Abstract
Recent studies in animal models of genital chlamydial disease revealed that early recruitment of dendritic cells and specific T helper type‐1 (Th1) cells into the genital mucosae is crucial for reducing the severity of the acute phase of a cervico‐vaginal infection and arresting ascending disease. These immune effectors are therefore important for preventing major complications of genital chlamydial infection. Other in vitro studies showed that intercellular adhesion molecule‐1 (ICAM‐1) plays a role in the antichlamydial action of specific CD4+ and CD8+ T cells. In the present study, we investigated the clinicopathological consequences of ICAM‐1 deficiency during chlamydial genital infection in ICAM‐1 knockout (ICAM‐1KO) mice, and analysed the cellular and molecular immunological bases for any observed pathology or complication. Following a primary genital infection of female ICAM‐l–/– and ICAM‐1+/+ mice, the intensity of the disease during the first 3 weeks (as assessed by shedding of chlamydiae in the genital tract) was significantly greater in ICAM‐1KO mice than in ICAM‐1+/+ mice (P < 0·0001), although both ICAM‐l–/– and ICAM‐1+/+ mice subsequently cleared the primary infection. There was greater ascending disease during the initial stage of the infection, and a higher incidence of tubal disease (hydrosalpinx formation) after multiple infections in ICAM‐l–/– mice. Analysis of the cellular and molecular bases for the increased acute and ascending disease in ICAM‐l–/– mice revealed that the high affinity of ICAM‐1 for leucocyte function antigen type‐1 is a property that promotes rapid activation of specific Th1 cells, as well as their early recruitment into the genital mucosa. Moreover, ICAM‐1 was more important for naive T‐cell activation than primed Th1 cells, although its absence delayed or suppressed immune T‐cell activation by at least 50%. Taken together, these results indicated that ICAM‐1 is crucial for rapid T‐cell activation, early recruitment and control of genitally acquired Chlamydia trachomatis.
Introduction
The obligate intracellular bacterium, Chlamydia trachomatis, is the aetiological agent of the most common bacterial sexually transmitted disease (STD) in the United States and several other industrialized nations, including the United Kingdom and Germany. A recent World Health Organisation report revealed that 90 million of 500 million annual new global STDs are attributed to C. trachomatis.1,2 In the United States, four million reported annual cases of genital chlamydial infections cost over two billion dollars.2,3 Of major pathophysiological significance is the potential for cervical disease in women to spread into the upper genital tract, leading to serious complications, such as pelvic inflammatory disease (PID), Fallopian tube scarring, ectopic pregnancy and infertility.4 The frequent insidious infections and the increasing incidence of severe irreversible complications which may be the first symptoms of an infection support the mounting concern that genital chlamydial disease poses a major threat to human reproduction, well‐being and national budgets. Control and prevention strategies of high priority are frequent screening programmes to ensure early detection and treatments2,5 and the administration of an efficacious vaccine.
The search for a chlamydial vaccine has led to the development of several animal models for understanding the pathogenesis and immunobiology of the disease, including the definition of the relevant immune effectors that mediate chlamydial immunity and the protective antigens that elicit such responses. Recent studies in animal models of cervical and ascending chlamydial disease suggested that rapid and early elicitation and recruitment of certain immune effectors [i.e. dendritic and T helper type 1 (Th1) cells] into the local genital mucosae are crucial for reducing the intensity of and terminating a cervico‐vaginal infection, arresting ascending disease,6–9 and are therefore important for preventing major complications of the infection. The requirement for Th1 cells and their noted cytokine, interferon‐γ (IFN‐γ), in the acquisition of anti‐chlamydial immunity in mice is well established.7,10–13 The activation, recruitment and retention of Th1 cells (and/or the precursors) in the genital mucosa involve obligatory intimate interaction with accessory, infected and non‐infected cells via cell surface molecules that include the gene products of the major histocompatibility complex, addressins, co‐receptors and co‐stimulatory and adhesion molecules.14,15 A detailed knowledge of the precise role of each of these cellular and molecular entities in the overall host response against Chlamydia is important for understanding the host factors that govern vaccine efficacy, and the acquisition and maintenance of immunity.
The intercellular adhesion molecules (ICAM‐1, ‐2 and ‐3) are prominent members of the immunoglobulin supergene family that function as important addressins, along with their receptors (i.e. the β2 integrins such as the leucocyte function antigen type 1, LFA‐1), in host defence and pathological conditions. These functions include accessory, co‐stimulatory and adhesion molecules in antigen presentation, and T‐cell homing, activation, cytotoxicity, recruitment and retention in tissues.14–17 Mice defective in ICAM‐1 due to targeted inactivation of the gene in embryonic stem cells suffer increased circulatory neutrophil counts and reduced activation and migration of leucocytes to sites of inflammation.18,19 Genetically engineered LFA‐1 knockout mice have reduced T‐cell responses to mitogens, suppressed alloreactivity and diminished capacity to mount anti‐tumour or anti‐viral immune responses.20,21 Also, leucocyte adhesion deficiency (LAD) is a human genetic disease characterized by defective expression or complete absence of β2 integrins due to a mutation(s) in the gene encoding the common β subunit (CD18) in LFA‐1, Mac‐1 and pl50,95 molecules.22 LAD patients suffer severe recurrent bacterial infections, leucocytosis, progressive periodontitis and hypoplasia of lymphoid tissues.
Among the three major ligands for LFA‐1, ICAM‐1 exhibits the highest affinity while ICAM‐3 has the lowest affinity.23,24 ICAM‐1 and ‐3 (but not ICAM‐2) are involved in signal transduction following LFA‐1 binding,25 suggesting that in addition to binding interactions, each ICAM may play different roles in lymphocyte activation and function. However, the relative roles of the ICAMs in host defence have not been assessed in a relevant disease system in vivo; therefore, the effect of the differential affinities on the functional properties of each ICAM in T‐cell activation, recruitment and function in protective immunity remain unclear. Previous studies investigating the role of lymphoepithelial interaction in anti‐chlamydial immunity indicated a significant role for the integrins and their counter‐receptors in the intracellular inhibition of C. trachomatis in mice.26–28 Thus, as compared with cytokine‐induced chlamydial inhibition in epithelial cells, direct epithelial–T‐cell interaction enhanced chlamydial inhibition by cytokine‐secreting murine T lymphocyte clones in vitro.26,27 Antibody blocking studies indicated that the enhanced anti‐chlamydial action under the condition of epithelial–lymphocyte interaction was associated with the ICAM‐1/LFA‐1 adhesion molecule binding pathway.29 Increased ICAM‐1 expression by chlamydial‐infected target cells also enhanced their lysis by specific CD8+ cytotoxic T lymphocytes in vitro.28 To clarify the role of the ICAM‐1/LFA‐1 pathway in chlamydial immunity in vivo, the growth of C. trachomatis was analysed in genetically engineered ICAM‐1 knockout (KO) mice to determine how ICAM‐1 deficiency affects certain cellular and molecular immune parameters that are required for chlamydial immunity. The results revealed that the high affinity of ICAM‐1 for LFA‐1 is important for the rapid and early T‐cell activation, which is required for controlling the early or acute phase of genital chlamydial infection.
