Abstract
The β1 integrins are a family of heterodimeric adhesion receptors involved in cell‐to‐cell contacts and cell‐to‐extracellular matrix interactions. Through their adhesive role, integrins participate in transduction of outside/inside signals and contribute to trigger a multitude of cellular events such as differentiation, cell activation, and motility. The fibronectin integrin receptors, α4β1 and α5β1, can function as costimulatory molecules in T‐cell receptor (TCR)‐dependent T‐cell activation. In the current study the Jurkat T‐cell line was used as a model system to investigate the TCR‐independent role of cell adhesion to fibronectin in the activation of Zap‐70, a central molecule in the signalling events in T cells. Upon adhesion to plastic immobilized fibronectin but not to bovine serum albumin (BSA) the phosphorylation of p125FAK, a protein kinase that localizes to focal adhesion sites, was induced. Moreover, clustering of fibronectin receptors led to the detection of a p125FAK/Zap‐70 complex. Finally, while the complex between fak‐B, another protein kinase localized to focal adhesion sites, and Zap‐70 was detected in cells plated either on BSA or on fibronectin, the formation of the p125FAK/Zap‐70 complex appeared specifically induced following fibronectin‐mediated integrin clustering. These data suggest the existence of a high degree of specificity when the members of the β1 integrin family mediate signalling pathways in T cells.
Introduction
The integrin family of cell surface receptors has long been known to play an essential role in the physical aspects of cell adhesion: these molecules represent the principal receptors for extracellular matrix (ECM) proteins but also serve as transmembrane bridges between the ECM and the actin containing filaments of the cytoskeleton. Co‐ordinate regulation of integrin‐mediated functions is of fundamental importance not only to cell adhesion and migration, but also to the overall cellular architecture, survival and function.1 Engagement of integrins by their extracellular ligands transduce signals mediating cytoplasmic alkalinization, increase in intracellular Ca2+, and tyrosine phosphorylation.2–4 In many cell types there is a prominent tyrosine phosphorylation of proteins of 105–130 000 MW following integrin cross‐linking.5,6 A large body of experimental data support the concept that the autophosphorylation of a protein kinase, p125FAK, that localizes to the focal adhesion contacts, is involved in the reorganization of the cytoskeleton and in the regulation of cell shape, and this enzymatic step is considered to be one of the key regulatory points in signal transduction following cell adhesion.7–9 In fact, association of the cytoplasmic components tensin and paxillin to the focal adhesion depends upon the autophosphorylation of p125FAK. On the other hand, after cell activation p125FAK is bound by src family kinase pp60src, pp59fyn and pp51csk through SH2 domains and these domains also link p125FAK to the Grb2 adaptor protein and then to the Ras pathway10 and thus integrins' ligation transduce external stimuli from the ECM to the nucleus.
Several members of the integrin family that regulate cell adhesion to ECM are also expressed on the T‐cell surface and, apart from a role in cell adhesion, recent observations have shown that they participate in transduction of outside/inside signals and trigger a multitude of cellular events such as activation, differentiation and functional responses11 of T cells. While it is known that binding of T cells to fibronectin (FN) via β1 integrins leads to the generation of costimulatory signals and that T‐cell adhesion to various ECM proteins can potentiate the response to substimulatory amounts of anti‐CD3 or anti‐T‐cell receptor (TCR),5,12 the signals that are required to induce cytoskeletal changes needed for increased adhesion remain largely unknown. Studies with human CD4+ peripheral T cells suggest that α4β1 and α5β1 account for all of the adhesion to FN.5,12,13 Engagement of TCR by antigen activates several protein kinases of which Zap‐70 is one of the most relevant.14–19 We have recently demonstrated that strong adhesion of T cells to FN up‐regulated the levels of nuclear factor‐κB (NF‐κB) and that the increased NF‐κB binding activity was inhibited by calphostin C, an inhibitor of protein kinase C (PKC).20 Using FN and the Jurkat T‐cell line as a prototype model, in the current study we demonstrate that FN stimulates tyrosine phosphorylation of p125FAK and that cross‐linking of β1 integrins by insoluble FN results in a p125FAK/Zap‐70 complex and in the induction of tyrosine phosphorylation.
