, Furman M, Garcia J, Balk S. CD161 (NKR‐P1A) costimulation of CD1d‐dependent activation of human T cells expressing invariant Vα24JαQ T cell receptor α chains. J Exp Med. 1998;188:867. doi: 10.1084/jem.188.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawano T, Cui J, Koezuka Y, et al. Natural killer‐like nonspecific tumor cell lysis mediated by specific ligand‐activated Vα14 NKT cells. Proc Natl Acad Sci USA. 1998;95:5690. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salcedo TW, Azzoni L, Wolf SF, Perussia B. Modulation of perforin and granzyme messenger RNA expression in human natural killer cells. J Immunol. 1993;151:2511. [PubMed] [Google Scholar]
- 33.Chouaib S, Chemini J, Bani L, et al. Interleukin 12 induces the differentiation of major histocompatibility complex class‐I primed cytotoxic T‐lymphocyte precursors into allospecific cytotoxic effectors. Proc Natl Acad Sci USA. 1994;91:12659. doi: 10.1073/pnas.91.26.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]