Materials and methods
Chlamydia stocks and antigens
Stocks of C. trachomatis agent of mouse pneumonitis (MoPn) used to infect mice in vivo were prepared by propagating elementary bodies (EB) in McCoy cells, as previously described.30 Stocks were titred by infecting McCoy cells with varying dilutions of EBs, and the infectious titre was expressed as inclusion‐forming units per millilitre (IFU/ml). Chlamydial antigen was prepared by growing MoPn in HeLa cells and purifying EBs over renografin gradients, followed by inactivation under ultraviolet (UV) light for 3 hr.
Animals, infection and analysis of the course of the disease
Female ICAM‐1–/– and ICAM‐1+/+ mice on (C57BL/6J) background, 5–8‐week‐old, were obtained from The Jackson Laboratory, Bar Harbor, MA. All animals were fed with food and water ad libitum, and maintained in laminar flow racks under pathogen‐free conditions of 12‐hr light and 12‐hr darkness. Mice were infected intravaginally with 107 IFU of MoPn per mouse in a volume of 30 µl of phosphate‐buffered saline (PBS) while under phenobarbitol anaesthesia. The course of the infection was monitored by periodic (every 3 days) cervico‐vaginal swabbing of individual animals. Chlamydia was isolated from the swabs in tissue culture according to standard methods and inclusions were visualized and enumerated by immunofluorescence.30 The animals were monitored for at least 4–6 weeks, a time period that spans the course of MoPn infection in mice.8 Infected mice did not show any clinical evidence of overt pathology beside the shedding of chlamydiae in their genital tracts, suggesting that the inoculum was not lethal for the animals. Experiments were repeated to include 10 or 12 animals per experimental group.
Assessment of ascending infection
MoPn was isolated from the upper genital tracts of mice at different times after infection as follows: mice were infected intravaginally with 107 IFU of MoPn per mouse as previously described.30 At the indicated times after infection, a portion of the reproductive system between the uterus and the ovaries of each mouse was removed, teased with forceps and tissue homogenates were collected in 1 ml PBS. Chlamydia was isolated from the homogenate in tissue culture according to a standard immunofluorescence staining method.30
Cytokines, monoclonal antibodies (mAbs) and other reagents
Enzyme‐linked immunosorbent assay (ELISA) kits for quantifying the amounts of murine cytokines in biological and culture fluids were purchased from BioSource International, Camarillo, CA. Chlamydial isolation from cervico‐vaginal swabs in tissue culture was assayed by staining infected monolayers of McCoy cells with fluorescein isothiocyanate (FITC)‐labelled, genus‐specific anti‐chlamydial antibodies (Kallestad Diagnostics, Chaska, MN) to detect chlamydial inclusions by direct immunofluorescence.30
Fluorescence‐activated cell sorting (FACS) analysis
Single cell preparations from the indicated organs and tissues of ICAM‐1–/– and ICAM‐1+/+ mice were stained with FITC‐labelled mAbs directed against murine CD3, CD4, CD8, CD54 (ICAM‐1), CD71 (transferrin receptor), CD102 (ICAM‐2), MadCAM‐1, natural killer (NK), major histocompatibility complex class II (MHC‐II) and Mac‐1 antigens, according to the manufacturer's protocols (BioSource International). Stained cells were analysed on a FACScan Flow Cytometer (Becton‐Dickinson, Sunnyvale. CA) using controls stained with isotype‐matched irrelevant antibodies. The results are expressed as the proportion (%) of positively stained cells in the cell preparation.
Assessment of chlamydia‐specific Th1 cell response and recruitment into the genital tract
The level of Th1 response and recruitment into the genital mucosa was determined by measuring the response of chlamydial‐specific, IFN‐γ‐secreting T cells in the genital tract tissues of infected mice, as previously described.7 Briefly, immune T cells were prepared from the genital tract tissues of infected mice by the collagenase digestion method31,32 as follows: at the indicated time after infection, animals in each group were killed and the genital tract between the vagina and ovaries (i.e. the cervix, uterus and Fallopian tubes) was excised and placed in sterile HEPES‐buffered RPMI‐1640 culture medium (Atlanta Biologicals, Norcross, GA). Explants were transferred to 7 ml of 0·6 mg/ml filter‐sterilized type I collagenase (Atlanta Biologicals). The tissues were minced, incubated at 37° for 45–60 min, then teased with forceps, and passed through a cell strainer. Following washing, the cells were enriched for T cells by the nylon wool adherence method.10,32 Purified genital tract cells contained at least 97% CD3+ cells, as determined by FACS analysis.
The level of response of chlamydial‐specific Th1 cells induced into genital tissues was assessed by seeding purified T cells into 96‐well tissue culture plates (Costar, Cambridge, MA) at 2 × 105 cells per well with syngeneic antigen‐presenting cells (APC; 2 × 105 cells per well), in the presence or absence of UV‐inactivated MoPn EBs as antigen at 10 µg/ml. Antigen‐presenting cells were γ‐irradiated (2000 rads) spleen cells from syngeneic wild‐type mice. After 3 or 5 days of incubation in humidified incubators at 37° and 5% CO2, the supernatants were collected and stored at – 70° until assayed for IFN‐γ content. It was previously shown that culture‐derived IFN‐γ by this procedure possesses biological activity as determined by the ability of IFN‐γ‐containing supernatants to protect L929 cells from infection by encephalomyocarditis virus.10
The amounts of IFN‐γ contained in supernatants derived from culture‐stimulated cells and controls were measured using a specific ELISA kit (CytoscreenTM Immunoassay Kit; BioSource International) according to the supplier's instructions. The concentration of the cytokine in each sample was obtained by extrapolation from a standard calibration curve generated simultaneously. Data were calculated as the mean values (±SD) of triplicate cultures for each experiment. The results were derived from at least three independent experiments.