Materials and methods
Materials
FN was purified on gelatin–Sepharose as described.21 Phorbol myristate acetate (PMA) and sodium orthovanadate were purchased from Sigma (Sigma Chemical Co., St Louis, MO). Leupeptin and aprotinin were obtained from Boehringer Mannheim GmbH (Mannheim, Germany). CNBr‐activated Sepharose 4B was purchased from Pharmacia Biotech Inc. (Uppsala, Sweden).
Antibodies
Rabbit polyclonal antibodies against peptides at the C‐terminus22 of murine p125FAK were generously provided by Dr G. Tarone, University of Torino. One of these antibodies is specific for p125FAK and does not recognize other kinase‐related members; the second polyclonal antibody, raised against a closely localized peptide at the C‐terminus,22 could not discriminate between p125FAK and the closely related fak‐B member of the FAK family (Dr G. Tarone, personal communication). Anti‐phosphotyrosine mouse monoclonal antibody (mAb) PY20 and the anti Zap‐70 mAb were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A mAb (4B4) against the β1 integrin subunit was purchased from Ortho (Ortho Diagnostic Systems, Raritan, NJ). Horseradish peroxidase‐conjugated goat anti‐rabbit and anti‐mouse immunoglobulin G (IgG) antibodies were purchased from Sigma.
Cell line
Jurkat T cells were originally obtained from the American Type Culture Collection (Rockville, MD) and maintained in RPMI‐1640 (BioWhittaker Inc., Walkersville, MD) supplemented with 10% fetal bovine serum (Gibco, Gaithersburg, MD), 100 U/ml penicillin/streptomycin and 2 mm l‐glutamine and grown to a density of 3–5×105/ml. Prior to the use in our assays, a clone of Jurkat cells was selected through several rounds of cell adhesion onto FN.
Adhesion assay
Twenty‐four‐well tissue‐culture plates were coated overnight at 4° with 0·3 ml of carbonate buffer, pH 9·4, containing 10 µg/ml FN. The wells were blocked with 0·5% bovine serum albumin and washed with phosphate‐buffered saline (PBS) prior to use. Jurkat cells were washed, resuspended in serum free RPMI‐1640 and added to FN‐coated wells (7×105 cells/well), centrifuged at 150 g and incubated at 37° for different times, time zero correspondng to the end of the centrifugation. Cells were collected and washed with cold PBS containing 1 mm sodium orthovanadate. For inhibition experiments, cells were plated onto FN as above, in the presence of function‐blocking or control antibodies. Adherent cells were analysed with an inverted microscope (DM IRB, Leica, Densheim, Germany) equipped with a differential interference contrast objective.
Preparation, of cell lysates, immunoprecipitation and immunoblotting
After stimulation, whole cell lysates from 1×107 cells were prepared for immunoprecipitation. Cells were washed once with cold PBS plus 1 mm sodium orthovanadate, resuspended in 300 ml of lysis buffer containing 50 mm Tris–HCl, pH 7·5, 50 mm NaCl, 1% Triton‐X‐100, 2 mm ethylenediamine tetra‐acetic acid (EDTA), 50 mm NaF, 1 mm phenylmethylsulphonyl fluoride (PMSF) 10 mg/ml aprotinin, 10 mg/ml leupeptin, and incubated on ice for 20 min. After lysis the samples were centrifuged at 12 000 g for 20 min and the supernatants were used for immunoprecipitation. Immune complexes were precipitated with protein A Sepharose beads and fractionated on 10% polyacrylamide gels under reducing conditions. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane, non‐specific sites were blocked with blocking buffer (1% low‐fat dry milk in PBS). The blots were then stained with anti‐phosphotyrosine or anti Zap‐70 antibodies, washed extensively, stained for 1 hr with secondary antibody and visualized by the enhanced chemiluminescence (ECL) system (Amersham).
Results and discussion
To study the signal events generated following triggering of FN integrin receptors an in vitro system using a clone of Jurkat T cells selected for the ability to strongly adhere onto FN was employed. FN‐coated wells readily promoted adhesion of Jurkat cells with a rapid time‐course and at 20 min 90% of the cells projected small processes, appeared flattened and spread out and lost homotypic aggregation (Fig. 1b). Cells plated onto BSA did not show any attachment to the substrate (Fig. 1a). α4β1 and α5β1 are the major β1 integrins expressed on Jurkat cells (23 and our unpublished observations). Although expression of this type of receptors is not always a predictor of integrin usage, in this case the addition of the β1 specific 4B4 mAb (Fig. 1c) or of both anti α4 and α5 mAbs (data not shown) could completely abrogate cell adhesion. These constitutively expressed integrins co‐operated in promoting adhesion of Jurkat cells, the α4β1 integrin displaying a higher efficiency when used on the intact FN ligand molecule, while the α5β1 integrin was more efficient when only the FN central cell‐binding domain was used as a substrate (unpublished data). Thus, binding of our subline of Jurkat cells to FN can be accounted for completely by the β1 integrins α4β1 and α5β1.