Measurement of efficiency of antigen presentation by splenic APCs from ICAM‐1–/– and ICAM‐1+/+ mice
The efficiency of antigen presentation by splenic APCs from ICAM‐1KO mice and wild‐types was compared by assessing the ability of γ‐irradiated whole spleen cells to present chlamydial antigens to naive or immune T cells from infected wild‐type mice. Spleen cells from naive or chlamydial‐infected wild‐type mice were enriched for T cells by the nylon wool adherence method.10,32 Purified splenic cells contained at least 97% CD3+ cells, as determined by FACS analysis. To assess antigen‐presenting function of γ‐irradiated splenic cells from either ICAM‐1KOs or control mice, 2 × 105 cells were co‐cultured with 2 × 105 nylon wool‐purified T cells in the presence or absence of chlamydial antigen (i.e. UV‐inactivated MoPn EBs at 10 µg/ml) in 96‐well tissue culture plates for 24, 48, 72, 96, or 120 hr. At the end of each incubation period, the supernatants were collected and assayed for interleukin‐2 (IL‐2) and/or IFN‐γ content by a quantitative ELISA (CytoscreenTM Immunoassay Kit; BioSource) according to the supplier's instructions. The concentration of the cytokine in each sample was obtained by extrapolation from a standard calibration curve generated simultaneously. Data were calculated as the mean values (± SD) of triplicate cultures for each experiment. The results were derived from at least three independent experiments.
Statistical analysis
The levels of IL‐2 or IFN‐γ in samples from different experiments were analysed and compared by performing a one‐ or two‐tailed t‐test, and the relationship between different experimental groupings was assessed by analysis of variance (anova). Minimal statistical significance was judged at P < 0·05.
Results
Analysis of genital chlamydial infection in ICAM‐1+/+ and ICAM‐1–/– mice
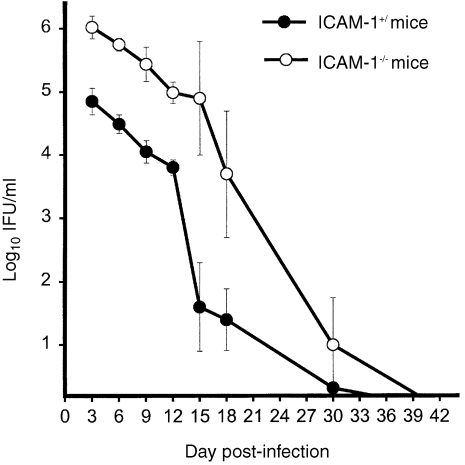
To evaluate the relative ability of ICAM‐1KO mice to resolve genital chlamydial infection as compared to wild‐type mice, the course of the disease was followed in both types of mice, by isolation of live chlamydiae from cervico‐vaginal swabs, during a 10‐week period. This period spans the course of the disease in immunocompetent wild‐type mice.8 It was found that ICAM‐1–/– mice suffered greater chlamydial burden than control mice during the first 3 weeks of intravaginal inoculation with C. trachomatis (Fig. 1). There was a one to two log difference in the number of inclusions isolated from the two groups of mice during this early stage of the infection. The difference in chlamydial shedding between ICAM‐1–/– and ICAM‐1+/+ mice at each time‐point during the early stages of the infection was statistically significant as assess by two‐tailed t‐test [P < 0·08, 0·05, 0·01, 0·006, 0·016 and 0·018 for days 3, 6, 9, 12, 15 and 18 post‐infection (p.i.), respectively]. However, by 42 days after the infection, both knockouts and wild‐type mice eventually cleared the primary infection without overt clinical signs of other complications. These results indicated that ICAM‐1 plays a role in the ability of the mice to control acute genital chlamydial infection during the early stage of the infection but may not be required for ultimate resolution of disease. Furthermore, it was previously shown that although there was a significant difference in cytokine and nitric oxide secretions by splenic and peritoneal exudate cells from ICAM‐1–/– and ICAM‐1+/+ mice, FACS analysis revealed no alterations in the proportions of the cells expressing CD3, CD4, CD8, LFA‐1, ICAM‐2, Mac‐1 and MHC class II antigens.29
Figure 1.
Course of genital chlamydial infection in ICAM‐1–/– and ICAM‐1+/+ mice. Female ICAM‐1–/– and ICAM‐1+/+ mice were infected intravaginally with MoPn. The course of the infection was monitored by periodic (every 3 days) cervico‐vaginal swabbing of individual animal. Chlamydia was isolated from the swabs in tissue culture according to standard methods and inclusions were visualized and enumerated by immunofluorescence.30 Results are expressed in IFU/ml. Experiments were repeated to give l0 or l2 animals per experimental group.
The finding that ICAM‐1 is required for controlling acute genital chlamydial disease during the early stage of the infection is of major significance in an attempt to understand the cellular and molecular factors that regulate the induction of relevant immune effectors responsible for protecting against certain complications of genital chlamydial infection. It was therefore necessary to investigate in detail whether ICAM‐1 is central to early T‐cell activation, recruitment and expression of effector function. Several hypotheses were propounded and tested as follows.
Severe acute disease in ICAM‐1–/– mice is due to delayed recruitment of Th1 cells into the genital tract
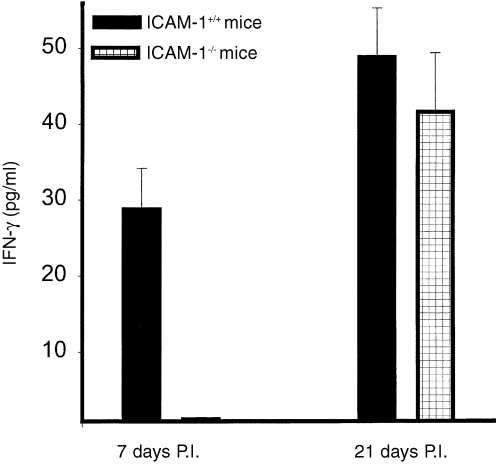
In initial studies, we tested the hypothesis that the greater acute disease observed during early stages of genital chlamydial infection in ICAM‐1–/– mice is due to delayed recruitment and retention of critical immune effectors relevant to protection and immunity against Chlamydia. We investigated the recruitment of one of these effectors, the Th1 cells, which are characterized by IFN‐γ secretion in response to specific antigenic stimulation. Figure 2 shows the recruitment of antigen‐specific IFN‐γ‐secreting Th1 cells into the genital tracts of ICAM‐1–/– and ICAM‐1+/+ mice during the acute (day 7 p.i) and later (day 27 p.i) stages of the infection. Th1 cells were essentially undetectable, or were barely detectable, in the genital tracts of ICAM‐1–/– mice during the first week of infection (P < 0·0001) but the cells became detectable and essentially indistinguishable from the level in control mice (P < 0·680) by 3–4 weeks thereafter, suggesting a slow or delayed recruitment. The results may indicate that the severe acute genital chlamydial disease in ICAM‐l–/– mice is due to delayed recruitment of Th1 into the genital tract, suggesting that ICAM‐1 is important for rapid T‐cell activation and recruitment. However, since both the low‐affinity ICAM‐3, as well as the high‐affinity ICAM‐1, could transduce signal leading to T‐cell activation,23,25 it was important to directly test whether the high‐affinity ICAM‐1 is important for rapid T‐cell activation.