Figure 1.
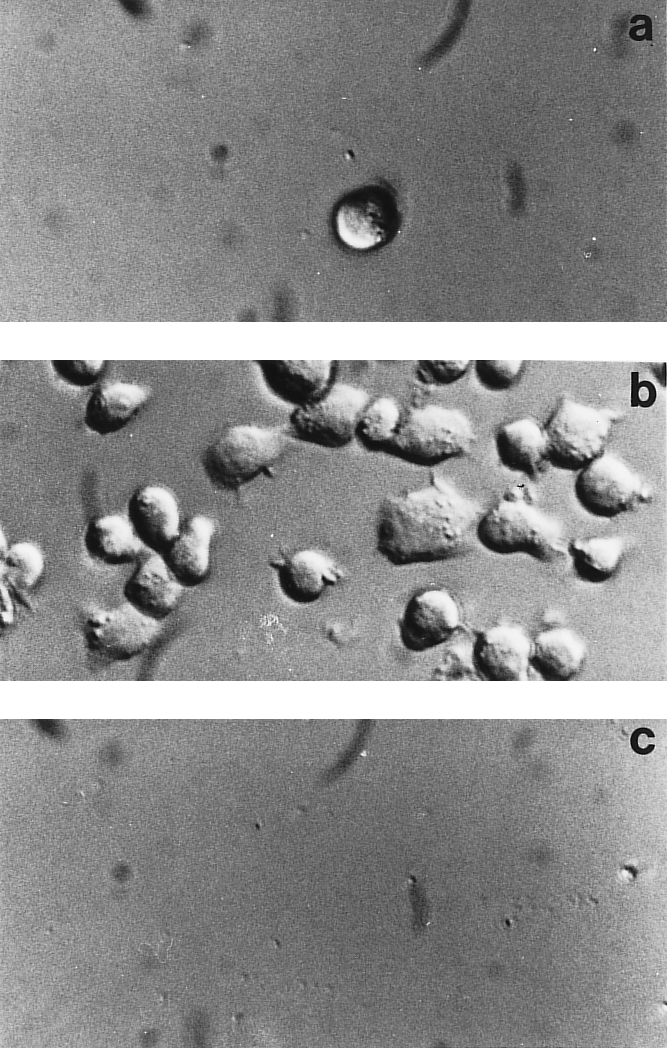
Cell adhesion to fibronectin. Jurkat cells were added to plastic‐immobilized BSA (10 µg/ml) (a) or FN (10 µg/ml) in the absence (b) or in the presence (c) of the anti β1 functional mAb 4B4. Adherent cells were analysed with an inverted microscope and micrographs were made using Nomarski optics.