Figure 2.
Recruitment of chlamydia‐reactive Th1 cells after chlamydial genital infection of ICAM‐1–/– and ICAM‐1+/+ mice. Nylon wool‐purified T cells were isolated at the indicated time‐points from genital tract tissues of infected female ICAM‐l–/– and ICAM‐1+/+ mice. The T cells were stimulated with APCs and chlamydial antigen for 5 days and the amounts of IFN‐γ in the culture supernatants were measured by ELISA as described in the Materials and Methods section. The concentrations of IFN‐γ are expressed as the mean (pg/ml) of results from different experiments. Control cultures that contained T cells and APCs but without chlamydial antigen did not show any measurable amounts of IFN‐γ and so the data are not presented in the results shown.
Role of ICAM‐1 in rapid T‐cell activation
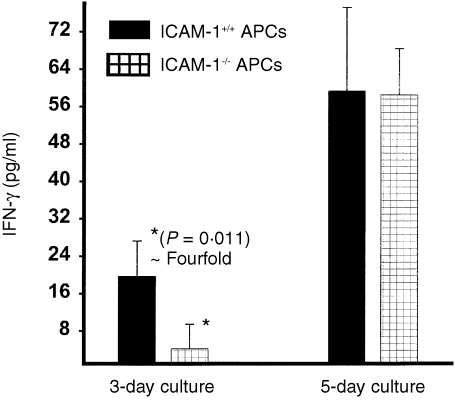
We tested the hypothesis that the high‐affinity ICAM‐1 is responsible for rapid delivery of the co‐stimulatory signal for relatively early T‐cell activation and function. Thus, the ability of splenic APCs from ICAM‐1–/– or ICAM‐1+/+ mice to activate chlamydial‐reactive immune Th1 cells rapidly were tested in short‐term (within 3 days) and long‐term (5 days) cultures. The results presented in Fig. 3 show that in short‐term cultures 105 APCs from ICAM‐1+/+ mice were not as efficient at activating chlamydial‐reactive immune Th1 cells to secrete IFN‐γ as equivalent number of APCs from ICAM‐1+/+ mice. There was at least fourfold higher IFN‐γ response by immune T cells in the presence of APCs from ICAM‐1+/+ mice than ICAM‐1–/– mice (P < 0·011). However, the ability of APCs from ICAM‐1–/– and ICAM‐1+/+ mice to activate immune T cells to secrete IFN‐γ in long‐term culture was essentially indistinguishable (P < 0·880). These results demonstrated that although ICAM‐1 is required for rapid activation of primed T cells, its absence only delays the activation process, which may then involve other low‐affinity ligands, such as ICAM‐3, previously shown to be involved in T‐cell activation signalling.23,25
Figure 3.
Role of ICAM‐1 in rapid T‐cell activation. Nylon wool‐purified immune splenic T cells (2 × 105/well) were isolated from chlamydia‐infected ICAM‐1+/+ mice and stimulated with 10 µg/ml of chlamydial antigen and l05 cells/well of APCs from either ICAM‐1–/– or ICAM‐1+/+ mice. After 3 or 5 days of incubation the amounts of IFN‐γ in the culture supernatants were measured by ELISA method. The concentrations of IFN‐γ are expressed as the mean (pg/ml) of results from different experiments. Cultures containing T cells and APCs but without chlamydial antigen did not show any measurable amounts of IFN‐γ and so the data are not presented.
Role of ICAM‐1 in the activation of naive vs. primed T cells
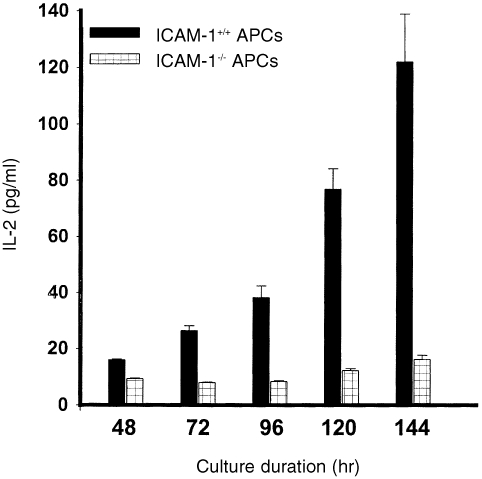
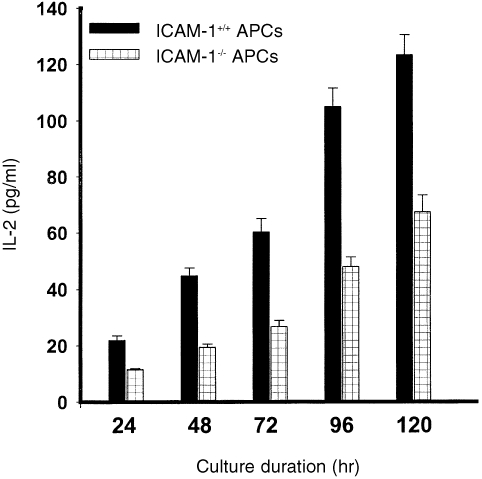
The dependence of naive T‐cell activation on co‐stimulatory signals, including those delivered via the ICAM‐1–LFA‐1 pathway,33 would suggest that ICAM‐1 deficiency could impair the ability of APCs to activate naive T cells even in long‐term cultures. In a kinetic study of the ability of APCs from ICAM‐1–/– and ICAM‐1+/+ mice to activate naive or primed T cells in cultures, we investigated the hypothesis that ICAM‐1‐deficient APCs would be impaired in their ability to activate naive Th1 cells in both short‐term and long‐term cultures. To increase the sensitivity of the assayed activation process, we measured a relatively early T‐cell activation signal, IL‐2 secretion, as an indication of a productive chlamydial antigen presentation and Th1 activation. Figure 4 reveals that APCs from ICAM‐1–/– mice were greatly impaired in their ability to activate naive T cells during a 6‐day culture period, as assessed by IL‐2 secretion. Even at day 6 in culture, the IL‐2 level measured in the presence of APCs from ICAM‐1–/– mice was less than 15% of the level measured in the presence of APCs from ICAM‐1+/+ mice (P < 0·0001). However, the results presented in Fig. 5 show that the ability of APCs from ICAM‐1–/– mice to activate primed T cells in long‐term culture was markedly improved with time in culture, although they were not as potent as APCs from ICAM‐1+/+ mice. Thus, with time in culture there was a dramatic improvement in IL‐2 secretion in the presence of APCs from ICAM‐1–/– mice (approximately 10 pg/ml at 24 hr to > 60 pg/ml at 120 hr), whereas in the presence of APCs from ICAM‐1+/+ mice IL‐2 levels were greater than 20 pg/ml at 24 hr and approximately 125 pg/ml at 120 hr (P < 0·011). These results clearly demonstrated that ICAM‐1 is crucial for the rapid activation of both naive and immune Th1 cells, and in addition there is an absolute requirement for ICAM‐1 for naive T‐cell activation against C. trachomatis.
Figure 4.
Role of ICAM‐1 in the activation of naive T cells. Nylon wool‐purified T cells were isolated from the spleens of naive ICAM‐1+/+ mice. The T cells (2 × l05/well) were stimulated with 10 µg/ml of chlamydial antigen and 2 × l05 cells/well of APCs from either ICAM‐1–/– or ICAM‐1+/+ mice. At the indicated time of incubation the amounts of IL‐2 in the culture supernatants were measured by ELISA as described in the Materials and Methods section. The concentrations of IL‐2 are expressed as the mean (pg/ml) of results from different experiments. Cultures containing T cells and APCs but without chlamydial antigen did not show any measurable amounts of IL‐2 and so the data are not presented.
Figure 5.
Role of ICAM‐1 in the activation of immune T cells. Nylon wool‐purified T cells were isolated from the spleens of genitally chlamydial‐infected ICAM‐1+/+ mice. The T cells (2 × l05/well) were stimulated with 10 µg/ml of chlamydial antigen and 2 × l05 cells/well of APCs from either ICAM‐1–/– or ICAM‐1+/+ mice. At the indicated times of incubation the amounts of IL‐2 in the culture supernatants were measured by ELISA as described in the Materials and Methods section. The concentrations of IL‐2 are expressed as the mean (pg/ml) of results from different experiments. Cultures containing T cells and APCs but without chlamydial antigen did not show any measurable amounts of IL‐2 and so the data are not presented.
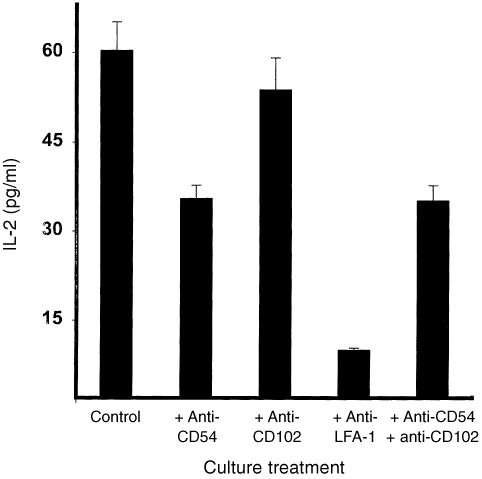
To assess the relative contributions of the different ligands of LFA‐1 (i.e. ICAM‐1, ‐2 and ‐3) to immune T‐cell activation, neutralizing mAbs directed against ICAM‐1, ‐2 or LFA‐1 were used to block the response obtained during the 3‐day culture period. The gene encoding the murine ICAM‐3 is yet to be cloned and so no antibodies are presently available against ICAM‐3. The results presented in Fig. 6 reveal that anti‐LFA‐1 antibodies which simultaneously block costimulation via the three ligands of LFA‐1 (i.e. ICAM‐1/LFA‐1, ICAM‐2/LFA‐1 and ICAM‐3/LFA‐1 pathways) essentially abrogated chlamydial‐specific T‐cell activation for IL‐2 secretion (i.e. approximately 90% suppression) (P < 0·0001). The presence of anti‐ICAM‐1 (CD54) antibodies suppressed T‐cell activation by approximately 50% (P < 0·006) but anti‐ICAM‐2 (CD 102) had no effect (P < 0·860). These results confirmed those from other experiments presented in Fig. 4 indicating that, although ICAM‐1 is absolutely required for significant naive Th1 activation, it contributes but is not indispensable for immune Th1 activation against C. trachomatis.
Figure 6.
Contribution of LFA‐1 and its ligands to immune T‐cell activation. Nylon wool‐purified T cells were isolated from the spleens of genitally infected ICAM‐1+/+ mice. The T cells (2 × 105/well) were stimulated with 10 µg/ml of chlamydial antigen, APCs from ICAM‐1+/+ mice, in the presence of neutralizing mAbs directed against LFA‐1, CD54 (ICAM‐1), CD102 (ICAM‐2) or a combination of anti‐CD54 and CD102. After 72 hr of incubation, the amounts of IL‐2 in the culture supernatants were measured by ELISA as described in the Materials and Methods section. The concentrations of IL‐2 are expressed as the mean (pg/ml) of results from different experiments. Cultures containing T cells and APCs but without chlamydial antigen did not show any measurable amounts of IL‐2 and so the data are not presented in the results shown.
High incidence of ascending infections and tubal abnormalities in chlamydia‐infected ICAM‐1–/– mice
Predictable clinico‐pathophysiological consequences of the delayed activation and recruitment of Th1 cells into the genital mucosa of chlamydial‐infected ICAM‐1–/– mice include a high incidence of ascending infection and tubal abnormalities, such as hydrosalpinx and infertility. Thus, in these studies, we analysed upper genital tract tissues between the uterus and the ovaries from ICAM‐1–/– and ICAM‐1+/+ mice for microbiological evidence of ascending infection, by isolating live chlamydiae from homogenized tissues in culture, as previously described.34 There was a greater incidence of ascending infection in ICAM‐1KO mice than controls during the 1st and 2nd weeks of infection (approximately 60‐and 90‐fold, respectively; Table 1) (P < 0·011 and 0·0001 for week 1 and 2, respectively), as assessed by the isolation of infectious chlamydiae from upper genital tracts of infected mice. These results suggested that the delayed T‐cell activation in ICAM‐1‐deficient mice causes greater incidence of ascending infection in the mice.
Table 1.
Ascending infection in ICAM‐l–/– and ICAM‐1+/+ mice after a primary genital infection with C. trachomatis
| Group* | Mean IFU/ml (range) |
|---|---|
| ICAM‐1+/+ mice | |
| Day 5 | 4·74 (0·0–16·40) |
| Day 10 | 1·54 (0·0–12·3) |
| ICAM‐1–/– mice | |
| Day 5 | 302·23 (94·16–614·10) |
| Day 10 | 142·52 (32·75–253·83) |
Each group contained at least six mice and the experiments were repeated three times.