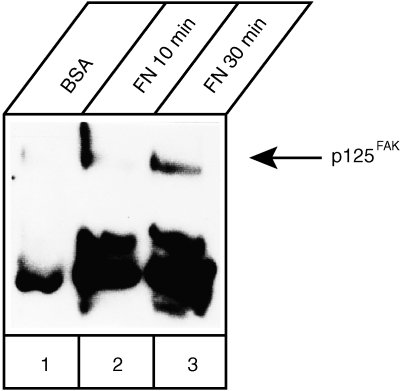
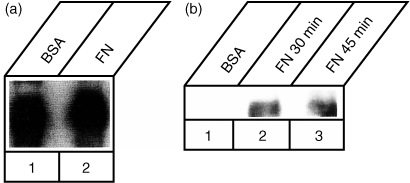
Several lines of evidence indicate that integrin ligation induces tyrosine phosphorylation of numerous intracellular substrates including p125FAK: this molecule is then involved in signal transduction to the cytoskeleton and to the nucleus.3 A rapid and sustained synergistic increase in tyrosine phosphorylation of p125FAK had been demonstrated in Jurkat cells after the simultaneous triggering of the TCR–CD3 complex and of the α4β1 and α5β1 integrin‐mediated binding to immobilized FN.24 Initially and to confirm that adhesion to FN was able to induce activation of p125FAK also in the present Jurkat subline, cells incubated on plastic surfaces coated with BSA or FN were solubilized and analysed for tyrosine phosphorylation of p125FAK. Cell lysates were immunoprecipitated with anti‐p125FAK antibodies and then analysed by Western blotting with the anti‐phosphotyrosine mAb PY20 (Fig. 2): already at 10 min of adhesion (lane 2) an increased phosphorylation, compared to BSA‐stimulated cells (lane 1), was detected that further increased at 30 min (lane 3). In the same blot other co‐immunoprecipitated bands of higher mobility were stained by mAb PY20. One phosphoprotein, absent from the BSA‐stimulated sample and migrating with an approximate molecular mass of about 70 000 MW, led us to hypothesize that it could represent Zap‐70. The strong band migrating around 50 000 MW very likely represented the heavy chain of the primary antibody. Solid phase cross‐linking of α4β1 using specific antibodies or the CS‐1 region of FN stimulated tyrosine phosphorylation of several proteins in Jurkat cells including PLCγ, p125FAK, paxillin, p59fyn/p56lck and MAP kinase.24,25 However, neither protein associations nor the involvement of Zap‐70 were investigated in that study and therefore it was not determined whether triggering via the α4β1 receptor was sufficient for the formation of a complex between p125FAK and Zap‐70. In a different study Rabinowich and colleagues were able to demonstrate that cross‐linking of either α4β1 or α5β1 in NK cells was sufficient to induce phosphorylation and physical association of p59fyn and Zap‐70 with p125FAK.26 The signal transduction pathways initiated by integrin ligation involve cytoskeletal‐dependent activation of tyrosine kinases and phosphorylation of a number of substrates.5,6 Tyrosine phosphorylation of p125FAK has been observed in a variety of cell types, suggesting that this kinase is part of a common pathway for integrin signalling. Moreover, related focal adhesion tyrosine kinases are phosphorylated when B or T cells are activated: Pyk‐2 or RAFTK, is tyrosine phosphorylated after β1 integrin stimulation in B cells,27 or after β3 integrin stumulation28 in cytotoxic lymphocytes; another member of this family, fak‐B,22 can become phosphorylated in T‐cell lines stimulated with interleukin‐2 (IL‐2) via a β2‐integrin dependent signal pathway.29,30 We assayed whether also in our T‐cell system activation involved fak‐B in a β1‐integrin dependent fashion and whether this kinase would associate with Zap‐70. Jurkat cells plated for 30 min onto BSA‐or FN‐coated dishes were lysed, and the cell lysates were incubated with polyvalent (i.e. able to recognize several members of the FAK family) or p125FAK‐specific antibodies. Blots of these precipitated complexes were immunodecorated with Zap‐70 antibodies. Fak‐B was constitutively associated with Zap‐70 (Fig. 3a) and stimulation with FN did not augment significantly the level of this complex. On the other hand, the association of p125FAK with Zap‐70 was specific for cells stimulated with FN for 30 min (Fig. 3b) and this association persisted almost unchanged also at 45 min
Figure 2.
Induction of p125FAK tyrosine phosphorylation following adhesion to fibronectin Phosphorylation of p125FAK was examined in Jurkat cells adherent to plastic‐immobilized fibronectin (10 µg/ml) for 10 or 30 min or incubated onto BSA‐treated dishes for 30 min. Aliquots of cell lysate containing equivalent amounts of proteins as determined by the Lowry assay were used for immunoprecipitation. p125FAK was incubated with specific antibodies and immunocomplexes were attached to protein A; then, immunoprecipitated complexes were fractionated on a 10% polyacrylamide gel under reducing conditions and immunoblotted with an anti‐phosphotyrosine mAb.
Figure 3.
Coprecipitation of the p125FAK/Zap‐70 complex following adhesion to FN. (a) Association of FAK‐related members with Zap‐70 was examined in Jurkat cells plated for 30 min onto BSA‐ or FN‐coated (10 µg/ml) dishes. Cell lysates were incubated with the anti‐p125FAK antibodies and immunocomplexes were attached to protein A–Sepharose, fractionated on a 10% polyacrylamide gel, transferred to nitrocellulose and immunoblotted with an anti Zap‐70 antibody. (b) Association of p125FAK with Zap‐70 was examined in Jurkat cells plated for 30 min onto BSA‐ (10 µg/ml) and for 30 or 45 min onto FN‐coated (10 µg/ml) dishes. Cell lysates were incubated with anti‐p125FAK specific antibodies and immunocomplexes were processed as in (a). The samples contained equal protein amounts by protein assay and equal quantities of p125FAK by Western blotting.
In our T‐cell model p125FAK was tyrosine phosphorylated after a brief period of adhesion to FN and Zap‐70 was co‐immunoprecipitated with p125FAK. It has also been reported that p125FAK is slightly phosphorylated in BSA‐stimulated Jurkat and H9 cells but that its levels increase after adhesion to FN.25 Furthermore, in a different experimental system, a CD4+ clone stimulated with CD3 or the chemokine RANTES, p125FAK associated with two separate 70 000 MW proteins, paxillin and Zap‐70·31 In that case the trimolecular complex was preformed but the levels of tyrosine phosphorylation were markedly increased after stimulation with the chemokine. Although we could not detect Zap‐70 in our BSA‐stimulated cells, the phosphorylation and the association with p125FAK were readily detected after 10 min adhesion; these results are in agreement with the possibility that in the antigen‐independent activation of T‐cell adhesion to FN can lead to activation of Zap‐70. p125FAK was found to associate with non‐receptor kinases such as p59fyn and p60src32 via their SH2 binding domains. However, soluble fusion proteins containing the tandem SH2 domains of Zap‐70 did not interact with any other phosphoprotein in vitro except TCRζ and CD3ε.33,34 Therefore, a mechanism involving other elements such as paxillin will be required to explain the observed physical association between p125FAK and Zap‐70 (32 and present report).
In this study we investigated the signal transduction mechanisms induced by integrin‐mediated cell–ECM interactions in T lymphocytes. The overall results suggest that in antigen‐independent conditions the cross‐linking of both α4β1 and α5β1 integrins by immobilized FN induces a signalling cascade, not necessarily dependent upon both integrins, which involved the activation of a molecular complex between p125FAK and Zap‐70. Recognition of fibronectin is rather more complex than previously supposed: multiple binding sites to which α4β1 binds, not only the classic IIICS peptide, but III‐CS, RGD, and at least one synergistic site within the central cell binding domain have been described.35–37 Thus, discriminating between downstream effects mediated by each individual integrin is not easily attained using fragments of fibronectin (i.e. the central cell‐binding domain and the IIICS peptide to discriminate between binding to the α4β1 and α5β1 integrins), that have previously been considered ‘specific’ for either integrin. An alternative approach using solid phase cross‐linking of individual integrins with integrin‐specific mAbs has also been used to correlate activation of integrins with downstream events.24,25 However, regardless of the ‘artificial way’ of integrin activation, it can not avoid cross‐talks between the mAb‐activated integrin and other integrins expressed by the cell under study.
In conclusion, while apparently more unique signalling pathways are activated by cross‐linking α4β1 and α5β1 integrins with FN in T cells such as the PKC‐dependent modulation of the p50 and c‐Rel components of NF‐κB20 and the down‐regulation of GATA‐3 (unpublished), other signalling proteins are shared with several receptor‐mediated signalling pathways (p125FAK and Zap‐70). Therefore, the generalized T‐cell activation with enhanced cytokine gene expression and cytokine release along with induction of cell proliferation even in the absence of a specific antigen‐mediated activation38 detected during the course of inflammatory processes, could depend upon cross‐talks between quite different but converging receptor‐signalling pathways.
Acknowledgments
This work was supported by the Associazione Italiana Ricerca sul Cancro (C.P., A.C.), by the MURST Cofinanziamento 1997–99% (C.P, A.C.), by the ‘Fondo di Ricerca Interdipartimentale’ (C.P, A.C.) and CNR Target Project on Biotechnology (C.P.).