To examine whether the high incidence of ascending disease in ICAM‐1–/– mice was accompanied by clinical evidence of upper genital tract diseases, we performed gross pathological studies on infected ICAM‐1–/– and ICAM‐1+/+ mice to compare the incidence of hydrosalpinx formation. The mice were infected four times over a period of 54 weeks to increase the incidence of tubal abnormalities.8,35 The reproductive system (between the uteri and ovaries) was examined visually for hydrosalpinx formation and haemorrhagic lesions. The results summarized in Table 2 reveal that a greater number of ICAM‐1–/– mice (83·33%) suffered tubal abnormalities marked by unilateral or bilateral hydrosalpinx affecting the uterine horns or Fallopian tubes than control mice. One of the six ICAM‐1+/+ mice presented with a unilateral night‐side hydrosalpinx. The incidence of tubal disease in ICAM‐1–/– mice was statistically greater than that in ICAM‐1–/– mice, as analysed by z‐test (P < 0·0046). These results indicated that the delayed T‐cell activation associated with ICAM‐1 deficiency may foster ascending infection that ultimately could lead to obstructive disease, tubal damage and possibly infertility in infected mice.
Table 2.
Incidence of tubal abnormalities in ICAM‐1–/– and ICAM‐1+/+ mice after multiple genital infections with C. trachomatis
| Group/animal # | Observed pathology* |
|---|---|
| ICAM‐1−/− mice: | |
| #1 | Bilateral hydrosalpinx |
| #2 | Bilateral hydrosalpinx |
| #3 | Left tubal hydrosalpinx and inflamed ovaries |
| #4 | Left tubal hydrosalpinx |
| #5 | No abnormalities observed |
| #6 | Right tubal hydrosalpinx |
| ICAM‐1+/+ mice: | |
| #1 | No abnormalities observed |
| #2 | No abnormalities observed |
| #3 | No abnormalities observed |
| #4 | No abnormalities observed |
| #5 | Right tubal hydrosalpinx |
| #6 | No abnormalities observed |
For gross pathological studies, ICAM‐1−/− and ICAM‐1+/+ mice were infected four times over a period of 54 weeks to increase the incidence of tubal abnormalities. The reproductive system (between the uteri and ovaries) was examined visually for hydrosalpinx formation and haemorrhagic lesions on one or both uterine tubes (i.e. unilateral or bilateral hydrosalpinx). Table 2 summarizes the results obtained from six mice in each group.
Discussion
The physiological importance of certain biological molecules in host defence and survival is usually better appreciated when the encoding genes are amenable to manipulations that allow the measurement of the effect in the intact individual. The application of the gene knockout technology to animal models of specific diseases furnishes a unique approach to the molecular analysis of the role of specific biomolecules in certain diseases in the intact animal. In this study, we have applied gene knockout technology in a murine model of genital chlamydial disease to analyse the role of ICAM‐1 in the immune control of genital chlamydial infection. This study has revealed (for the first time) that the high‐affinity binding of ICAM‐1 to its receptor, LFA‐1, is important for rapid T‐cell activation and function, and also for recruitment and retention of T cells in the genital mucosa. Of particular significance is the role of ICAM‐1 in protecting against ascending chlamydial disease that is pathognomonic for some of the severe complications of chlamydial infection, including PID, tubal scarring and other obstructive diseases.36
Among the three counter‐receptors for LFA‐1, ICAM‐1 exhibits the highest affinity for LFA‐1 while ICAM‐3 has the lowest affinity.23,24 From an immunological standpoint, high‐affinity receptor‐ligand binding could promote the rapid and selective activation and expansion of high affinity receptor‐bearing cells over low affinity bearers. This selective activation and expansion process is responsible for the phenomenon of affinity maturation during humoral immune responses, in which the average affinity of antibodies produced against an antigen increases with subsequent challenge due to preferential binding and possibly rapid activation and expansion of high‐affinity immunoglobulin‐bearing B cells over low‐affinity binding clonotypes.37 Thus, the high‐affinity ICAM‐1/LFA‐1 pathway appeared to bestow a temporal advantage to ICAM‐1‐bearing APCs to rapidly activate T cells. Moreover, although both ICAM‐1 and ‐3 are capable of signalling for T‐cell activation and function, it appears that the high‐affinity binding of ICAM‐1 induces rapid delivery of the co‐stimulatory signal upon engagement of the primary signal, leading to early T‐cell activation and function. This proposition is consistent with the results showing that ICAM‐1 promotes rapid T‐cell activation leading to early control and prevention of the dissemination of chlamydiae or development of complications of chlamydial genital infection. However, as revealed in Table 2, the role of ICAM‐1 in leucocyte recruitment could also significantly contribute to the greater disease in ICAM‐1KO mice. Besides, certain other, less defined, differences between ICAM‐1 and other ligands of LFA‐1 may also contribute to the observed effects. Furthermore, although the general role of ICAM‐1 in T‐cell activation has been noted in previous studies, the current report represents the first demonstration that the effect of thehigh‐affinity ICAM‐1 is to promote rapid T‐cell activation. However, the finding that ICAM‐1KO mice eventually cured their genital chlamydial infection may indicate that either ICAM‐1 is only relevant for controlling acute disease at the early stage of the infection or that ICAM‐1 may not be indispensable for ultimate resolution of disease. In addition, the results could also suggest that in the absence of ICAM‐1 other equally effective, albeit slower, mechanisms could activate T cells to control chlamydial infection. In this context, secretion of other less potent pro‐inflammatory cytokines, such as IL‐6 and IL‐1β, may lead to delayed effect and clearance. It is conceivable that the greater acute disease suffered by ICAM‐1KO mice at the early stage of genital chlamydial infection increased the incidence of ascending infection, leading to the development of tubal complications, such as hydrosalpinx seen in mice exposed to multiple genital infections. Thus, predictably, the infertility rate in chlamydial‐infected ICAM‐1KO mice is likely to be high as well.