Glossary
Abbreviations
- ECM
extracellular matrix
- FN
fibronectin
References
- 1.Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120:577. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz MA, Schaller MD, Ginsberg MH. Integrins – emerging paradigms of signal‐transduction. Annu Rev Cell Dev Biol. 1995;11:549. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 4.Yamada KM, Geiger B. Molecular interactions in cell adhesion complexes. Curr Opin Cell Biol. 1997;9:76. doi: 10.1016/s0955-0674(97)80155-x. [DOI] [PubMed] [Google Scholar]
- 5.Nojima Y, Rothstein DM, Sugita K, Schlossman SF, Morimoto C. Ligation of VLA‐4 on T cells stimulates tyrosine phosphorylation of a 105‐kD protein. J Exp Med. 1992;175:1045. doi: 10.1084/jem.175.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kornberg LJ, Shelton Earp H, Turner CE, Prockop C, Juliano RL. Signal transduction by integrins: increased protein tyrosine phosphorilation caused by clustering of β1 integrins. Proc Natl Acad Sci USA. 1991;88:8392. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nojima Y, Tachibana K, Sato T, Schlossman SF, Morimoto C. Focal adhesion kinase (pp125FAK) is tyrosine phosphorylated after engagement of α4β1 and α5β1 integrins on human T‐lymphoblastic cells. Cell Immunol. 1995;16:8. doi: 10.1006/cimm.1995.1002. [DOI] [PubMed] [Google Scholar]
- 8.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds BA, Parsons JT. pp125FAK, a structurally distinctive protein‐tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA. 1992;89:5192. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanks SK, Calalb MB, Harper MCV, Patel SK. Focal adhesion protein‐tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci USA. 1992;89:8487. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlaepfer DD, Broome MA, Hunter T. Fibronectin‐stimulated signaling and focal adhesion kinase‐c‐src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997;17:1702. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dustin ML, Springer TA. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu Rev Immunol. 1991;145:59. doi: 10.1146/annurev.iy.09.040191.000331. [DOI] [PubMed] [Google Scholar]
- 12.Shimitzu Y, Van Sventer GA, Horgan KJ, Shaw S. Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA‐4 and VLA‐5 with fibronectin or VLA‐6 with laminin. J Immunol. 1990;145:59. [PubMed] [Google Scholar]
- 13.O'Rourke AM, Mescher MF. Cytotoxic T‐lymphocyte activation involves a cascade of signalling and adhesion events. Nature. 1992;358:25. doi: 10.1038/358253a0. [DOI] [PubMed] [Google Scholar]
- 14.Arpaia E, Shahar M, Dadi H, Cohen A, Roifman CM. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap‐70 kinase. Cell. 1994;76:947. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 15.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP‐70: a 70 kD protein‐tyrosine kinase that associates with the TCR z chain. Cell. 1994;71:649. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 16.Chan AC, Kadlecek TA, Elder ME, et al. ZAP‐70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264:1599. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- 17.Elder ME, Lin D, Clever J, et al. Human severe combined immunodeficiency due to a defect in zap‐70, a T cell tyrosine kinase. Science. 1994;264:1596. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 18.Qian D, Mollenauer MN, Weiss A. Dominant‐negative zeta‐associated protein 70 inhibits T cell antigen receptor signaling. J Exp Med. 1996;183:611. doi: 10.1084/jem.183.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelfand EW, Weinberg K, Mazer BD, Kadlecek TA, Weiss A. Absence of zap‐70 prevents signaling through the antigen receptor on peripheral blood T cells but not on thymocytes. J Exp Med. 1995;182:1057. doi: 10.1084/jem.182.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bearz A, Tell G, Colombatti A, Formisano S, Pucillo C. Fibronectin binding promotes a PKC‐dependent modulation of NF‐KB in human T cells. Biochem Biophys Res Comm. 1998;243:732. doi: 10.1006/bbrc.1997.8017. [DOI] [PubMed] [Google Scholar]