The present and previous studies have shown that ICAM‐1 is absolutely required for naive Th1 activation, and significantly contributes (although not indispensably) to immune Th1 activation against C. trachomatis.28,29 It is interesting that dendritic cells, recently shown to be important in controlling ascending chlamydial infection,6 have a high proclivity for activating primary T‐cell immune responses, especially specific Th1 cells against several antigens,38–40 including chlamydial antigens.41,42 Dendritic cells are highly potent APC, whose robust capacity for efficient presentation of antigens for T‐cell activation is due to their high costimulatory ability that is associated with an elevated density expression of co‐stimulators such as ICAM‐1, LFA‐3 and B7 molecules.43,44 Since the motile dendritic cells are also the agents of antigen transportation across the stratified epithelia of the vagina and cervix to the draining mucosal inductive sites for immune induction,45 the presence of high ICAM‐1‐bearing dendritic cells in the genital mucosa would lead to rapid activation of Th1 cells following genital chlamydial infection, thereby reducing chlamydial multiplication, preventing ascending disease and averting major complications of the infection. However, local factors that suppress ICAM‐1 expression are likely to delay Th1 cell activation, leading to ascending disease and development of complication of chlamydial infection. In this respect, hormonal fluctuations associated with the female menstrual cycle have been shown to affect the epithelial thickness, endocytic activity of epithelial cells, the distribution of dendritic cells in the genital tract and the effectiveness of mucosal vaccines.45–47 Susceptibility to genital reinfection by C. trachomatis may be governed by the effects of these factors on local immune effectors such as dendritic cells and T cells, and their interactions in the epithelium. Thus, the estrous cycle has been shown to influence the development of infertility following chlamydial infection.48
The significance of ICAM‐1 in immunity against chlamydial disease can be realized from recent advisory public policy recommendations on PID, suggesting that anti‐chlamydial vaccine‐designing strategies could focus on preventing ascending infection that is pathognomonic for complications of genital chlamydial disease.36,49 Thus, rational manipulation of certain host conditions and factors capable of regulating local or regional ICAM‐1 expression could enhance the rapid protective T‐cell response to a vaccine, thereby preventing ascending infection, and averting major complications of genital chlamydial infection. Beside possible pharmacological manipulation of ICAM‐1 expression in mucosal tissues as a strategy for boosting rapid Th1 response to control chlamydial infection, recruitment of Th1 cells is also a mechanism for up‐regulating local ICAM‐1 expression in the epithelium. Th1 cells secrete IFN‐γ that induces ICAM‐1 on epithelial cells, leading to enhanced epithelial–T‐lymphocyte interactions.50 Thus, the anti‐chlamydial action of Th1 cytokines, such as IFN‐γ, is at multiple levels, including up‐regulation of co‐stimulatory and adhesion molecules, and induction of anti‐microbial processes, such as tryptophan catabolism, iron deprivation and nitric oxide secretion.51,52
These findings from this study provide important insights into the cellular and molecular immunological interactions leading to protective immunity against genital chlamydial disease, which may form the basis for designing a rational intervention strategy against C. trachomatis infections.
Acknowledgments
This study was supported by PHS Grants AI41231, RR03034, GM08248, RR011598 from the National Institutes of Health. We thank Drs Harlan Caldwell, Gordon B. Bailey, Linda Perry and Nerimiah Emmett for their critiques and excellent suggestions.
Glossary
Abbreviations
- ICAM‐1
‐2 and ‐3, intercellular adhesion molecule‐type‐1, ‐2 and ‐3
- ICAM‐1KO
ICAM‐1 knockout, IFU, infection‐forming units
- LFA‐1
leucocyte function antigen‐type 1
- MoPn
Chlamydia trachomatis agent of mouse pneumonitis
- PID
pelvic inflammatory disease
References
- 1.WHO (World Health Organization) Global Prevalence and Incidence of Selected Curable Sexually Transmitted Diseases: Overview and Estimates. Geneva: WHO; 1996. [Google Scholar]
- 2.Schachter J, Grayston JT. Epidemiology of human chlamydial infections. In: Stephens RS, Byrne GI, Christiansen, G, et al., editors. Chlamydial Infections. San Franciso, CA: ICS; p. 3. [Google Scholar]
- 3.D.C. & P.H.S Chlamydia trachomatis Genital Infections – United States, 1995. Morbidity Mortality Weekly Report. 1997;46:193. [PubMed] [Google Scholar]
- 4.Paavonen J, Wolner‐hanssen P. Chlamydia trachomatis: a major threat to reproduction. Hum Reprod. 1989;4:111. doi: 10.1093/oxfordjournals.humrep.a136855. [DOI] [PubMed] [Google Scholar]
- 5.Scholes D, Stergachis A, Heidrich FE, Andrilla H, Holmes KK, Stamm WE. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med. 1996;334:1362. doi: 10.1056/NEJM199605233342103. [DOI] [PubMed] [Google Scholar]
- 6.Stagg AJ, Tuffrey M, Woods C, Wunderink E, Knight SC. Protection against ascending infection of the genital tract by Chlamydia trachomatis is associated with recruitment of major histocompatibility complex class II antigen‐presenting cells into uterine tissue. Infect Immun. 1998;66:3535. doi: 10.1128/iai.66.8.3535-3544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igietseme JU, Uriri IM, Kumar SN, et al. Route of infection that induces a high intensity of gamma interferon‐secreting T cells in the genital tract produces optimal protection against Chlamydia trachomatis infection in mice. Infect Immun. 1998;66:4030. doi: 10.1128/iai.66.9.4030-4035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patton DL, Rank RG. Animal models for the study of pelvic inflammatory disease. In: Quinn TC, editor. Sexually Transmitted Diseases. New York: Raven Press, Ltd; 1992. p. 85. [Google Scholar]
- 9.Cain TK, Rank RG. Local Th1‐like responses are induced by intravaginal infection of mice with the mouse pneumonitis (MoPn) biovar of Chlamydia trachomatis. Infect Immun. 1995;63:1784. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Igietseme JU, Ramsey KH, Magee DM, Williams DM, Kincy TJ, Rank RG. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar‐specific, Th1 lymphocyte clone. Regional Immunol. 1993;5:317. [PubMed] [Google Scholar]
- 11.Cotter TW, Ramsey KH, Miranpuri GS, Poulsen CE, Byrne GI. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor‐deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su H, Caldwell HD. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 15.Hunkapillar T, Hood L. Diversity of the immunoglobulin gene superfamily. Adv Immunol. 1989;44:1. doi: 10.1016/s0065-2776(08)60639-2. [DOI] [PubMed] [Google Scholar]
- 16.Larson TS, Springer TA. Structure and function of leukocyte integrins. Immunol Rev. 1990;114:181. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 17.Harvey JE, Hogg N, Landis RC. LFA‐1 and the ICAMs. In: Shimizu Y, editor. Lymphocyte Adhesion Molecules. Austin: R.G. Landes Co; 1993. p. 26. [Google Scholar]
- 18.Xu H, Gonzalo JA, St Pierre Y, et al. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1‐deficient mice. J Exp Med. 1994;180:95. doi: 10.1084/jem.180.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sligh JE., Jr Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1993;90:8529. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shier P, Otulakowski G, Ngo K, et al. Impaired immune responses toward alloantigens and tumor cells but normal thymic selection in mice deficient in the β2 integrin leukocyte function‐associated antigen‐1. J Immunol. 1996;157:5375. [PubMed] [Google Scholar]
- 21.Schmits R, Kundig TM, Baker DM, et al. LFA‐1‐deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J Exp Med. 1996;183:1415. doi: 10.1084/jem.183.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac‐1, LFA‐1 and p150,95 glycoproteins. Annu Rev Med. 1987;38:175. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 23.De Fougerolles A, Qin X, Springer TA. Characterization of the function of intercellular adhesion molecule (ICAM) ‐3 and comparison with ICAM‐1 and ICAM‐2 in immune responses. J Exp Med. 1994;179:619. doi: 10.1084/jem.179.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Binnerts ME, Van Kooyk Y, Simmons DL, Figdor CG. Distinct binding of T lymphocytes to ICAM‐1, ‐2 or ‐3 upon activation of LFA‐1. Eur J Immunol. 1994;24:2155. doi: 10.1002/eji.1830240933. [DOI] [PubMed] [Google Scholar]
- 25.Hayflick JS, Kilgannon P, Gallatin WM. The intercellular adhesion molecule (ICAM) family of proteins: new members and novel functions. Immunologic Res. 1998;17:313. doi: 10.1007/BF02786454. [DOI] [PubMed] [Google Scholar]
- 26.Igietseme JU, Wyrick PB, Goyeau D, Rank RG. An in vitro model for immune control of chlamydial growth in polarized epithelial cells. Infect Immun. 1994;62:3528. doi: 10.1128/iai.62.8.3528-3535.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igietseme JU. The molecular mechanism of T cell control of Chlamydia in mice: role of nitric oxide. Immunology. 1996;87:1. [PMC free article] [PubMed] [Google Scholar]
- 28.Beatty PR, Stephens RS. CD8+ T lymphocyte‐mediated lysis of Chlamydia‐infected L cells using an endogenous antigen pathway. J Immunol. 1994;153:4588. [PubMed] [Google Scholar]
- 29.Igietseme JU, Uriri IM, Hawkins R, Rank RG. Integrin‐mediated epithelial‐T cell interaction enhances nitric oxide production and increased intracellular inhibition of Chlamydia. J Leuk Biol. 1996;59:656. doi: 10.1002/jlb.59.5.656. [DOI] [PubMed] [Google Scholar]
- 30.Ramsey KH, Soderberg LSF, Rank RG. Resolution of chlamydial genital infection in B‐cell‐deficient mice and immunity to reinfection. Infect Immun. 1988;56:1320. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Igietseme JU, Streilein JW, Miranda F, Feinerman SJ, Atherton SS. Mechanism of protection against Herpes Simplex Virus Type 1‐induced retinal necrosis by in vitro‐activated T lymphocytes. J Virol. 1991;65:763. doi: 10.1128/jvi.65.2.763-768.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Igietseme JU, Rank RG. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen‐specific T cells in the genital tract. Infect Immun. 1991;59:1346. doi: 10.1128/iai.59.4.1346-1351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter LL, Swain SL. From naive to memory: development and regulation of CD4+ T cell responses. Immunologic Res. 1998;18:1. doi: 10.1007/BF02786509. [DOI] [PubMed] [Google Scholar]
- 34.Igietseme JU, Perry LL, Ananaba GA, et al. Chlamydial infection in inducible nitric oxide synthase knockout mice. Infect Immun. 1998;66:1282. doi: 10.1128/iai.66.4.1282-1286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolner‐hanssen P, Patton DL, Stamm WE, Holmes KK. Severe salpingitis in pig‐tailed macaques after repeated cervical infections followed by a single tubal inoculation with Chlamydia trachomatis. In: Oriel D, Ridgway G, Schachter J, Taylor‐robinson D, Ward M, editors. Chlamydia Infections. New York: Cambridge University Press; 1986. p. 371. [Google Scholar]
- 36.Rice PA, Schachter J. Pathogenesis of pelvic inflammatory disease. What are the questions? JAMA. 1991;266:2587. [PubMed] [Google Scholar]
- 37.Foote J, Eisen HN. Kinetics and affinity limits on antibodies produced during immune responses. Proc Natl Acad Sci USA. 1995;92:1254. doi: 10.1073/pnas.92.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL‐12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071. [PubMed] [Google Scholar]
- 39.Heufler C, Koch F, Stanzl U, et al. Interleukin‐12 is produced by dendritic cells and mediates T helper 1 development as well as interferon‐gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 40.Macatonia SE, Taylor PM, Knight SC, Askonas BA. Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell responses in vitro. J Exp Med. 1989;169:1255. doi: 10.1084/jem.169.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su H, Messer R, Whitmire W, Fischer E, Portis JC, Caldwell HD. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable chlamydiae. J Exp Med. 1998;188:809. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ojcius DM, Bravo De Alba Y, Kanellopoulos JM, et al. Internalization of Chlamydia by dendritic cells and stimulation of Chlamydia‐specific T cells. J Immunol. 1998;160:12970. [PubMed] [Google Scholar]
- 43.King PD, Ibrahim MAA, Katz DR. Adhesion molecules: co‐stimulators and co‐mitogens in dentritic cell–T cell interaction. Adv Exp Med Biol. 1993;329:53. doi: 10.1007/978-1-4615-2930-9_9. [DOI] [PubMed] [Google Scholar]
- 44.Jenkins MK, Desilva DR, Johnson JG, Norton SD. Costimulating factors and signals relevant for antigen presenting cell function. Adv Exp Med Biol. 1993;329:87. doi: 10.1007/978-1-4615-2930-9_15. [DOI] [PubMed] [Google Scholar]
- 45.Neutra MR, Pringault E, Kraehenbuhl J‐P. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu Rev Immunol. 1996;14:275. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]
- 46.Parr ME, Parr EL. Mucosal immunity in the female and male reproductive tracts. In: Ogra PL, Mestecky J, Lamm ME, editors. Handbook of Mucosal Immunology. New York: Academic Press; 1994. p. 667. [Google Scholar]
- 47.Young WG, Newcomb GM, Hosking AR. The effect of atrophy, hyperplasia, and keratinization accompanying the estrous cycle on Langerhans' cells in mouse vaginal epithelium. Am J Anat. 1995;174:173. doi: 10.1002/aja.1001740207. [DOI] [PubMed] [Google Scholar]
- 48.Pal S, Hui W, Peterson EM, De La Maza LM. Factors influencing the induction of infertility in a mouse model of Chlamydia trachomatis ascending genital infection. J Med Microbiol. 1998;47:599. doi: 10.1099/00222615-47-7-599. [DOI] [PubMed] [Google Scholar]
- 49.Soper DE. Pelvic inflammatory disease. Inf Dis Clin North Am. 1994;8:821. [PubMed] [Google Scholar]
- 50.Singer KH. Interactions between epithelial cells and T lymphocytes: role of adhesion molecules. J Leuk Biol. 1990;48:367. doi: 10.1002/jlb.48.4.367. [DOI] [PubMed] [Google Scholar]
- 51.Byrne GI, Lehmann LK, Landry GJ. Induction of tryptophan catabolism is the mechanism for gamma interferon‐mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986;53:347. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Igietseme JU, Ananaba GA, Candal DH, Lyn D, Black CM. Immune control of chlamydial growth in the human epithelial cell line RT4 involves multiple mechanisms that include nitric oxide induction, tryptophan catabolism and iron deprivation. Microbiol Immunol. 1998;42:617. doi: 10.1111/j.1348-0421.1998.tb02332.x. [DOI] [PubMed] [Google Scholar]