- 21.Vuento M, Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under nondenaturing conditions. Biochem J. 1979;183:331. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanner SB, Aruffo A, Chan P‐Y. Lymphocyte antigen receptor activation of a focal adhesion kinase‐related tyrosine kinase substrate. Proc Natl Acad Sci USA. 1994;91:10484. doi: 10.1073/pnas.91.22.10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szabo MC, Teague TK, McIntyre BW. Regulation of lymphocyte pseudopodia formation by triggering the integrin α4/β1. J Immunol. 1995;154:2112. [PubMed] [Google Scholar]
- 24.Maguire JE, Danahey KM, Burkly LC, Van Seventer GA. T cell receptor‐ and β1 integrin‐mediated signals synergize to induce phosphorylation of focal achesion kinase (pp. 125FAK) in human T cells. J Exp Med. 1995;182:2079. doi: 10.1084/jem.182.6.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato T, Tachibana K, Nojima Y, D'Avirro N, Morimoto C. Role of the VLA‐4 molecule in T cell costimulation. Identification of the tyrosine phosphorylation pattern induced by the ligation of VLA‐4. J Immunol. 1995;155:2938. [PubMed] [Google Scholar]
- 26.Rabinowich H, Manciules M, Heberman RB, Whiteside TL. β1 integrin‐mediated activation of focal adhesion kinase and its association with Fyn and Zap‐70 in human NK cells. J Immunol. 1996;157:3860. [PubMed] [Google Scholar]
- 27.Astier A, Avraham H, Manie SN, et al. The related adhesion focal tyrosine kinase is tyrosine‐phosphorylated after β1‐integrin stimulation in B cells and binds to p130cas. J Biol Chem. 1997;272:228. doi: 10.1074/jbc.272.1.228. [DOI] [PubMed] [Google Scholar]
- 28.Ma EA, Lou O, Berg NN, Ostergaard HL. Cytotoxic T lymphocytes express a b3 integrin which can induce the phosphorylation of focal adhesion kinase and the related PYK‐2. Eur J Immunol. 1997;27:329. doi: 10.1002/eji.1830270147. [DOI] [PubMed] [Google Scholar]
- 29.Kanner SB. Focal adhesion kinase‐related fakB is regulated by the integrin LFA‐1 and interacts with the SH3 domain of phospholipase Cγ1. Cell Immunol. 1996;171:164. doi: 10.1006/cimm.1996.0188. [DOI] [PubMed] [Google Scholar]
- 30.Brockdorf J, Kanner SB, Nielsen M, et al. Interleukin‐2 induces β2‐integrin‐dependent signal transduction involving the focal adhesion kinase‐related protein B (fakB) Proc Natl Acad Sci USA. 1998;95:6959. doi: 10.1073/pnas.95.12.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bacon KB, Szabo MC, Yssel H, Bolen JB, Schall TJ. RANTES induces tyrosine kinase activity of stably complexed p125FAK and ZAP‐70 in human T cells. J Exp Med. 1996;184:873. doi: 10.1084/jem.184.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp. 125FAK, directs SH2‐dependent binding of pp60 src. Mol Cell Biol. 1994;14:1680. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wange RL, Malek SN, Desiderio S, Samelson LE. Tandem SH2 domains of ZAP‐70 bind to T cell antigen receptor ζ and CD3ε from activated Jurkat T cells. J Biol Chem. 1993;268:19 797. [PubMed] [Google Scholar]
- 34.Yamasaki S, Takamatsu M, Iwashima M. The kinase, SH3, and SH2 domains of Lck play critical roles in T‐cell activation after zap‐70 membrane localization. Mol Cell Biol. 1996;16:7151.. doi: 10.1128/mcb.16.12.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin Z, Giacomello E, Gabriele E, et al. Cooperative activity of α4β1 and α4β7 integrins in mediating human B‐cell lymphoma adhesion and chemotaxis on fibronectin through recognition of multiple synergizing sites within the central cell‐binding domain. Blood. 1999;93:1221. [PubMed] [Google Scholar]
- 36.Dominiguez‐jimenez C, Sanchez‐aparicio P, Albar JP, Garcia‐pardo A. The α4β1 fibronectin ligands CS‐1, Hep II, and RGD induce different intracellular events in B lymphoid cells. Comparison with the effects to the endothelial ligand VCAM‐1. Cell Adhes Commun. 1996;4:251. doi: 10.3109/15419069609010770. [DOI] [PubMed] [Google Scholar]
- 37.Chi‐rosso G, Gotwals PJ, Yang J, et al. Fibronectin type III repeats mediate RGD‐independent adhesion and signalling through activated β1 integrins. J Biol Chem. 1997;272:31447. doi: 10.1074/jbc.272.50.31447. [DOI] [PubMed] [Google Scholar]
- 38.Sturmhofel K, Brando C, Martinon F, Shevach EM, Coligan JE. Antigen‐independent, integrin‐mediated T cell activation. J Immunol. 1995;154:2104. [PubMed] [Google Scholar]